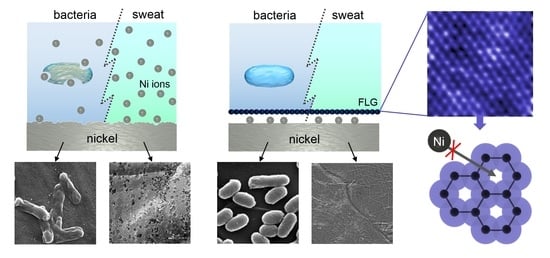
The Many Faces of Graphene as Protection Barrier. Performance under Microbial Corrosion and Ni Allergy Conditions
Abstract
:1. Introduction
2. Materials and Methods
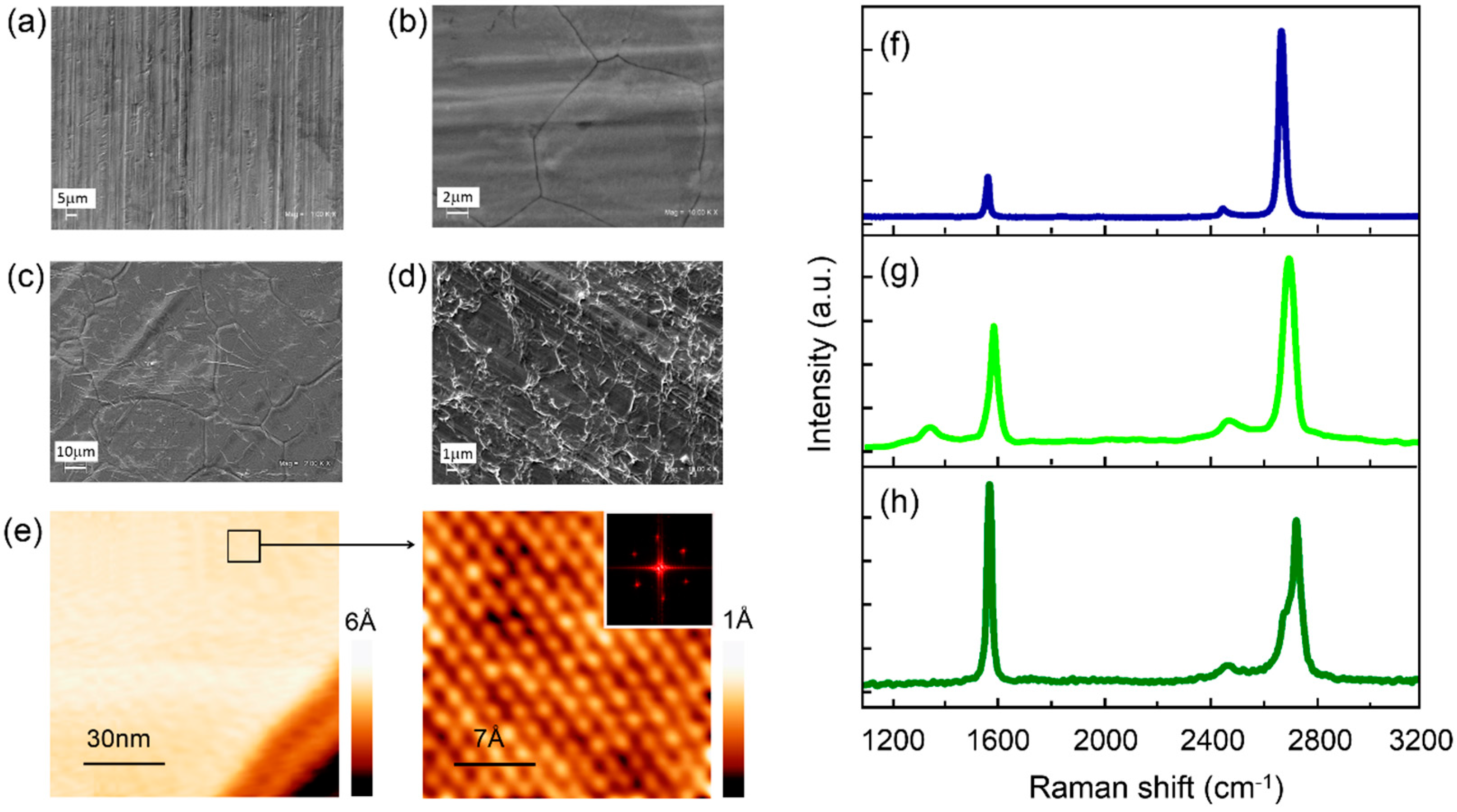
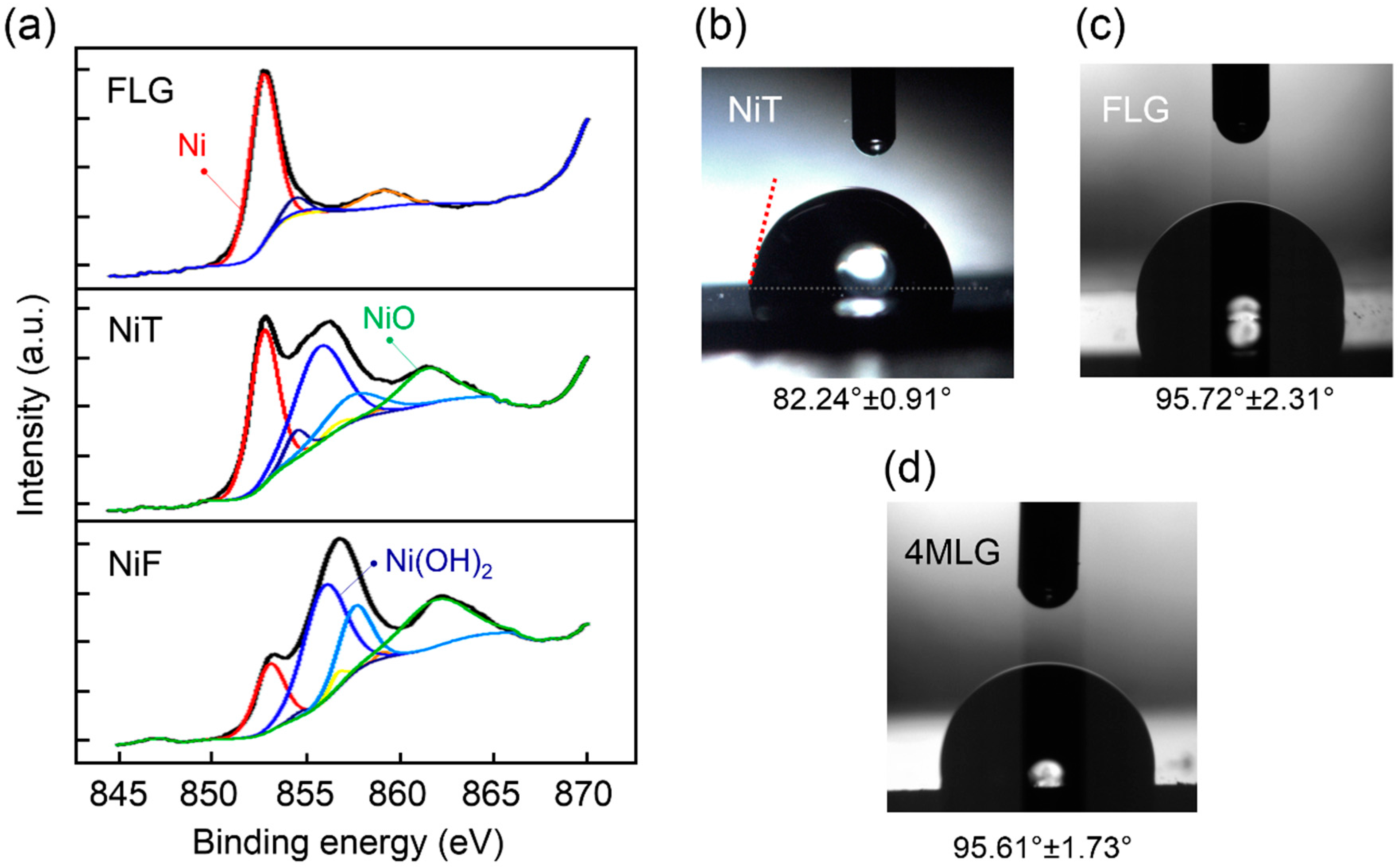
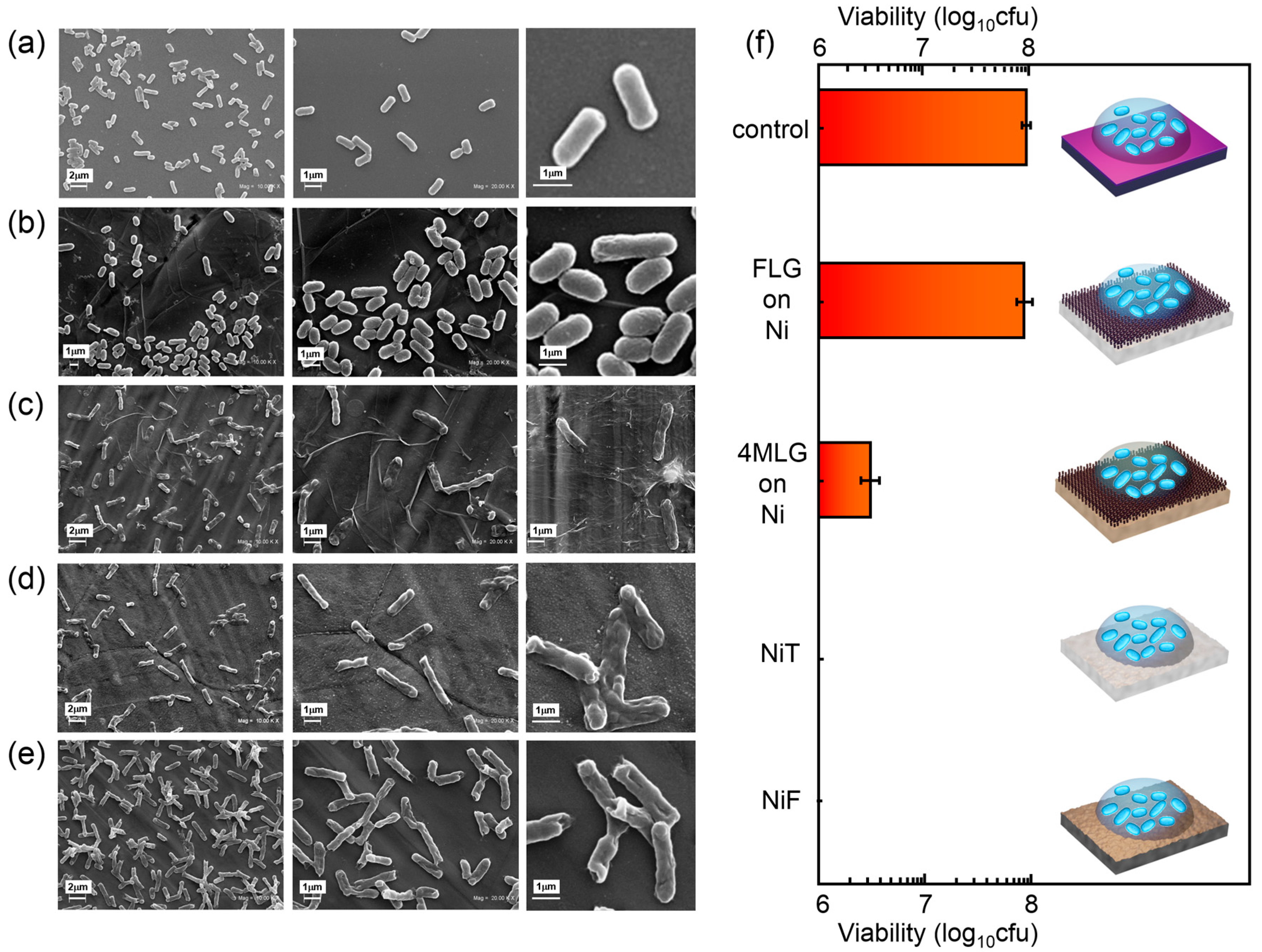
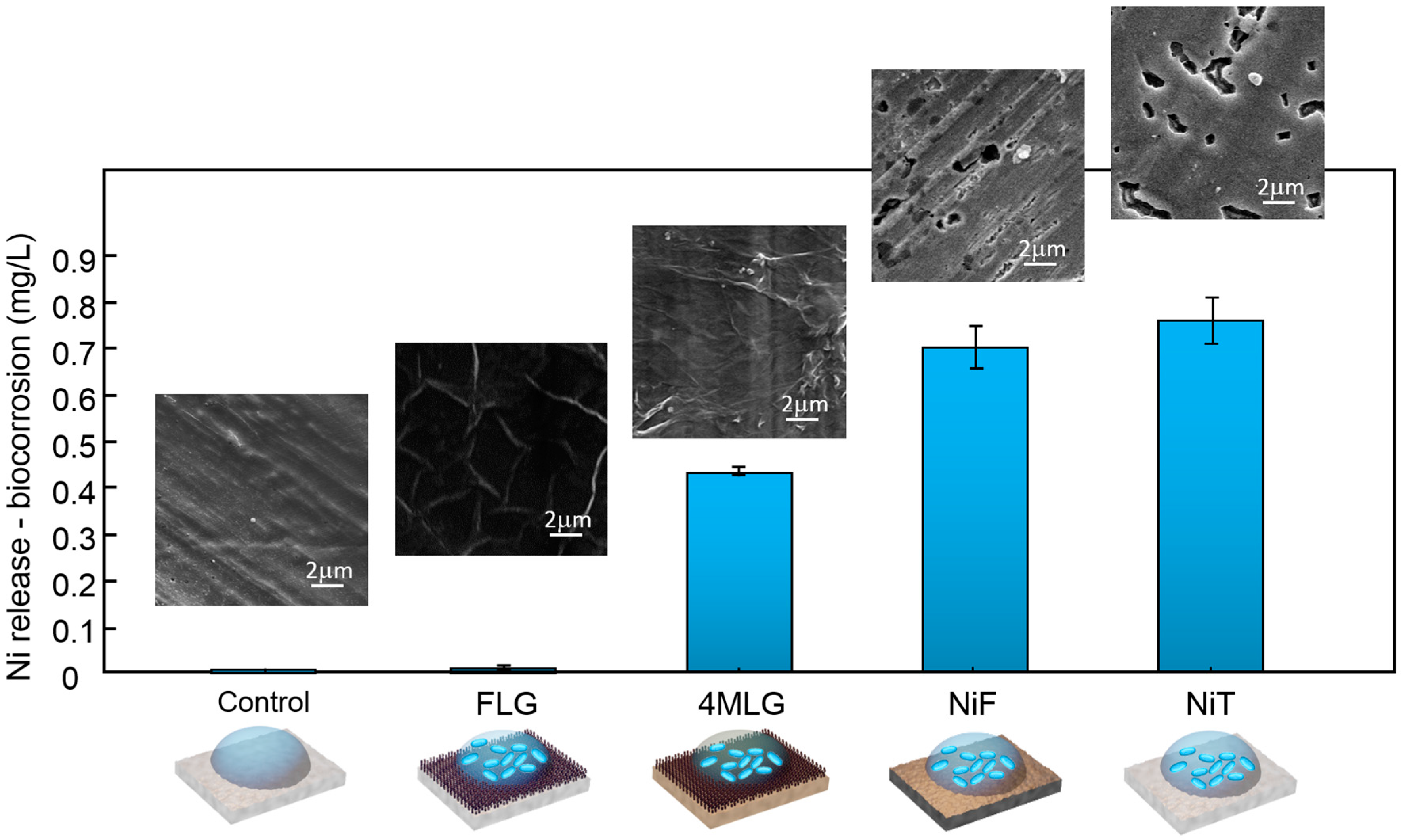
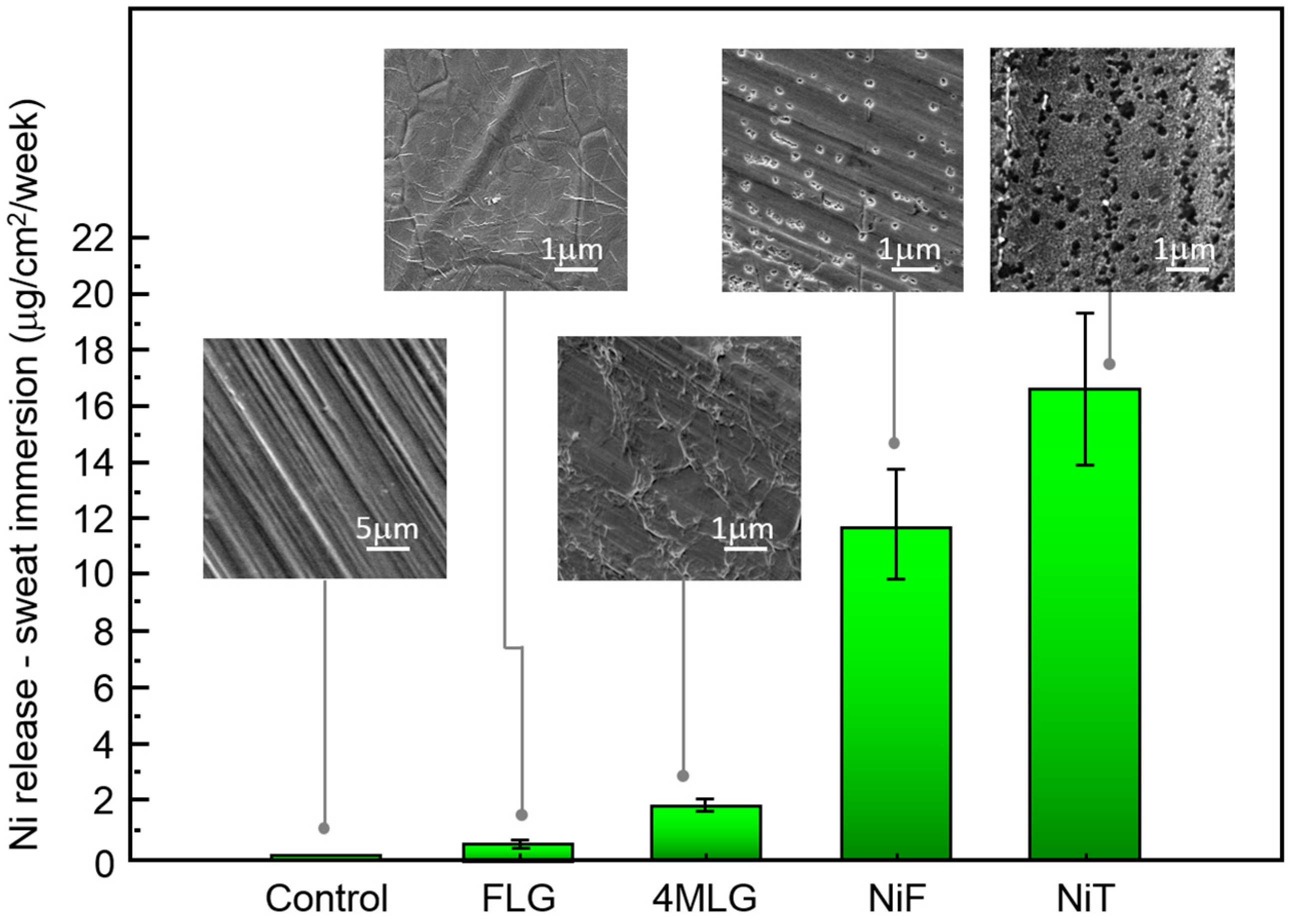
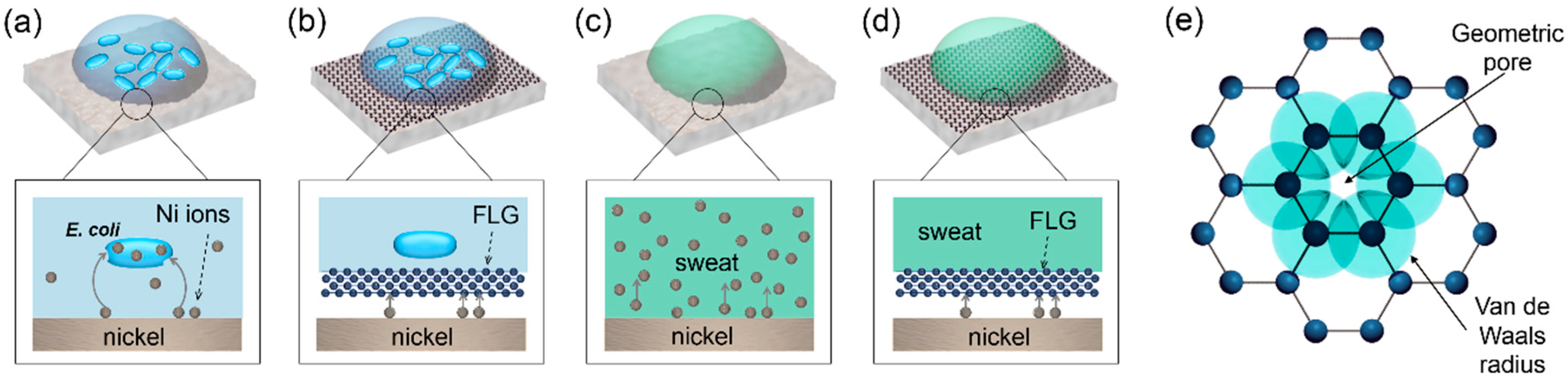
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rao, T. Microbial fouling and corrosion: Fundamentals and mechanisms. In Operational and Environmental Consequences of Large Industrial Cooling Water Systems; Springer: Boston, MA, USA, 2012; pp. 95–126. [Google Scholar]
- Li, K.; Whitfield, M.; van Vliet, K.J. Beating the bugs: Roles of microbial biofilms in corrosion. Corros. Rev. 2013, 31, 73–84. [Google Scholar]
- Almeida, C.; Azevedo, N.; Santos, S.; Keevil, W.; Vieira, M. Discriminating multi-species populations in biofilms with peptide nucleic acid fluorescence in situ hybridization (PNA FISH). PLoS ONE 2011, 6, e14786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videla, H.; Herrera, L. Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeterior. Biodegrad. 2009, 63, 896–900. [Google Scholar] [CrossRef]
- Zhang, W.; Lee, S.; McNear, K.L.; Chung, T.F.; Lee, S.; Lee, K.; Crist, S.; Ratliff, T.L.; Zhong, Z.; Chen, Y.P.; et al. Use of graphene as protection film in biological environments. Sci. Rep. 2014, 4, 4097–4105. [Google Scholar] [CrossRef] [PubMed]
- Scott-Fordsmand, J. Toxicity of nickel to soil organisms in Denmark. Rev. Environ. Contam. Toxicol. 1997, 148, 1–34. [Google Scholar]
- Poonkothai, M.; Shyamala, B. Nickel as an essential element and a toxicant. Int. J. Environ. Sci. 2012, 1, 285–288. [Google Scholar]
- Thyssen, J.; Johansen, J.; Menne, T.; Nielsen, N.; Linneberg, A. Nickel allergy in Danish women before and after nickel regulation. N. Engl. J. Med. 2009, 360, 2259–2260. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.; Ross–Hansen, K.; Menne, T.; Johansen, J. Patch test reactivity to metal allergens following regulatory interventions: A 33-year retrospective study. Contact Dermat. 2010, 63, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Moennich, J.; McKean, B.; Zirwas, M.; Taylor, J. Nickel allergy in the United States: A public health issue in need of a ‘nickel directive’. J. Am. Acad. Dermatol. 2009, 60, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Nosbaum, A.; Nosbaum, L.; Rival-Tringali, A.; Barth, X.; Damon, H.; Vital-Durand, D.; Claudy, A.; Faure, M. Nickel-induced systemic allergic dermatitis from a sacral neurostimulator. Contact Dermat. 2008, 59, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Lidén, C.; Carter, S. Nickel release from coins. Contact Dermat. 2001, 44, 160–165. [Google Scholar] [CrossRef]
- Spiewak, R.; Pietowska, J.; Curzytek, K. Nickel: A unique allergen—From molecular structure to European legislation. Expert Rev. Clin. Immunol. 2007, 3, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Roediger, B.; Weninger, W. How nickel turns on innate immune cells. Immunol. Cell Biol. 2011, 89, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.; Gawkrodger, J.; White, I.; Lidén, C. Coin exposure may cause allergic nickel dermatitis: A review. Contact Dermat. 2013, 68, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.; Menne, T.; Johansen, J. Identification of metallic items that caused nickel dermatitis in Danish patients. Contact Dermat. 2010, 63, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Suneja, T.; Flanagan, K.; Glaser, D. Blue-jean button nickel: Prevalence and prevention of its release from buttons. Dermatitis 2007, 18, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Svedman, C.; Ekqvist, S.; Möller, H.; Björk, J.; Pripp, C.; Gruvberger, B.; Holmström, E.; Gustavsson, C.; Bruze, M.A. Correlation found between contact allergy to stent material and restenosis of the coronary arteries. Contact Dermat. 2009, 60, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Goebeler, M. Nickel allergies: Paying the toll for innate immunity. J. Mol. Med. 2011, 89, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Wilkinson, J. Nickel allergy and hand eczema. In Nickel and the Skin: Immunology Toxicology; Maibach, H.I., Menne, T., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 133–164. [Google Scholar]
- European Parliament and Council Directive 94/27/EC. Official Journal of the European Communities. 1994. Available online: https://publications.europa.eu/en/publication-detail/-/publication/0a5a20d2-063e-4892-b94d-d82c9b223de0/language-en (accessed on 27 October 2017).
- European Parliament and Council Directive 1907/2006/EC. Official Journal of the European Communities. 2017. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=uriserv:OJ.C_.2017.011.01.0013.01.ENG (accessed on 27 October 2017).
- Memon, A.; Molokhia, M.; Friedmann, P. The inhibitory effects of topical chelating agents and antioxidants on nickel-induced hypersensitivity reactions. J. Am. Acad. Dermatol. 1994, 30, 560–565. [Google Scholar] [CrossRef]
- Wöhrl, S.; Kriechbaumer, N.; Hemmer, W.; Focke, M.; Brannath, W.; Götz, M.; Jarisch, R.A. Cream containing the chelator DTPA (diethylenetriaminepentaacetic acid) can prevent contact allergic reactions to metals. Contact Dermat. 2001, 44, 224–228. [Google Scholar] [CrossRef]
- Christensen, O.; Kristensen, M. Treatment with disulfiram in chronic nickel hand dermatitis. Contact Dermat. 1982, 8, 59–63. [Google Scholar] [CrossRef]
- Hopfer, S.; Linden, J.; Rezuke, W.; O’Brien, J.; Smith, L.; Watters, F.; Sunderman, F. Increased nickel concentrations in body fluids of patients with chronic alcoholism during disulfiram therapy. Res. Commun. Chem. Pathol. Pharmacol. 1987, 55, 101–109. [Google Scholar] [PubMed]
- Wall, L. Nickel penetration through rubber gloves. Contact Dermat. 1980, 6, 461–463. [Google Scholar] [CrossRef]
- Vemula, P.; Anderson, R.; Karp, J. Nanoparticles reduce nickel allergy by capturing metal ions. Nat. Nanotechnol. 2011, 6, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Hou, P.; Wang, J.; Chen, J.F. Controlled release active antimicrobial corrosion coatings with Ag/SiO2 core-shell nanoparticles. Mater. Chem. Phys. 2010, 120, 351–355. [Google Scholar] [CrossRef]
- Kwaadsteniet, M.; Botes, M.; Cloete, T.E. Application of nanotechnology in antimicrobial coatings in the water industry. Nano 2011, 6, 395–407. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Henriquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing bacterial interaction with copper surfaces through graphene and hexagonal–boron nitride coatings. ACS App. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.V.; Gadhamshetty, R.; Mukherjee, Z.; Chen, W.; Ren, H.; Cheng, M.; Koratkar, N. Passivation of microbial corrosion using a graphene coating. Carbon 2013, 56, 45–49. [Google Scholar] [CrossRef]
- Liu, H.; Kondo, H.; Ohno, T. Spintronic transport in armchair graphene nanoribbon with ferromagnetic electrodes: Half-metallic properties. Nanoscale Res. Lett. 2016, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Dedkov, Y.; Fonin, M. Electronic and magnetic properties of the grapheme-ferromagnet interface. New J. Phys. 2010, 12, 125004. [Google Scholar] [CrossRef]
- Gong, C.; Lee, G.; Shan, B.; Vogel, E.M.; Wallace, R.M.; Cho, K. First-principles study of metal-graphene interfaces. J. Appl. Phys. 2010, 108, 123711. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359–4366. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.S.; Tiwari, A. Graphene: The thinnest known coating for corrosion protection. JOM 2014, 66, 637–642. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Hofmann, M.; Chang, K.W.; Jhu, J.G.; Li, Y.Y.; Chen, K.Y.; Yang, C.C.; Chang, W.S.; Chen, L.C. Complete corrosion inhibition through graphene defect passivation. ACS Nano 2013, 8, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Dorta, F.; Jiménez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. Nanomolecular approach to reveal decreased adhesion of biofouling-producing bacteria to grapheme-coated material. J. Nanobiotechnol. 2015, 13, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, R.F.; Patan, N.K.; Al-Maadeed, M.A. Graphene oxide as antimicrobial against two gram-positive and two gram-negative bacteria in addition to one fungus. OnLine J. Biol. Sci. 2014, 14, 230–239. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Zhong, Q.; Zhong, Y.; Zhao, M.K. Controlled synthesis of surface-clean monolayer graphene. Key Eng. Mater. 2013, 562, 85–90. [Google Scholar] [CrossRef]
- Tian, J.; Hu, B.; Wei, Z.; Jin, Y.; Luo, Z.; Xia, M.; Pan, Q.; Liu, Y. Surface structure deduced differences of copper foil and film for graphene CVD growth. App. Surf. Sci. 2014, 300, 73–79. [Google Scholar] [CrossRef]
- Juang, Z.Y.; Wu, C.Y.; Lu, A.Y.; Su, C.Y.; Leou, K.C.; Chen, F.R.; Tsai, C.H. Graphene synthesis by chemical vapor deposition and transfer by a roll-to-roll process. Carbon 2010, 48, 3169–3174. [Google Scholar] [CrossRef]
- Barin, G.B.; Song, Y.; de Fátima Gimenez, I.; Souza Filho, A.G.; Barreto, L.S.; Kong, J. Optimized graphene transfer: Influence of polymethylmethacrylate (PMMA) layer concentration and baking time on graphene final performance. Carbon 2015, 84, 82–90. [Google Scholar] [CrossRef]
- Jin, W.; Hao, Q.; Peng, X.; Chu, P.K. Enhanced corrosion resistance and biocompatibilty of PMMA-coated ZK60 magnesium alloy. Mater. Lett. 2016, 173, 178–181. [Google Scholar] [CrossRef]
- De Jesús, J.; Carrazza, J.; Pereira, P.; Zaera, F. Hydroxylation of NiO films: The effect of water and ion bombardment during the oxidation of Ni foils with O2 under vacuum. Surf. Sci. 1998, 397, 34–47. [Google Scholar] [CrossRef]
- Bruinsma, G.; van der Mei, H.; Busscher, H. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Ramírez, C.; Gallegos, I.; Ihl, M.; Bifani, V. Study of contact angle, wettability and water vapor permeability in carboxymethylcellulose (CMC) based film with Murta leaves (Ugni molinae Turcz) extract. J. Food Eng. 2012, 109, 424–429. [Google Scholar] [CrossRef]
- Nan, L.; Xu, D.; Gu, T.; Song, X.; Yang, K. Microbiological influenced corrosion resistance characteristics of a 304L-Cu stainless steel against Escherichia coli. Mat. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Stoddart, P.R.; Palombo, E.A.; McArthur, S.L.; Wade, S.A. Inhibition or acceleration: Bacterial test media can determine the course of microbiologically influenced corrosion. Corros. Sci. 2014, 86, 149–158. [Google Scholar] [CrossRef]
- Chirino, B.; Strahsburger, E.; Agulló, L.; González, M.; Seeger, M. Genomic and functional analyses of the 2–aminophenol catabolic pathway and partial conversion of its substrate into picolinic acid in Burkholderia xenovorans LB400. PLoS ONE 2013, 8, e75746. [Google Scholar] [CrossRef] [PubMed]
- Luvara, G. Reference Test Method for Release of Nickel from Products Intended to Come into Direct and Prolonged Contact with the Skin; British Standards Institution: London, UK, 1999. [Google Scholar]
- Brugnoni, C.; Bianchi, R.; Binchini, A.; Stroosnijder, M. Influence of experimental test conditions on the Ni release of a Cu-Ni alloy in artificial sweat. In Materials for Medical Engineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Zhang, Y.; Gao, T.; Xie, S.; Dai, B.; Fu, L.; Gao, Y.; Chen, Y.; Liu, M.; Liu, Z. Different growth behaviors of ambient pressure chemical vapor deposition graphene on Ni(111) and Ni films: A scanning tunneling microscopy study. Nano Res. 2012, 5, 402–411. [Google Scholar] [CrossRef]
- Cao, H.; Yu, Q.; Colby, R.; Pandey, D.; Park, C.; Lian, J.; Zemlyanov, D.; Childres, I.; Drachev, V.; Stach, E.; et al. Large-scale graphitic thin films synthesized on Ni and transferred to insulators: Structural and electronic properties. J. Appl. Phys. 2010, 107, 044310. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T. Probing layer number and stacking order of few-layer graphene by Raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2008, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lambers, E.; Dykstal, C.; Seo, J.; Rowe, J.; Holloway, P. Room-temperature oxidation of Ni(110) at low and atmospheric oxygen pressures. Oxid. Met. 1996, 45, 301–321. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Z.; Liang, T.; Meng, Y.; Suzuki, K.; Miura, H. The effect of graphene coated nickel foam on the microstructures of NiO and their supercapacitor performance. J. Electroanal. Chem. 2017, 799, 595–601. [Google Scholar]
- Stalder, A.; Kulik, G.; Sage, D.; Barbieri, L.; Hoffmann, P. A Snake-based approach to accurate determination of both contact points and contact angles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 286, 92–103. [Google Scholar] [CrossRef]
- Busscher, H.; Weerkamp, A.H. Specific and non-specific interactions in bacteria adhesion to solid substrata. FEMS Microbiol. Lett. 1987, 46, 165–173. [Google Scholar] [CrossRef]
- Méndez-Vilas, A. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Research Center: Badajoz, Spain, 2013; pp. 1230–1246. [Google Scholar]
- Munz, M.; Giusca, C.E.; Myers-Ward, R.L.; Gaskill, D.K.; Kazakova, O. Thickness-dependent hydrophobicity of epitaxial graphene. ACS Nano 2015, 9, 8401–8411. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Alexandrova, R.; Tudose, R.; Mosoarca, M.; Costisor, O. Antimicrobial activity in vitro of four nickel complexes. Bulg. J. Agric. Sci. 2012, 18, 446–450. [Google Scholar]
- Fage, S.; Faurschou, A.; Thyssen, J. Copper hypersensitivity. Contact Dermat. 2014, 71, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Yasuyuki, M.; Kunihiro, K.; Kurissery, S.; Kanavillil, N.; Sato, Y.; Kikuchi, Y. Antibacterial properties of nine pure metals: A laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling 2010, 26, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Weik, T.; Roider, G.; Summer, B.; Thomsen, M. Influence of surface coating on metal ion release: Evaluation in patients with metal allergy. Orthopedics 2016, 39, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Ryabtseva, M.A.; Shesterikov, E.V.; Pichugin, V.F.; Peitsch, T.; Epple, M. The Release of nickel from nickel-titanium (NiTi) is strongly reduced by a sub-micrometer thin layer of calcium phosphate deposited by rf-magnetron sputtering. J. Mater. Sci. Mater. Med. 2010, 21, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Elhadiri, H.; Chegdani, K.; Sobh, M.; Cherkaoui, M.; Rifi, E.H. Study of the behavior of Moroccan coin ½ DH in a simulated sweat solution and 3% NaCl. J. Mater. Environ. Sci. 2014, 5, 1557–1564. [Google Scholar]
- Sreeprasad, T.; Berry, V. How do the electrical properties of graphene change with its functionalization? Small 2013, 9, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, C.; Montero-Silva, F.; Gentil, D.; Del Campo, V.; Henrique Rodrigues da Cunha, T.; Henríquez, R.; Häberle, P.; Garín, C.; Ramírez, C.; Fuentes, R.; et al. The Many Faces of Graphene as Protection Barrier. Performance under Microbial Corrosion and Ni Allergy Conditions. Materials 2017, 10, 1406. https://doi.org/10.3390/ma10121406
Parra C, Montero-Silva F, Gentil D, Del Campo V, Henrique Rodrigues da Cunha T, Henríquez R, Häberle P, Garín C, Ramírez C, Fuentes R, et al. The Many Faces of Graphene as Protection Barrier. Performance under Microbial Corrosion and Ni Allergy Conditions. Materials. 2017; 10(12):1406. https://doi.org/10.3390/ma10121406
Chicago/Turabian StyleParra, Carolina, Francisco Montero-Silva, Dana Gentil, Valeria Del Campo, Thiago Henrique Rodrigues da Cunha, Ricardo Henríquez, Patricio Häberle, Carolina Garín, Cristian Ramírez, Raúl Fuentes, and et al. 2017. "The Many Faces of Graphene as Protection Barrier. Performance under Microbial Corrosion and Ni Allergy Conditions" Materials 10, no. 12: 1406. https://doi.org/10.3390/ma10121406