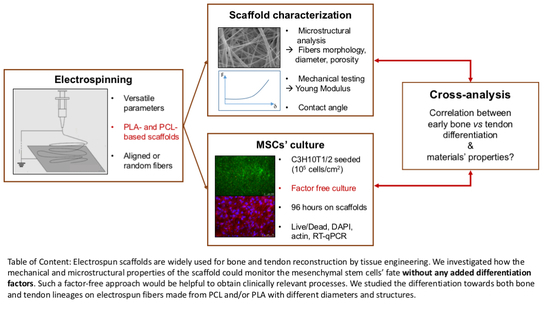
The Osteogenic and Tenogenic Differentiation Potential of C3H10T1/2 (Mesenchymal Stem Cell Model) Cultured on PCL/PLA Electrospun Scaffolds in the Absence of Specific Differentiation Medium
Abstract
:1. Introduction
2. Results
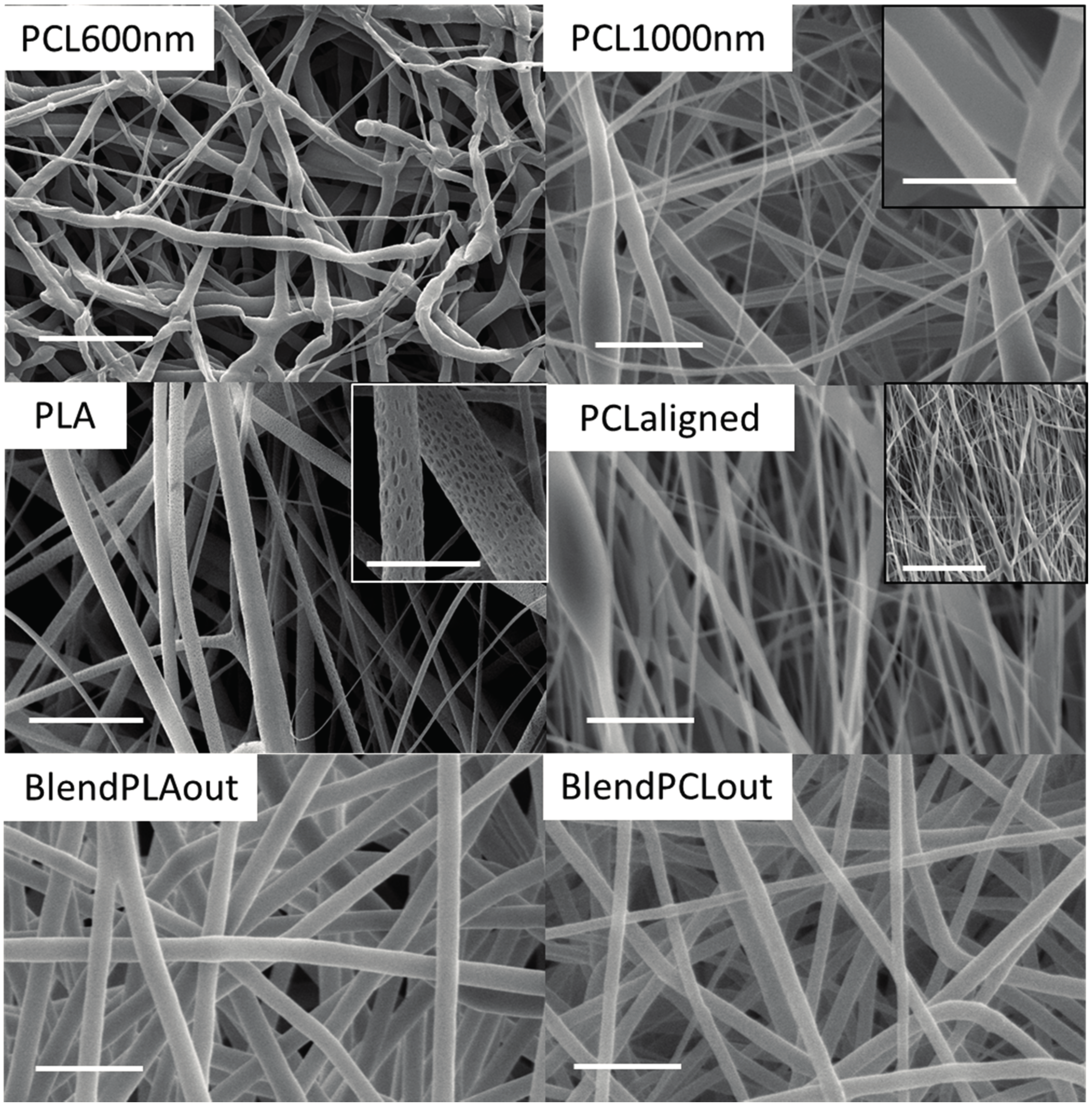
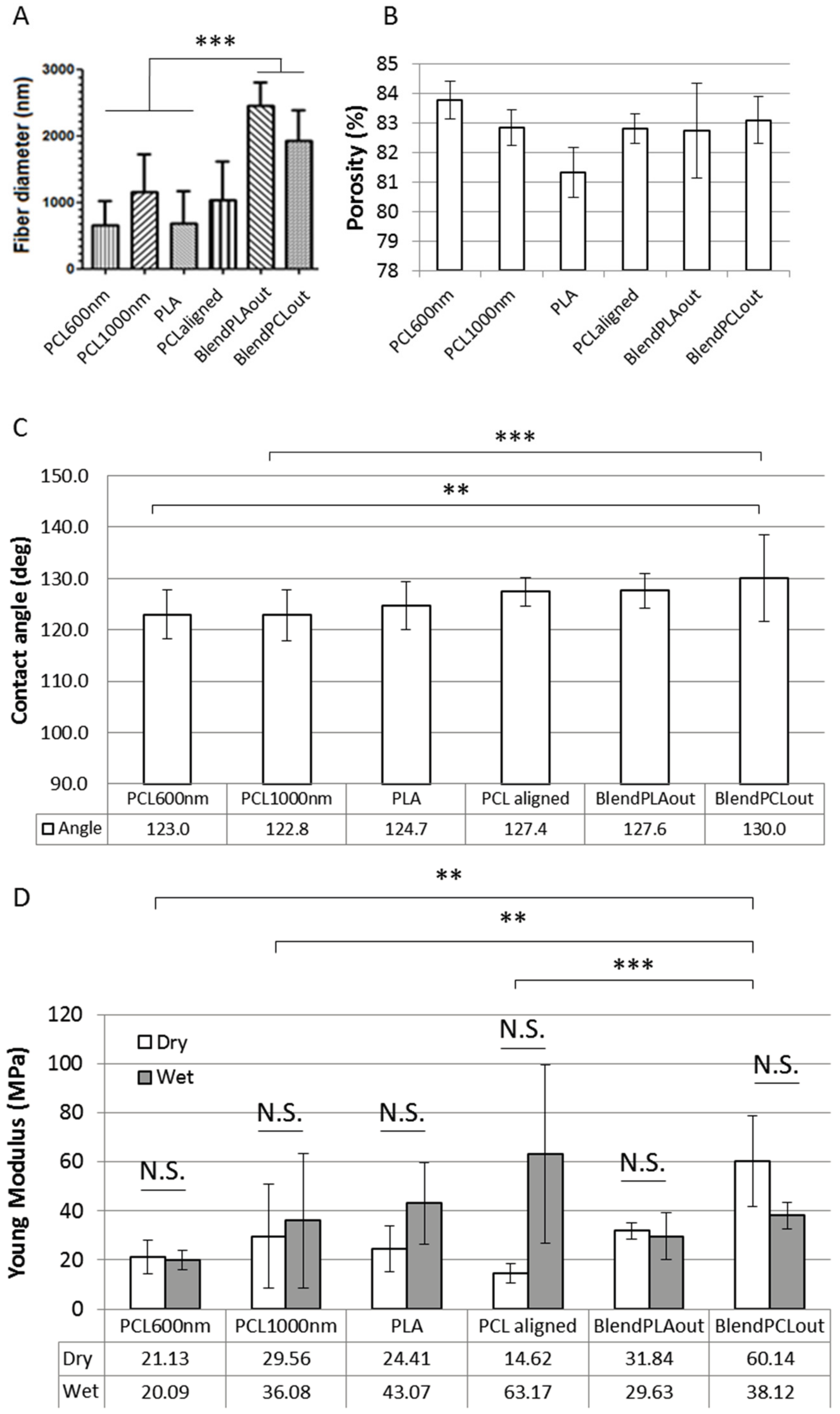
2.1. Electrospun Fiber Morphology
2.2. Surface Characterization
2.3. Mechanical Characterization of Electrospun Fibers
2.4. Cell—Scaffold Interactions
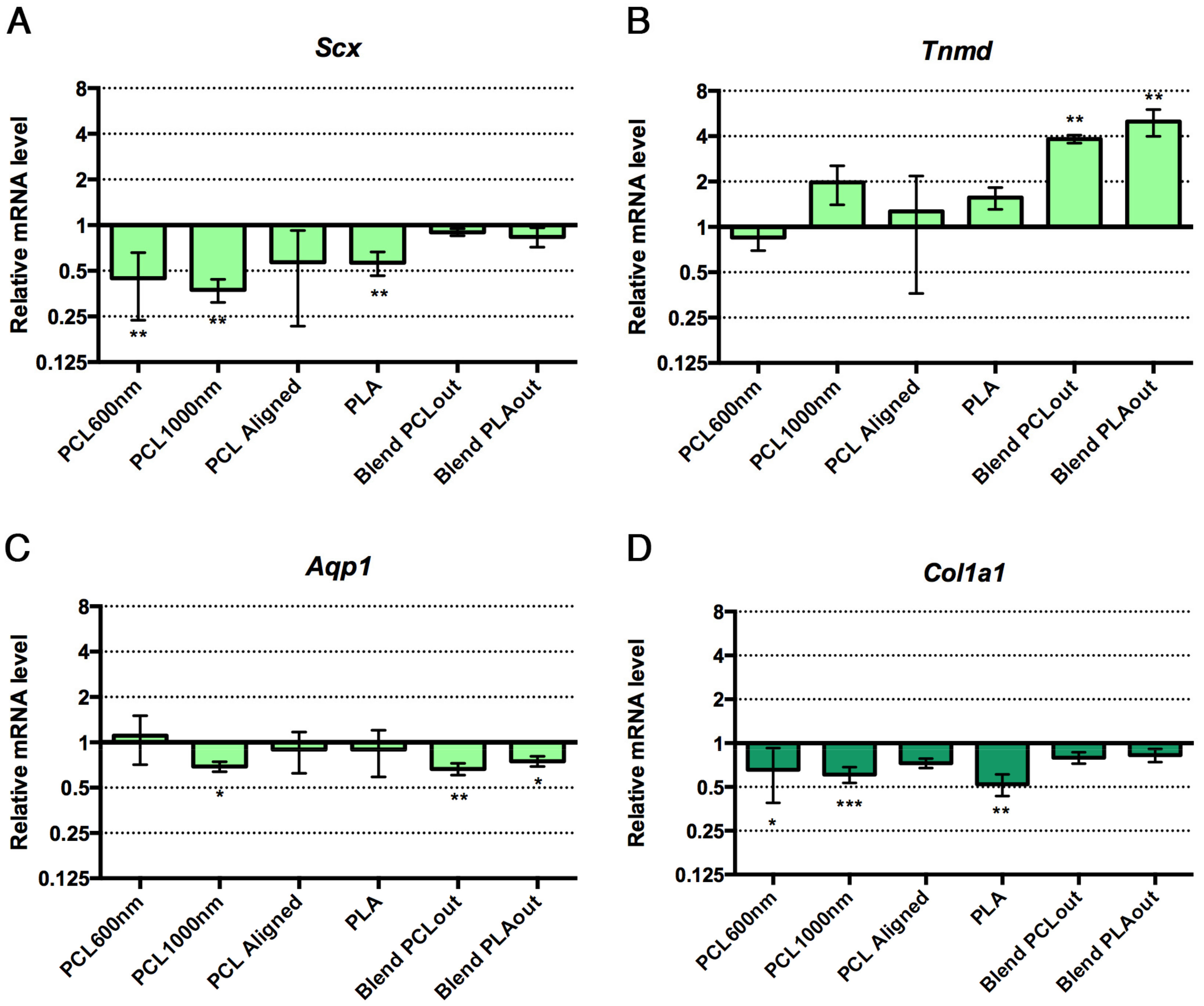
2.5. Cell Differentiation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Electrospinning Process
4.3. Morphological Characterization of the Scaffolds
4.4. Mechanical Characterization of the Scaffolds
4.5. Wettability
4.6. Cell Cultures on Electrospun Scaffolds
4.7. Cell—Material Interactions
4.8. Cell Viability
4.9. Gene Expression Analysis
4.10. Statistic Tests
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Szentivanyi, A.L.; Zernetsch, H.; Menzel, H.; Glasmacher, B. A review of developments in electrospinning technology: New opportunities for the design of artificial tissue structures. Int. J. Artif. Organs 2011, 34, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Katti, D.S.; Robinson, K.W.; Ko, F.K.; Laurencin, C.T. Bioresorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parameters. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 70, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.H.; Wang, K.; Yong, T.; Ramakrishna, S. Polymeric Nanofibers in Tissue Engineering. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 3527313893. [Google Scholar]
- Li, W.; Shanti, R.M.; Tuan, R.S. Electrospinning Technology for Nanofibrous Scaffolds in Tissue Engineering. Nanotechnol. Life Sci. 2006, 9, 135–187. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Saunders, R.L.; Hammer, D.A.; Burdick, J.A. Fiber alignment directs cell motility over chemotactic gradients. Biotechnol. Bioeng. 2013, 110, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hu, K.; Wei, Z. Comparison of cell behavior on pva / pva-gelatin electrospun nanofibers with random and aligned configuration. Sci. Rep. 2016, 6, 37960. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.; Arinzeh, T.L. Evaluating apatite formation and osteogenic activity of electrospun composites for bone tissue engineering. Biotechnol. Bioeng. 2014, 111, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Nanotechnology and Tissue Engineering: The Scaffold Based Approach. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 3527313893. [Google Scholar]
- Cardwell, R.D.; Kluge, J.A.; Thayer, P.A.; Guelcher, S.A.; Dahlgren, L.A.; Kaplan, D.L.; Goldstein, A.S. Static and Cyclic Mechanical Loading of Mesenchymal Stem Cells on Elastomeric, Electrospun Polyurethane Meshes. J. Biomech. Eng. 2015, 137, 71010. [Google Scholar] [CrossRef] [PubMed]
- Full, S.M.; Delman, C.; Gluck, J.M.; Abdmaulen, R.; Shemin, R.J.; Heydarkhan-Hagvall, S. Effect of fiber orientation of collagen-based electrospun meshes on human fibroblasts for ligament tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chainani, A.; Hippensteel, K.J.; Kishan, A.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. Part A 2013, 19, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Fu, Y.-C.; Nie, H.; Ho, M.-L.; Wang, C.-K.; Wang, C.-H. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol. Bioeng. 2008, 99, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Bonani, W.; Maniglio, D.; Motta, A.; Tan, W.; Migliaresi, C. Biohybrid nanofiber constructs with anisotropic biomechanical properties. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shi, B.; Ding, J.; Zhuang, X.; Zhang, X.; Fu, C.; Chen, X. A comparative study of preventing postoperative tendon adhesion using electrospun polyester membranes with different degradation kinetics. Sci. China Chem. 2015, 58, 1159–1168. [Google Scholar] [CrossRef]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: In vitro optimization studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Ladd, M.R.; Lee, S.J.; Stitzel, J.D.; Atala, A.; Yoo, J.J. Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering. Biomaterials 2011, 32, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult Mesenchymal Stem Cells for Tissue Engineering versus Regenerative Medicine. J. Cell. Physiol. 2007, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Guerquin, M.J.; Charvet, B.; Nourissat, G.; Havis, E.; Ronsin, O.; Bonnin, M.A.; Ruggiu, M.; Olivera-Martinez, I.; Robert, N.; Lu, Y.; et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J. Clin. Investig. 2013, 123, 3564–3576. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.J. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin. Orthop. Relat. Res. 2013, 471, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The Role of Growth Factors in the Repair of Bone. J. Bone Jt. Surg. 2006, 84, 1032–1044. [Google Scholar] [CrossRef]
- Cooper, J.O.; Bumgardner, J.D.; Cole, J.A.; Smith, R.A.; Haggard, W.O. Co-cultured tissue-specific scaffolds for tendon/bone interface engineering. J. Tissue Eng. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Reznikoff, C.A.; Brankow, D.W.; Heidelberger, C. Establishment and Characterization of a Cloned Line of C3H Mouse Embryo Cells Sensitive to Postconfluence Inhibition of Division. Cancer Res. 1973, 33, 3231–3239. [Google Scholar] [PubMed]
- Aubin, J.E. Bone stem cells. J. Cell. Biochem. Suppl. 1998, 30, 73–82. [Google Scholar] [CrossRef]
- Havis, E.; Bonnin, M.-A.; Olivera-Martinez, I.; Nazaret, N.; Ruggiu, M.; Weibel, J.; Durand, C.; Guerquin, M.-J.; Bonod-Bidaud, C.; Ruggiero, F.; et al. Transcriptomic analysis of mouse limb tendon cells during development. Development 2014, 141, 3683–3696. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Song, H.; Hu, J.; Tang, Q.; Zhu, T.; Shen, W.; Chen, J.; Liu, H.; Heng, B.C.; et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials 2015, 44, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Keun Kwon, I.; Kidoaki, S.; Matsuda, T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: Structural characteristics, mechanical properties and cell adhesion potential. Biomaterials 2005, 26, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Bigerelle, M.; Giljean, S.; Anselme, K. Existence of a typical threshold in the response of human mesenchymal stem cells to a peak and valley topography. Acta Biomater. 2011, 7, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Yoshimoto, H.; Vacanti, J.P. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004, 10, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Nanobioengineered electrospun composite nanofibers and osteoblasts for bone regeneration. Artif. Organs 2008, 32, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tae, G.; Kim, Y.H.; Park, I.S.; Kim, S.-H.; Kim, S.H. The effect of gelatin incorporation into electrospun poly(l-lactide-co-ɛ-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials 2008, 29, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Semnani, D.; Morshed, M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J. Appl. Polym. Sci. 2007, 106, 2536–2542. [Google Scholar] [CrossRef]
- Asran, A.S.; Razghandi, K.; Aggarwal, N.; Michler, G.H.; Groth, T. Nanofibers from Blends of Polyvinyl Alcohol and Polyhydroxy Butyrate as Potential Scaffold Material for Tissue Engineering of Skin. Biomacromolecules 2010, 11, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadehmoghadam, S.; Dong, Y.; Barbhuiya, S.; Guo, L.; Liu, D.; Umer, R.; Qi, X.; Tang, Y. Electrospinning: Current Status and Future Trends. In Nano-Size Polymers; Springer: Cham, Switzerland, 2016; pp. 89–154. [Google Scholar]
- Elamparithi, A.; Punnoose, A.M.; Paul, S.F.D.; Kuruvilla, S. Gelatin electrospun nanofibrous matrices for cardiac tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 20–27. [Google Scholar] [CrossRef]
- Lin, S.T.; Kimble, L.; Bhattacharyya, D. Polymer Blends and Composites for Biomedical Applications. In Biomaterials for Implants and Scaffolds; Springer: Berlin/Heidelberg, Germany, 2017; pp. 195–235. [Google Scholar]
- Semnani, D.; Naghashzargar, E.; Hadjianfar, M.; Dehghan Manshadi, F.; Mohammadi, S.; Karbasi, S.; Effaty, F. Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 149–157. [Google Scholar] [CrossRef]
- Bedoui, F.; Widjaja, L.K.; Luk, A.; Bolikal, D.; Murthy, N.S.; Kohn, J. Anomalous increase in modulus upon hydration in random copolymers with hydrophobic segments and hydrophilic blocks. Soft Matter 2012, 8, 2230. [Google Scholar] [CrossRef]
- Wanasekara, N.; Chen, M.; Chalivendra, V.; Bhowmick, S. Investigation of the Young’s Modulus of Fibers in an Electrospun PCL Scaffold Using AFM and its Correlation to cell Attachment. In MEMS and Nanotechnology; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-8824-9. [Google Scholar]
- Robledo, R.F.; Rajan, L.; Li, X.; Lufkin, T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002, 16, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Szpalski, C.; Wetterau, M.; Barr, J.; Warren, S.M. Bone tissue engineering: Current strategies and techniques—Part I: Scaffolds. Tissue Eng. Part B Rev. 2012, 18, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Bashur, C.A.; Shaffer, R.D.; Dahlgren, L.A.; Guelcher, S.A.; Goldstein, A.S. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng. Part A 2009, 15, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar] [PubMed]
- Kim, B.S.; Kim, E.J.; Choi, J.S.; Jeong, J.H.; Jo, C.H.; Cho, Y.W. Human collagen-based multilayer scaffolds for tendon-to-bone interface tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4044–4054. [Google Scholar] [CrossRef] [PubMed]
- Seidi, A.; Ramalingam, M.; Elloumi-Hannachi, I.; Ostrovidov, S.; Khademhosseini, A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011, 7, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Yang, F.; Cheng, X.; Frank Walboomers, X.; Jansen, J.A. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur. Cells Mater. 2010, 19, 180–192. [Google Scholar] [CrossRef]
- Repanas, A.; Wolkers, W.F.; Gryshkov, O.; Kalozoumis, P.; Mueller, M.; Zernetsch, H.; Korossis, S.; Glasmacher, B. Coaxial Electrospinning as a Process to Engineer Biodegradable Polymeric Scaffolds as Drug Delivery Systems for Anti-Inflammatory and Anti- Thrombotic Pharmaceutical Agents. Clin. Exp. Pharmacol. 2015, 5, 192. [Google Scholar] [CrossRef]
- Wang, D.; Christensen, K.; Chawla, K.; Xiao, G.; Krebsbach, P.H.; Franceschi, R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999, 14, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-Z.; Yang, W.; Yang, F.; Kersten-Niessen, M.; Jansen, J.A.; Both, S.K. Effects of Continuous Passaging on Mineralization of MC3T3-E1 Cells with Improved Osteogenic Culture Protocol. Tissue Eng. Part C Methods 2014, 20, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ye, X.; Yu, X.; Xu, Q.; Pan, K.; Lu, S.; Yang, P. Co-culture with periodontal ligament stem cells enhanced osteoblastic differentiation of MC3T3-E1 cells and osteoclastic differentiation of RAW264.7 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 14596–14607. [Google Scholar] [PubMed]
- Cardwell, R.D.; Dahlgren, L.A.; Goldstein, A.S. Electrospun fibre diameter, not alignment, affects mesenchymal stem cell differentiation into the tendon/ligament lineage. J. Tissue Eng. Regen. Med. 2014, 8, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Mashinchian, O.; Turner, A.; Matthew, J. Regulation of stem cell fate by nanomaterial substrates. Nanomedicine 2015, 10, 829–847. [Google Scholar] [CrossRef] [PubMed]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Faia-Torres, A.B.; Charnley, M.; Goren, T.; Guimond-Lischer, S.; Rottmar, M.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Osteogenic differentiation of human mesenchymal stem cells in the absence of osteogenic supplements: A surface-roughness gradient study. Acta Biomater. 2015, 28, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, C.R.; Coulais, C.; Namboothiry, M.; Carroll, D.L.; Hantgan, R.R.; Guthold, M. The mechanical properties of individual, electrospun fibrinogen fibers. Biomaterials 2009, 30, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Rajaraman, R.; Rounds, D.E.; Yen, S.P.S.; Rembaum, A. A scanning electron microscope study of cell adhesion and spreading in vitro. Exp. Cell Res. 1974, 88, 327–339. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
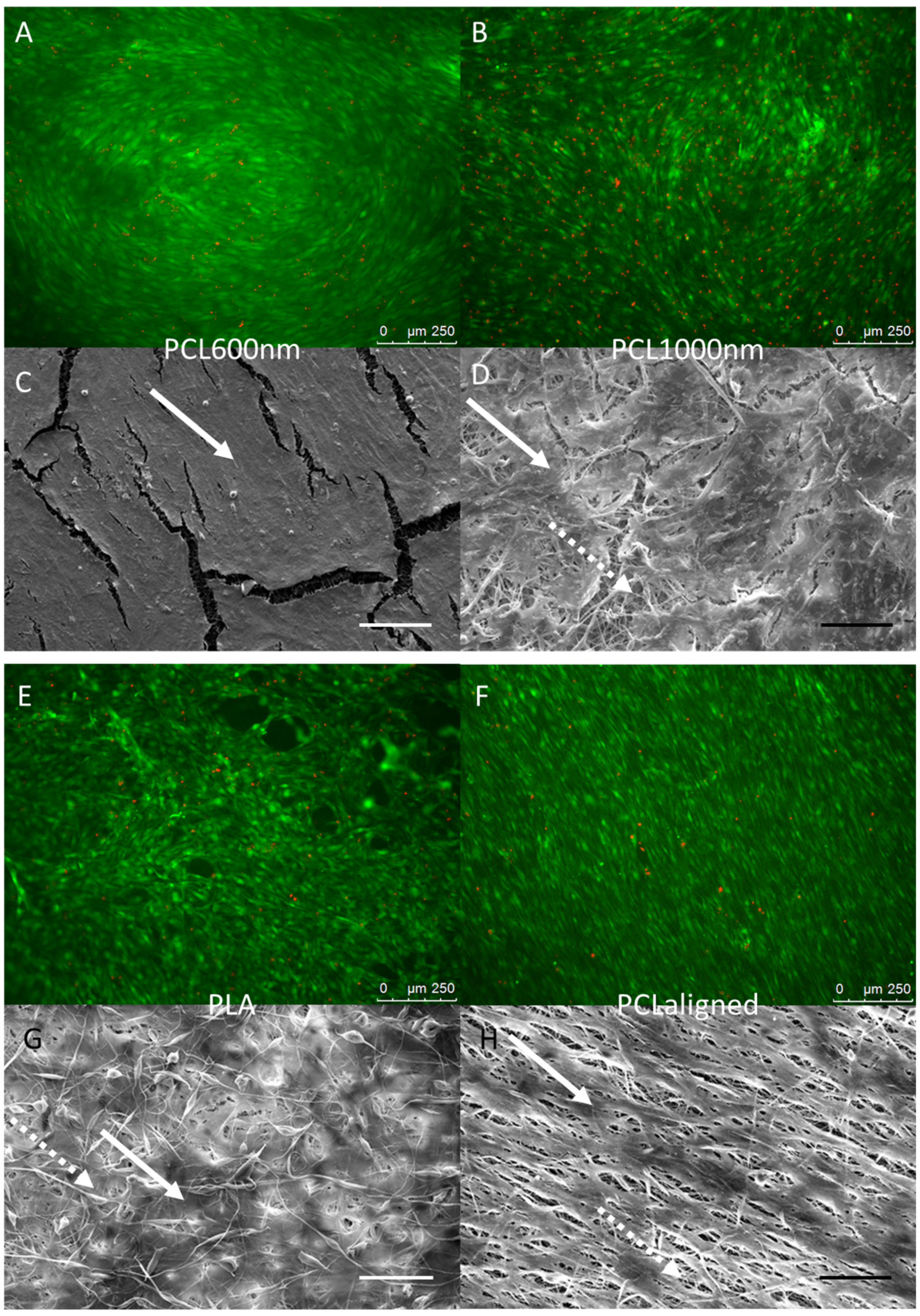
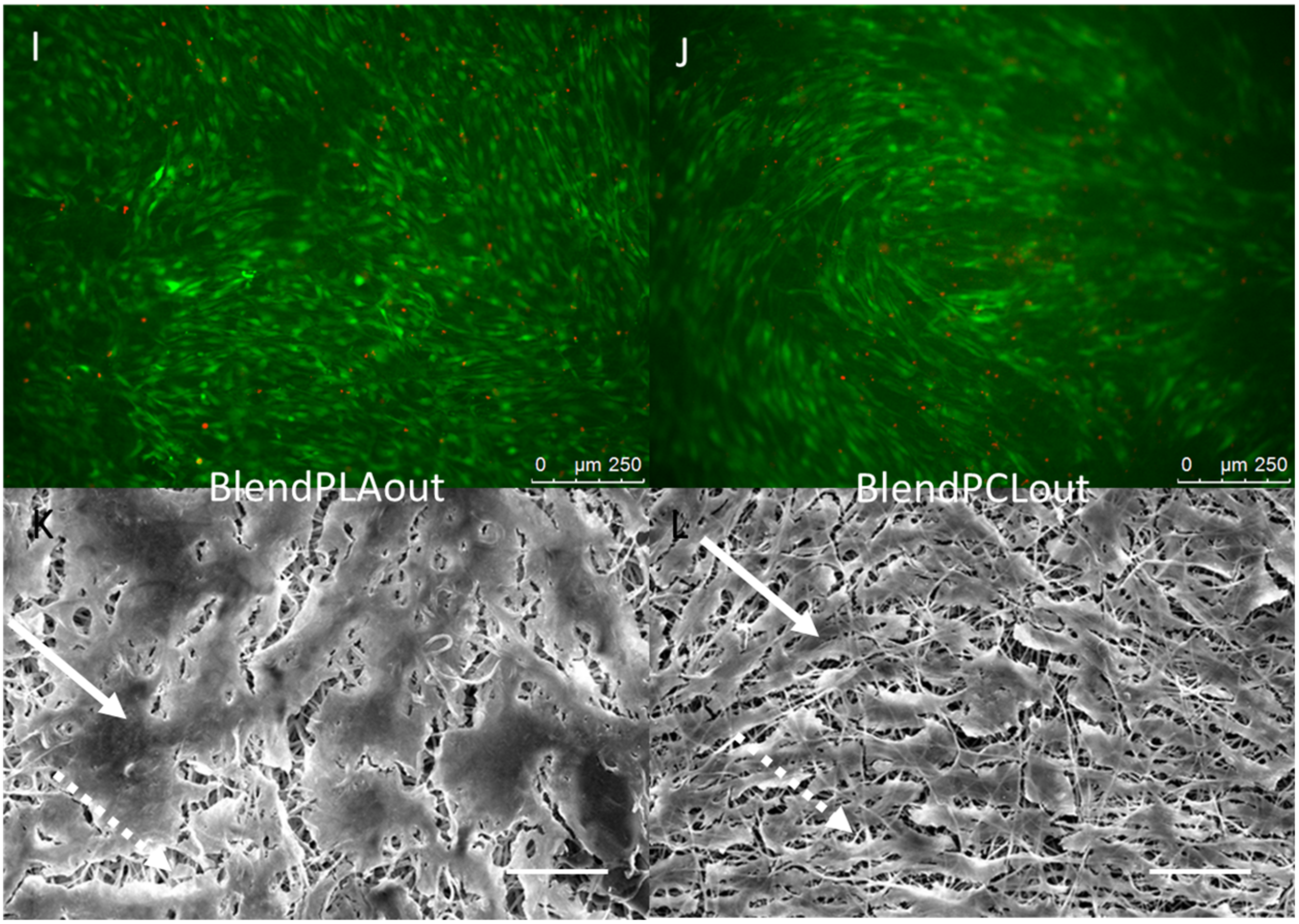


| Scaffold | Abbreviation | Polymer | Concentration | Solvent | Flow | Speed |
|---|---|---|---|---|---|---|
| Rate | ||||||
| Pure polycaprolactone | PCL600nm | PCL | 15 wt/wt % | 60 wt % DCM 40 wt % DMF | 16,6 µL/min | / |
| Random PCL | PCL1000nm | PCL | 100 mg/mL | TFE | 50 µL/min * | 250 rpm |
| Pure polylactic acid | PLA | PLA | 10 wt/wt % | 70% DCM 30% acetone | 67 µL/min | 50 rpm |
| Aligned PCL | PCLaligned | PCL | 100 mg/mL | TFE | 50 µL/min * | 1000 rpm |
| PCL/PLA coaxial blend (PCL outside) | BlendPCLout | PCL (133 mg/mL) & PLA (66 mg/mL) | TFE | 50 µL/min * | 250 rpm | |
| PCL/PLA coaxial blend (PLA outside) | BlendPLAout | TFE | 50 µL/min * | |||
| Scaffold | Contact Angle (°) | Young’s Modulus (MPa) | Fiber Diameter (nm) | Porosity µ + δ (%) | Cell Response | ||
|---|---|---|---|---|---|---|---|
| Dry | Wet | Spreading | Differentiation | ||||
| PCL600nm | 123 | 21 | 20 | 665 | 83.8 | +++ | Bone |
| PCL1000nm | 123 | 30 | 36 | 1159 | 82.8 | ++ | Bone |
| PLA | 124.7 | 24 | 43 | 681 | 81.3 | + | Unclear |
| PCLaligned | 127 | 15 | 63 | 1032 | 82.8 | ++ Alignment | Bone |
| BlendPLAout | 128 | 32 | 30 | 2461 | 82.7 | ++ | Tendon |
| BlendPCLou | 130 | 60 | 38 | 1928 | 83.1 | ++ | Tendon |
| Gene Symbol | Gene Name | NCBI Reference Sequence | Primer Sequences |
|---|---|---|---|
| Aqp1 | Aquaporin1 | NM_007472.2 | Fwd 5′-CAATTCACTTGGCCGCAATGACCT-3′ Rev 5′-TACCAGCTGCAGAGTGCCAATGAT-3′ |
| Bglap | Bone gamma carboxyglutamate protein/Osteocalcin | NM_007541.3 | Fwd 5′-CAGCGGCCCTGAGTCTGA-3′ Rev 5′-TTATTGCCCTCCTGCTTGGA-3′ |
| Col1a1 | Collagen 1 alpha 1 | NM_007742.4 | Fwd 5′-TGGAGAGAGCATGACCGATG-3′ Rev 5′-GAGCCCTCGCTTCCGTACT-3′ |
| Dlx5 | Distal-less homeobox 5 | NM_010056.3 | Fwd 5′-CGTCTCAGGAATCGCCAACT-3′ Rev5′-AGTCAGAATCGGTGGCCG-3′ |
| Rplp0 | Ribosomal protein, large, P0/36B4 | NM_007475.5 | Fwd 5′-ACCTCCTTCTTCCAGGCTTT-3′ Rev 5′-CTCCCACCTTGTCTCCAGTC-3′ |
| Runx2 | Runt related transcription factor 2 | NM_001271627.1 | Fwd 5′-GGTCCCCGGGAACCAA-3′ Rev 5′-GGCGATCAGAGAACAAACTAGGTTT-3′ |
| Scx | Scleraxis | NM_198885.3 | Fwd 5′-CCTTCTGCCTCAGCAACCAG-3′ Rev 5′-GGTCCAAAGTGGGGCTCTCCGTGACT-3′ |
| Tnmd | Tenomodulin | NM_022322.2 | Fwd 5′-AACACTTCTGGCCCGAGGTAT-3′ Rev 5′-AAGTGTGCTCCATGTCATAGGTTTT-3′ |
| Rn18s | 18S ribosomal RNA | NR_003278.3 | Fwd 5′-GGCGACGACCCATTCG-3′ Rev 5′-ACCCGTGGTCACCATGGTA-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudequin, T.; Gaut, L.; Mueller, M.; Huepkes, A.; Glasmacher, B.; Duprez, D.; Bedoui, F.; Legallais, C. The Osteogenic and Tenogenic Differentiation Potential of C3H10T1/2 (Mesenchymal Stem Cell Model) Cultured on PCL/PLA Electrospun Scaffolds in the Absence of Specific Differentiation Medium. Materials 2017, 10, 1387. https://doi.org/10.3390/ma10121387
Baudequin T, Gaut L, Mueller M, Huepkes A, Glasmacher B, Duprez D, Bedoui F, Legallais C. The Osteogenic and Tenogenic Differentiation Potential of C3H10T1/2 (Mesenchymal Stem Cell Model) Cultured on PCL/PLA Electrospun Scaffolds in the Absence of Specific Differentiation Medium. Materials. 2017; 10(12):1387. https://doi.org/10.3390/ma10121387
Chicago/Turabian StyleBaudequin, Timothée, Ludovic Gaut, Marc Mueller, Angela Huepkes, Birgit Glasmacher, Delphine Duprez, Fahmi Bedoui, and Cécile Legallais. 2017. "The Osteogenic and Tenogenic Differentiation Potential of C3H10T1/2 (Mesenchymal Stem Cell Model) Cultured on PCL/PLA Electrospun Scaffolds in the Absence of Specific Differentiation Medium" Materials 10, no. 12: 1387. https://doi.org/10.3390/ma10121387