Novel Development of Phosphate Treated Porous Hydroxyapatite
Abstract
:1. Introduction
2. Results
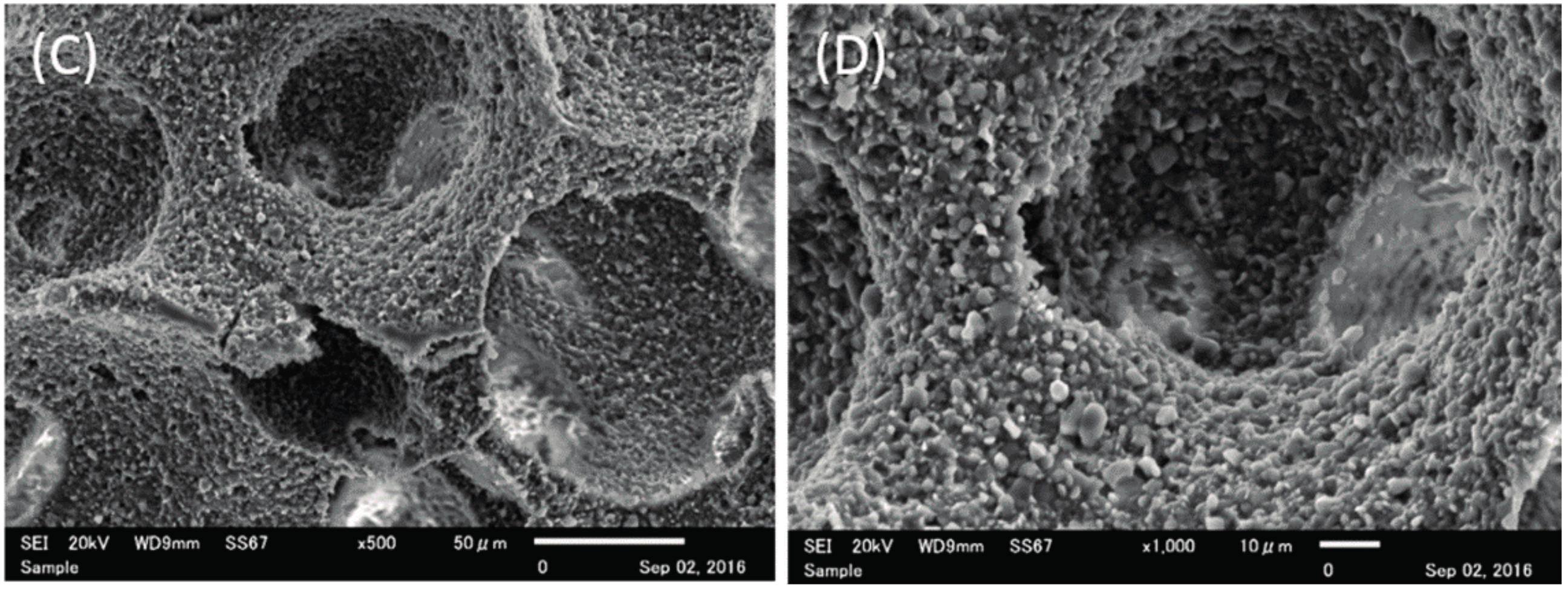
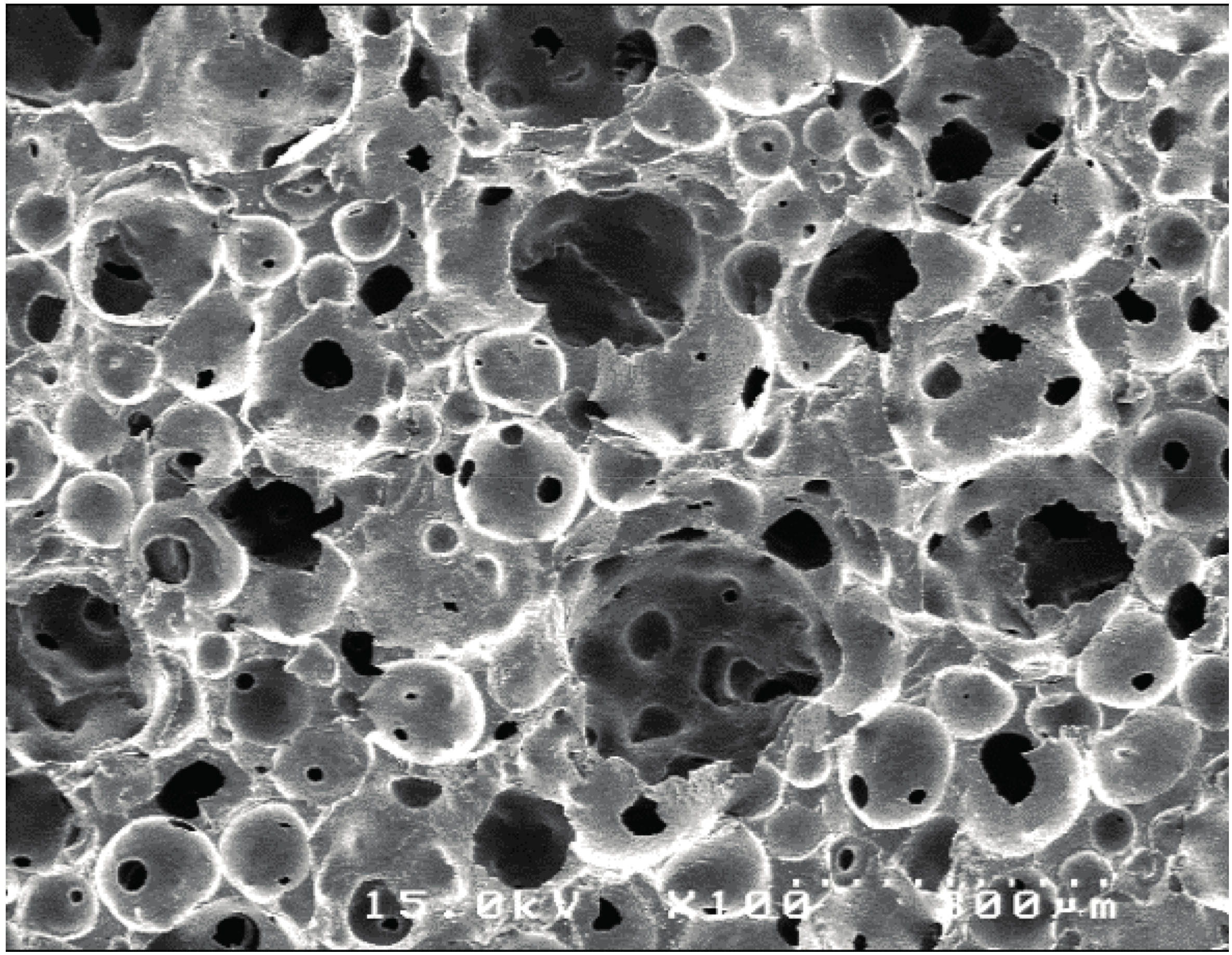
2.1. Structure Characteristics Properties of Porous Hydroxyapatite (HA) and Phosphate Treated HA
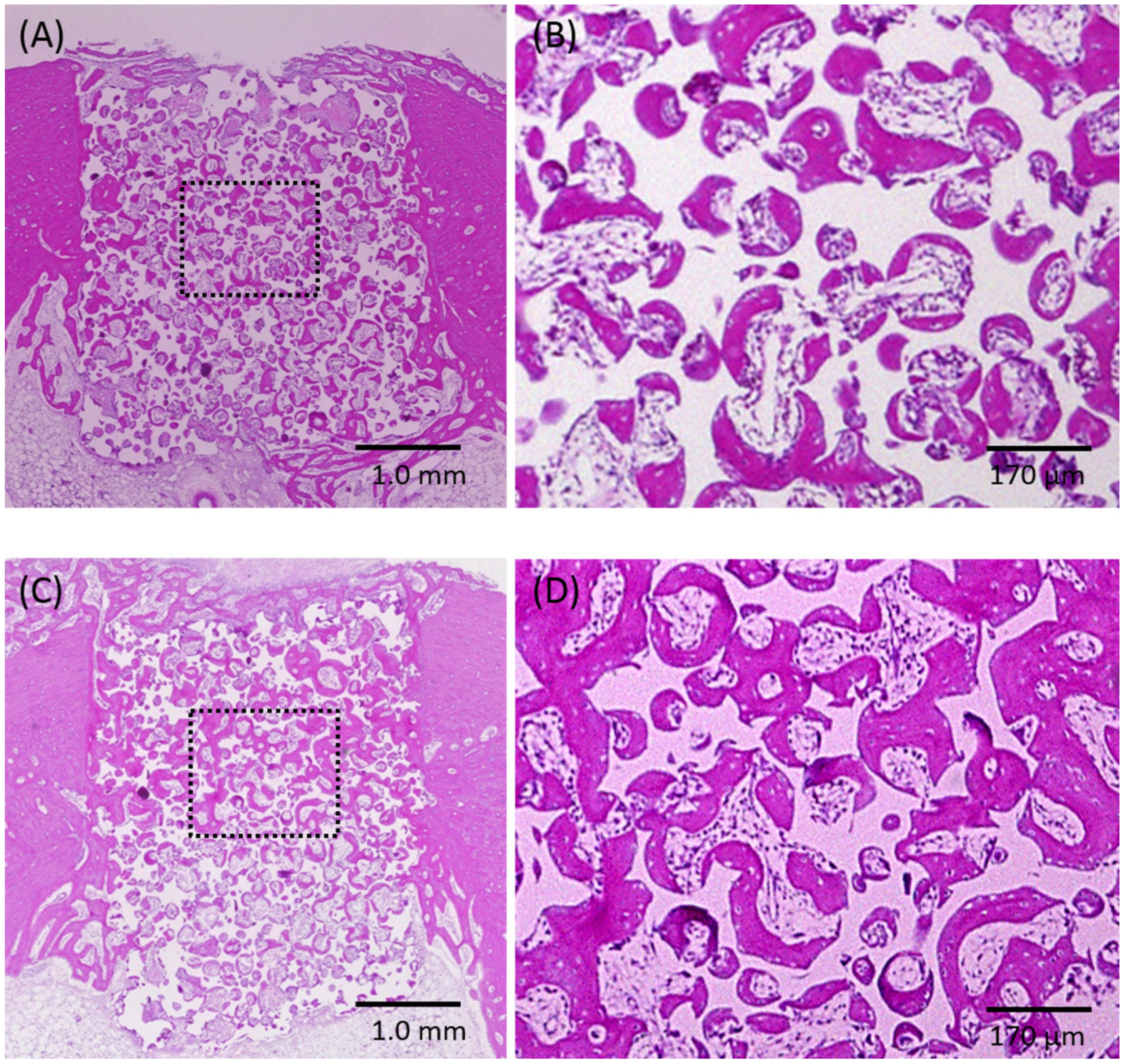
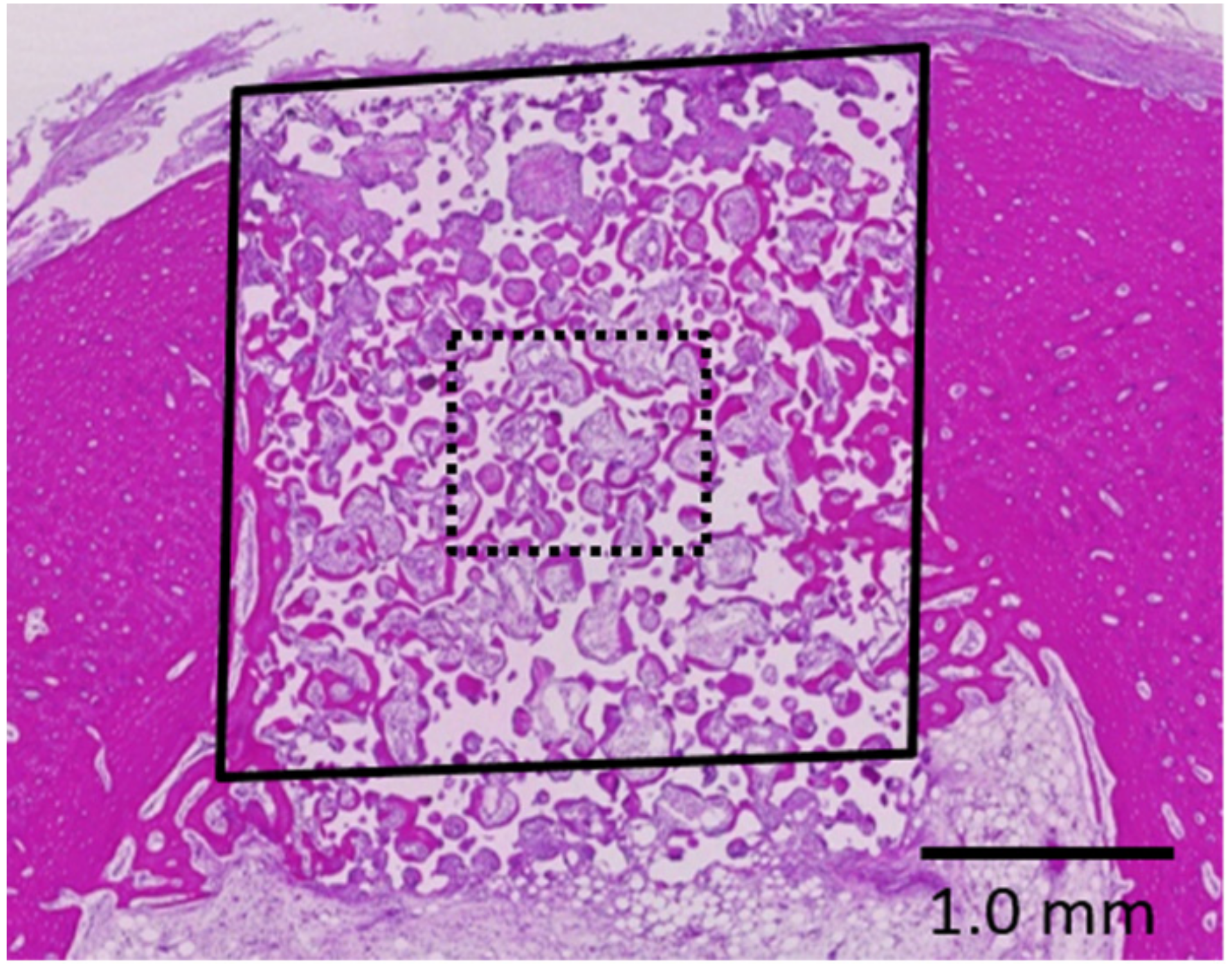
2.2. Histological Observations and Histomorphometric Analyses
3. Discussion
4. Methods
4.1. Fabrication of Phosphoric Acid-Treated Porous HA
4.2. Structure Characterization
4.2.1. Scanning Electron Microscope (SEM) Observation
4.2.2. Porosity Measurements
4.2.3. Measurement of Mechanical Strength
4.3. Evaluation of Bone Formation In Vivo
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. 2002, 59, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Doi, K.; Hayashi, K.; Morita, K.; Matsuura, A.; Teixeira, E.R.; Akagawa, Y. Comparative evaluation of bone regeneration using spherical and irregularly shaped granules of interconnected porous hydroxylapatite. A beagle dog study. J. Prosthodont. Res. 2011, 55, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Kubo, T.; Makihara, Y.; Oue, H.; Morita, K.; Oki, Y.; Kajihara, S.; Tsuga, K. Osseointegration aspects of placed implant in bone reconstruction with newly developed block-type interconnected porous calcium hydroxyapatite. J. Appl. Oral Sci. 2016, 24, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.; Li, J.; Wilson, M.E.; Rogers, T.; Close, J.; Huang, L.; Kumta, P.N.; Sfeir, C. Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: Influence of the synthesis parameters on transfection efficiency. Biomaterials 2007, 28, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, Z.; Rouabhia, M. Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. J. Bone Miner. Metab. 2011, 29, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Vieira, S.I.; Olhero, S.M.; Torres, P.M.; Pina, S.; da Cruz e Silva, O.A.; Ferreira, J.M. Synthesis, mechanical and biological characterization of ionic doped carbonated hydroxyapatite/β-tricalcium phosphate mixtures. Acta Biomater. 2011, 7, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Murata, H.; Takeshita, H.; Sakabe, T.; Tsuji, Y.; Kubo, T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int. Orthop. 2006, 30, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xu, G.H.; Yu, X.Z.; Zhang, W.J.; Xiao, Z.Y.; Yao, K.D. Fabrication and biological characteristics of β-tricalcium phosphate porous ceramic scaffolds reinforced with calcium phosphate glass. J. Mater. Sci. Mater. Med. 2009, 20, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Kokubo, T.; Nakamura, T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials 2005, 26, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; So, K.; Akiyama, N.; Matsushita, T.; Kokubo, T.; Nakamura, T. A porous bioactive titanium implant for spinal interbody fusion: An experimental study using a canine model. J. Neurosurg. Spine 2007, 7, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Okazaki, Y.; Hiasa, K.; Yasuda, K.; Nogami, K.; Mizumachi, W.; Hirata, I. Bioactive surface modification of hydroxyapatite. Biomed. Res. Int. 2013, 626452. [Google Scholar] [CrossRef] [PubMed]
- Jammet, P.; Souyris, F.; Baldet, P.; Bonnel, F.; Huguet, M. The effect of different porosities in coral implants: An experimental study. J. Cranio-Maxillofac. Surg. 1994, 22, 103–108. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Morrison, S.J.; Klawitter, J.J. Tissue reaction to three ceramics of porous and non-porous structures. J. Biomed. Mater. Res. 1972, 6, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Pernelle, K.; Imbert, L.; Bosser, C.; Auregan, J.C.; Cruel, M.; Ogier, A.; Jurdic, P.; Hoc, T. Microscale mechanical and mineral heterogeneity of human cortical bone governs osteoclast activity. Bone 2017, 94, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.T.; Cooper, L.F. Comparison of bone graft matrices for human mesenchymal stem cell-directed osteogenesis. J. Biomed. Mater. Res. A 2004, 68, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Bertazzo, S.; Zambuzzi, W.F.; Campos, D.D.; Ogeda, T.L.; Ferreira, C.V.; Bertran, C.A. Hydroxyapatite surface solubility and effect on cell adhesion. Colloids Surf. B Biointerfaces 2010, 78, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Semel, F.J.; Lados, D.A. Porosity analysis of PM materials by helium pycnometry. Powder Metall. 2006, 49, 173–182. [Google Scholar] [CrossRef]

| Group | The Porosity (%) | The Compressive Strength (MPa) |
|---|---|---|
| Porous HA | 75.31 ± 1.62 | 6.98 ± 0.79 ** |
| Phosphate treated HA | 77.71 ± 4.68 * | 2.72 ± 0.81 |
| Group | Whole Area | Central Area |
|---|---|---|
| Porous HA | 37.2 ± 4.5 | 37.4 ± 3.9 |
| Phosphate treated HA | 44.5 ± 7.7 | 47.8 ± 7.6 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, K.; Abe, Y.; Kobatake, R.; Okazaki, Y.; Oki, Y.; Naito, Y.; Prananingrum, W.; Tsuga, K. Novel Development of Phosphate Treated Porous Hydroxyapatite. Materials 2017, 10, 1405. https://doi.org/10.3390/ma10121405
Doi K, Abe Y, Kobatake R, Okazaki Y, Oki Y, Naito Y, Prananingrum W, Tsuga K. Novel Development of Phosphate Treated Porous Hydroxyapatite. Materials. 2017; 10(12):1405. https://doi.org/10.3390/ma10121405
Chicago/Turabian StyleDoi, Kazuya, Yasuhiko Abe, Reiko Kobatake, Yohei Okazaki, Yoshifumi Oki, Yoshihito Naito, Widyasri Prananingrum, and Kazuhiro Tsuga. 2017. "Novel Development of Phosphate Treated Porous Hydroxyapatite" Materials 10, no. 12: 1405. https://doi.org/10.3390/ma10121405




