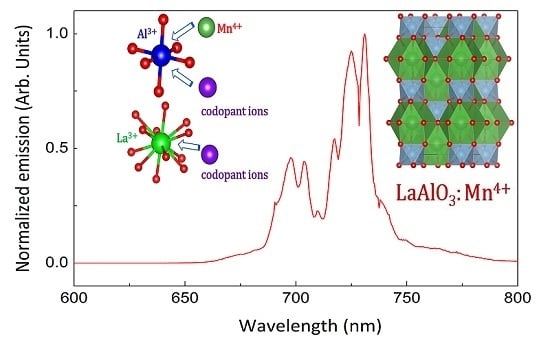
LaAlO3:Mn4+ as Near-Infrared Emitting Persistent Luminescence Phosphor for Medical Imaging: A Charge Compensation Study
Abstract
:1. Introduction
2. Results and Discussion
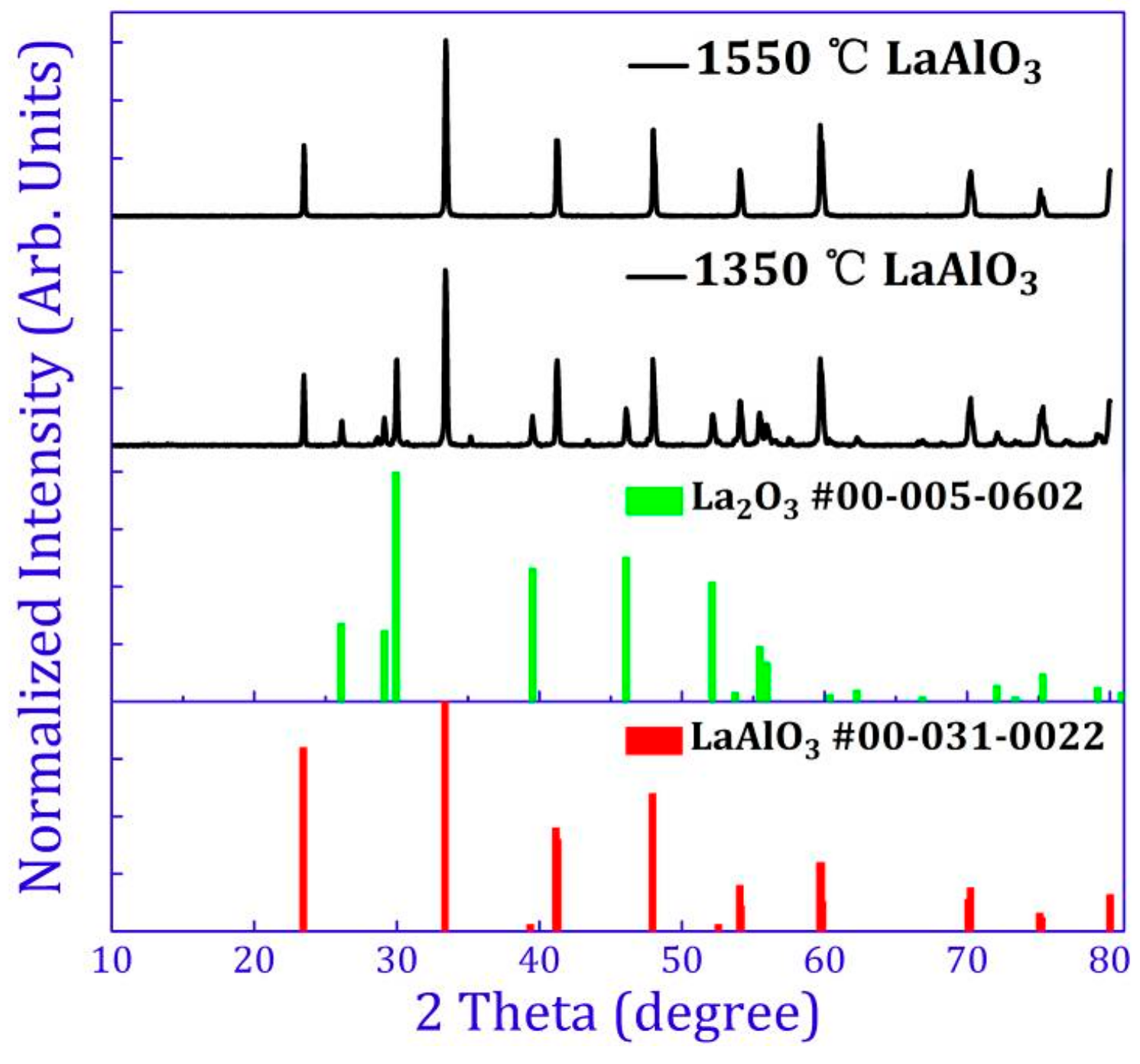
2.1. Crystal Structure
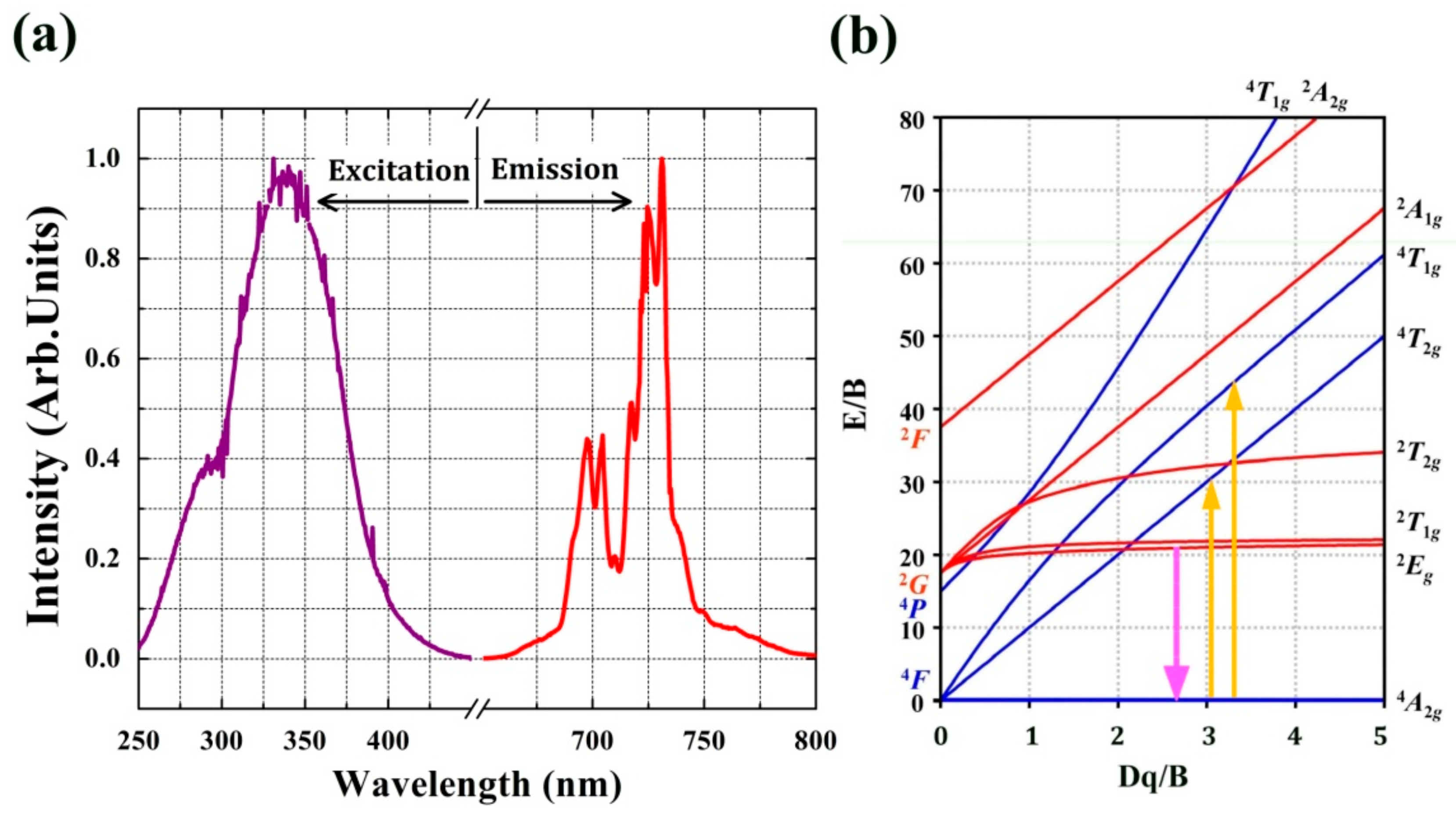
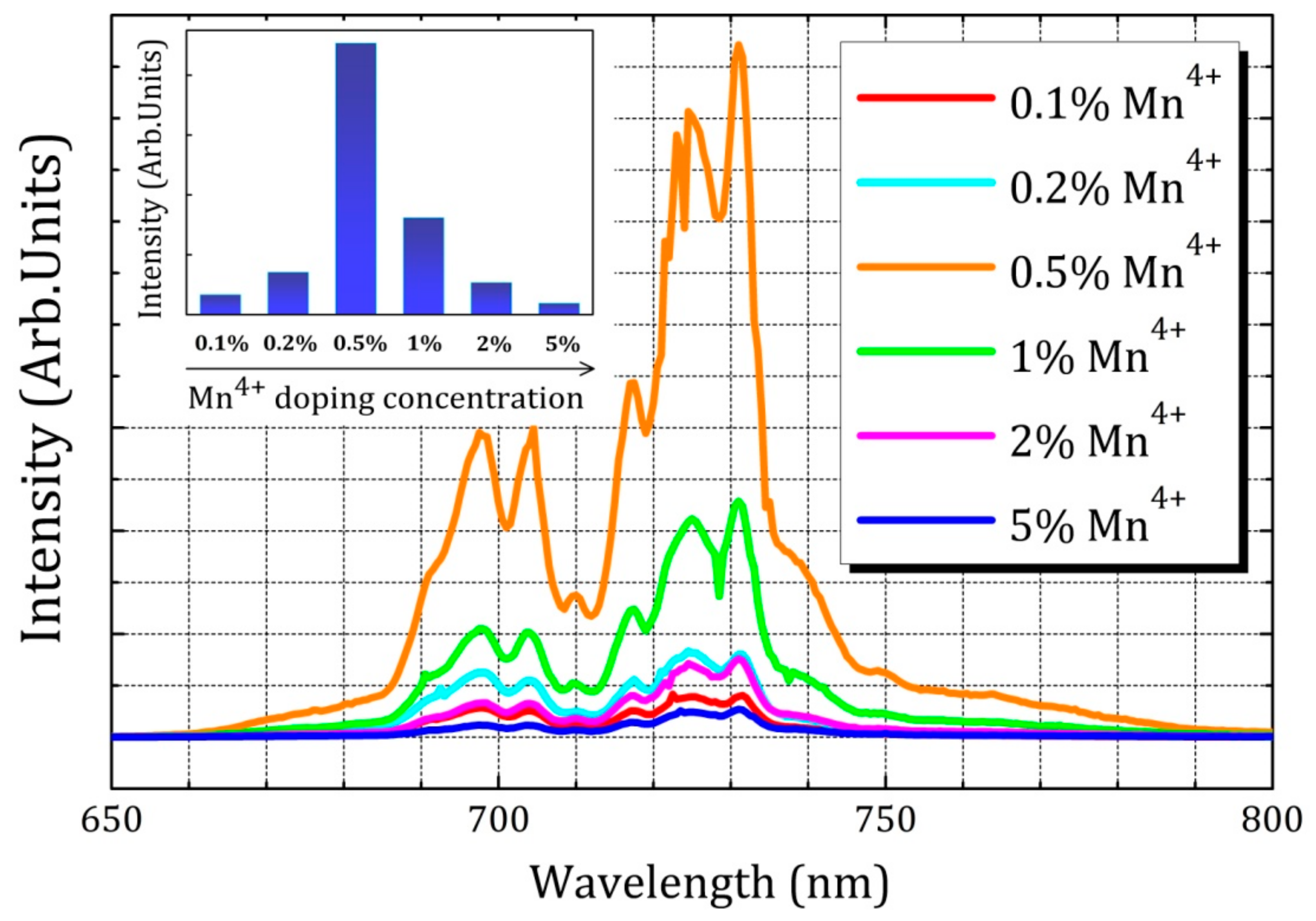
2.2. Luminescence Properties
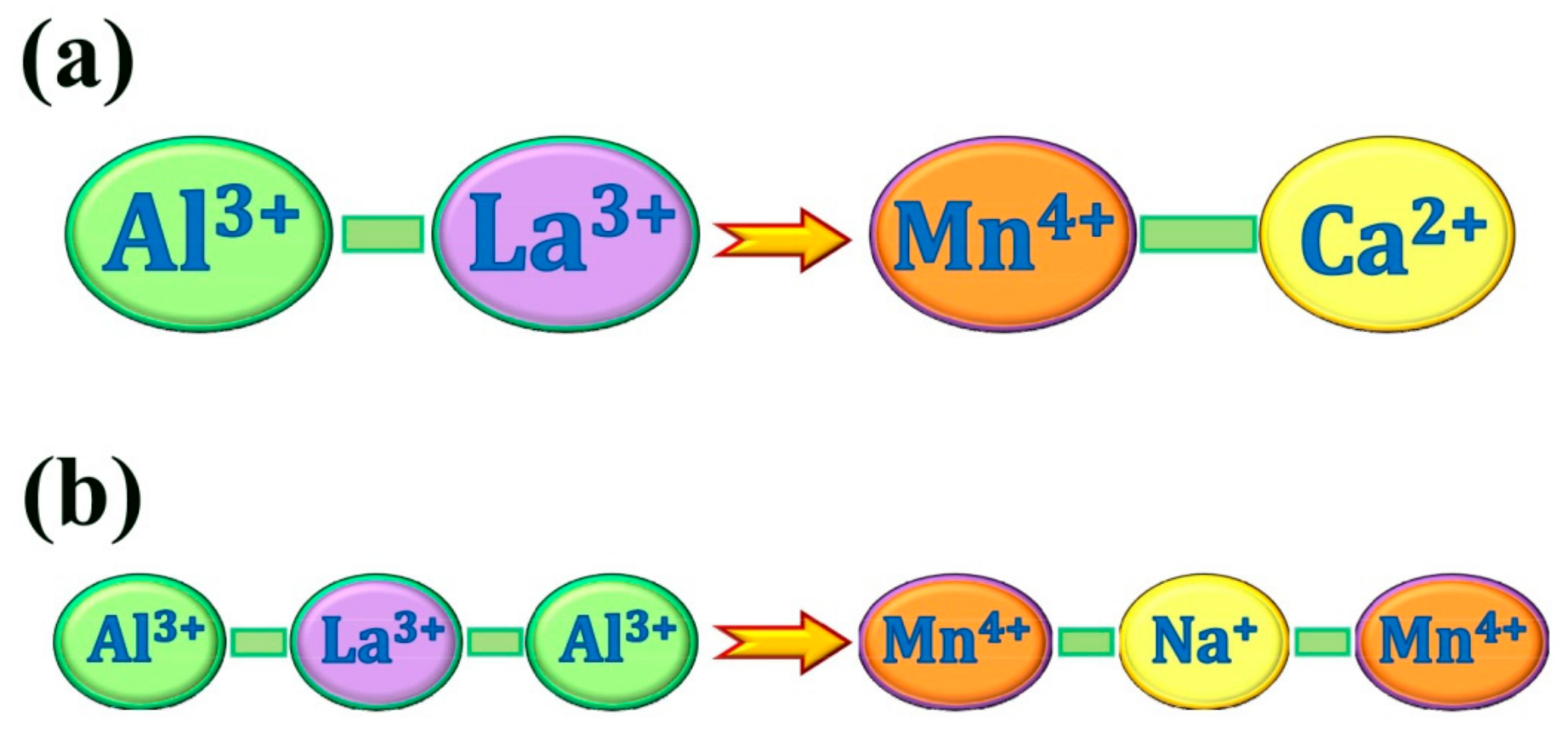
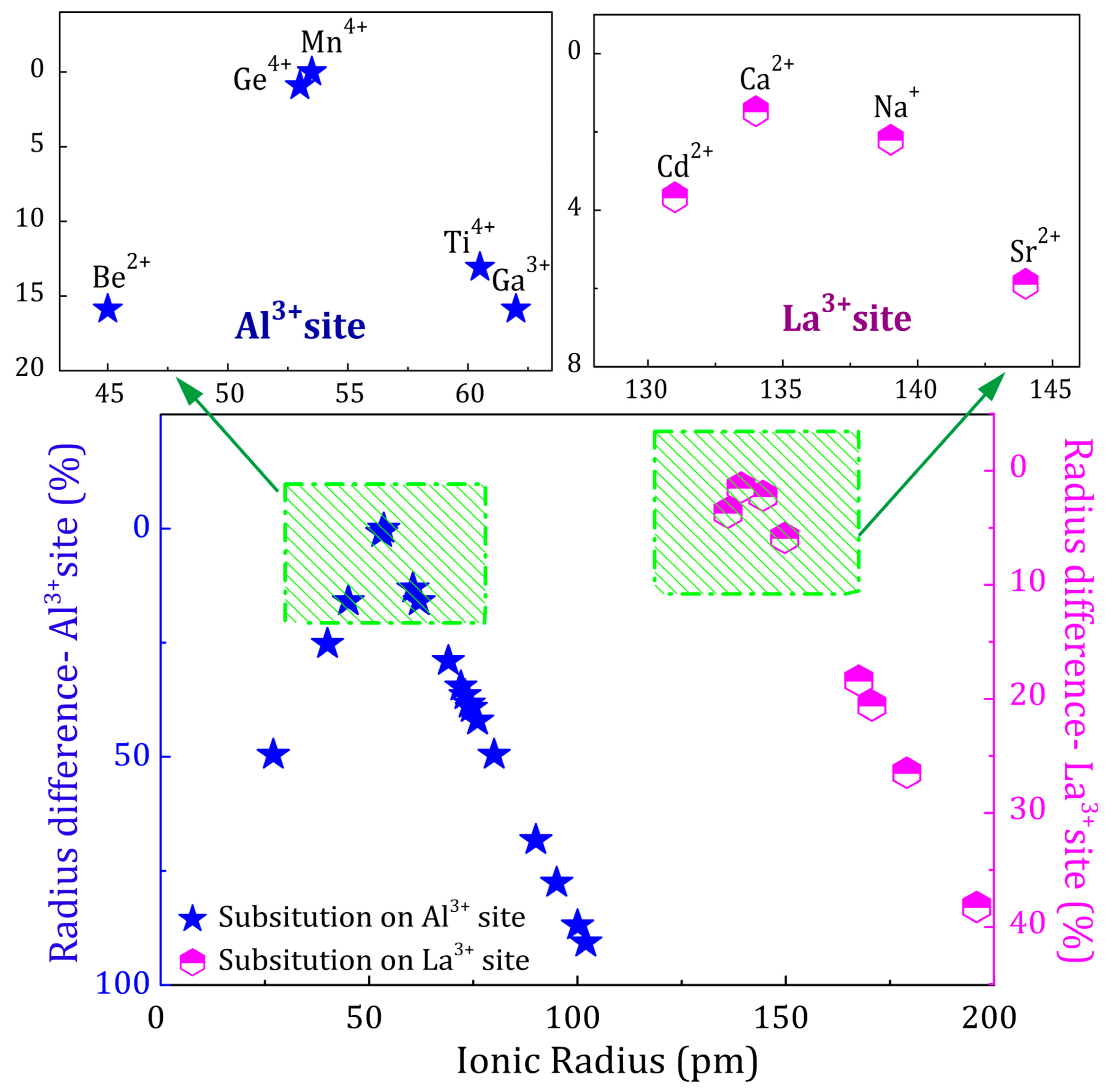
2.3. Charge Compensation Strategy
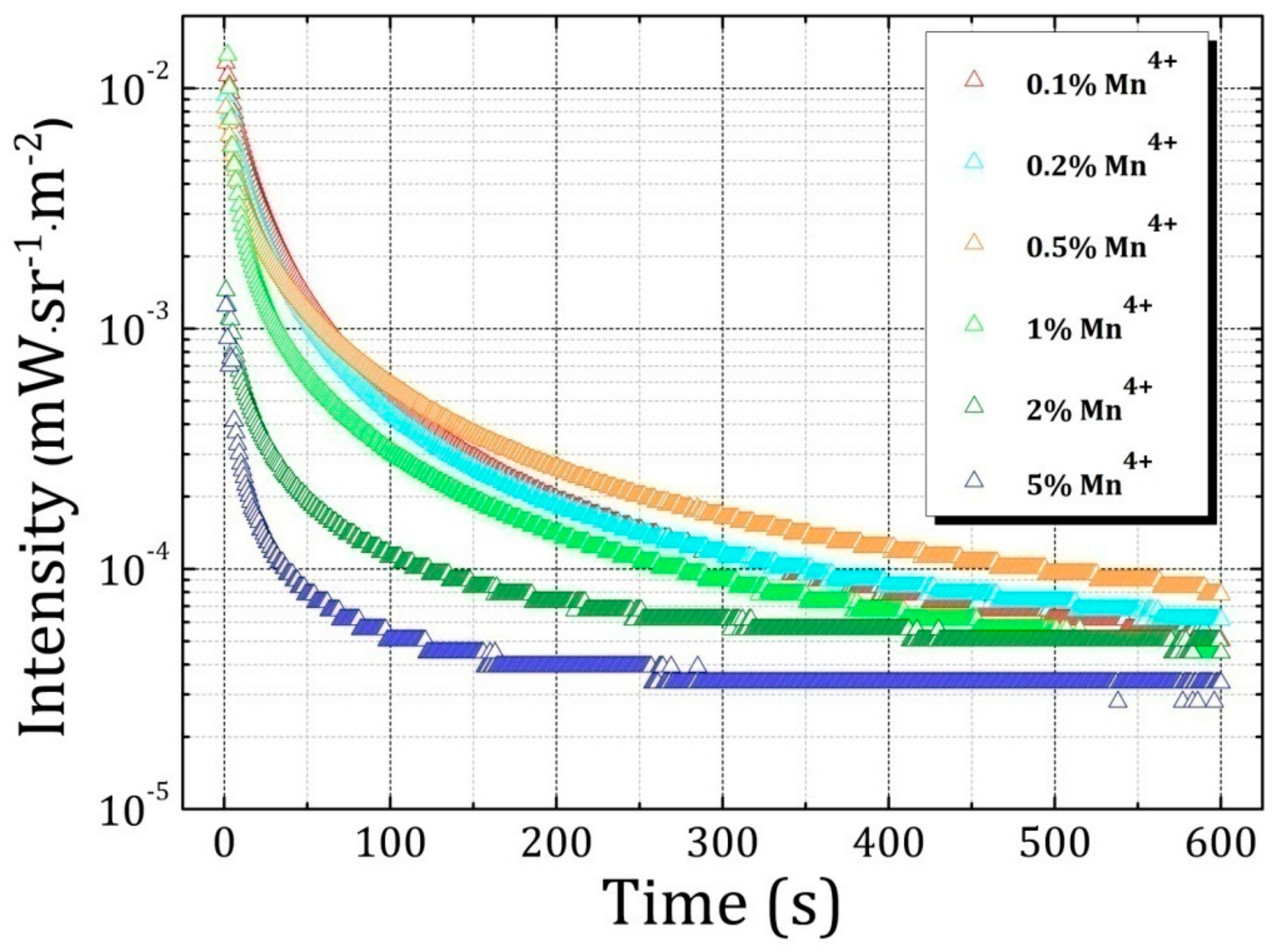
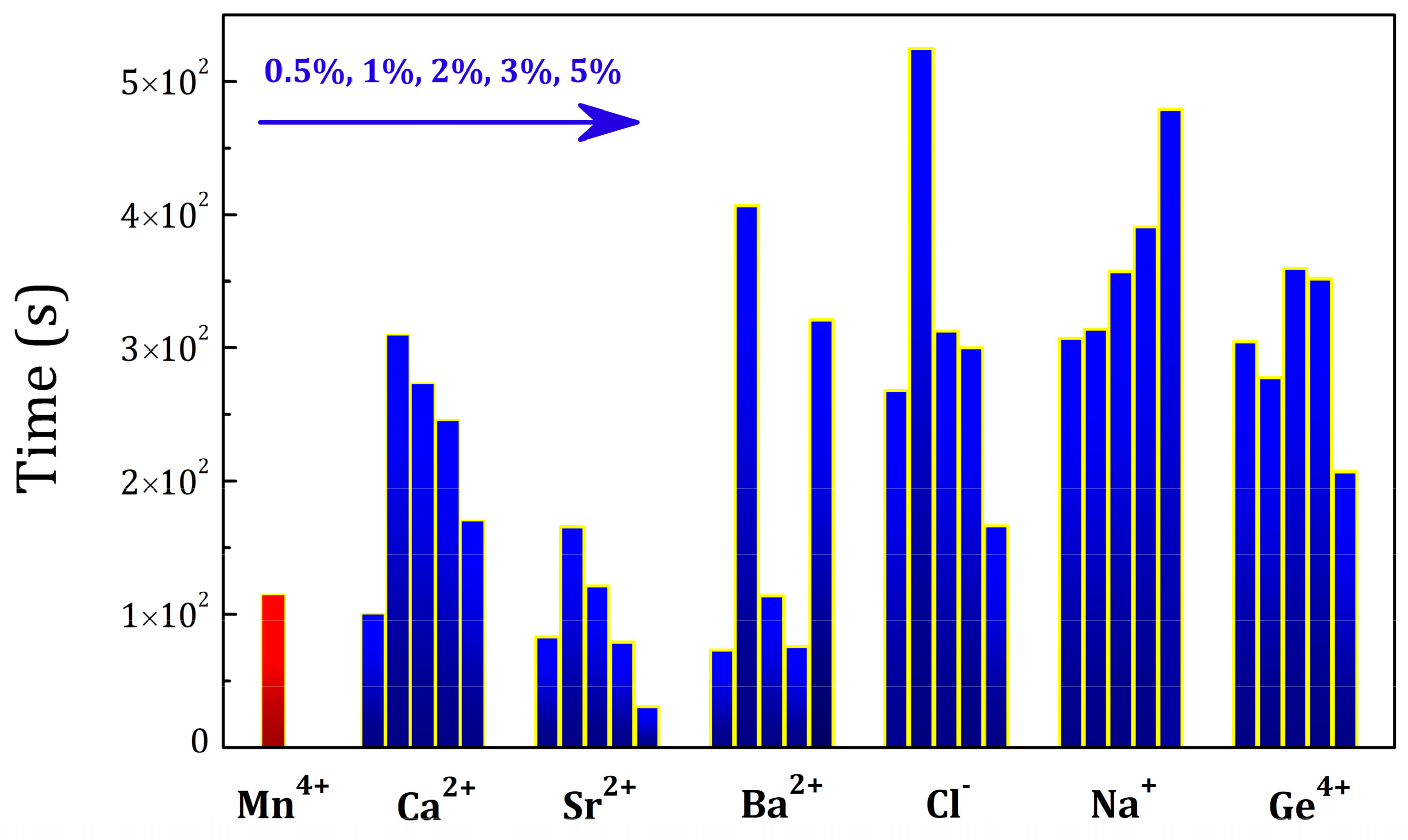
2.4. Influence of Various Co-Dopants
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Aitasalo, T.; Dereń, P.; Hölsä, J.; Jungner, H.; Krupa, J.-C.; Lastusaari, M.; Legendziewicz, J.; Niittykoski, J.; Stręk, W. Persistent luminescence phenomena in materials doped with rare earth ions. J. Solid State Chem. 2003, 171, 114–122. [Google Scholar] [CrossRef]
- Smet, P.F.; Van den Eeckhout, K.; De Clercq, O.Q.; Poelman, D. Persistent phosphors. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2015; Volume 48, pp. 1–108. [Google Scholar]
- Viana, B.; Sharma, S.; Gourier, D.; Maldiney, T.; Teston, E.; Scherman, D.; Richard, C. Long term in vivo imaging with Cr3+ doped spinel nanoparticles exhibiting persistent luminescence. J. Lumin. 2016, 170, 879–887. [Google Scholar] [CrossRef]
- Li, Y.; Gecevicius, M.; Qiu, J. Long persistent phosphors- from fundamentals to applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Matsuzawa, T. Mechanism of long phosphorescence of SrAl2O4:Eu2+, Dy3+ and CaAl2O4:Eu2+, Nd3+. J. Lumin. 1997, 72, 287–289. [Google Scholar] [CrossRef]
- De Chermont, Q.L.M.; Chanéac, C.; Seguin, J.; Pellé, F.; Maîtrejean, S.; Jolivet, J.-P.; Gourier, D.; Bessodes, M.; Scherman, D. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 9266–9271. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Nie, S. Cell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatings. J. Am. Chem. Soc. 2007, 129, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ohulchanskyy, T.Y.; Kumar, R.; Ågren, H.; Prasad, P.N. Ultrasmall monodisperse NaYF4:Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Red and near infrared persistent luminescence nano-probes for bioimaging and targeting applications. RSC Adv. 2014, 4, 58674–58698. [Google Scholar] [CrossRef]
- Maldiney, T.; Viana, B.; Bessière, A.; Gourier, D.; Bessodes, M.; Scherman, D.; Richard, C. In vivo imaging with persistent luminescence silicate-based nanoparticles. Opt. Mater. 2013, 35, 1852–1858. [Google Scholar] [CrossRef]
- Bessière, A.; Jacquart, S.; Priolkar, K.; Lecointre, A.; Viana, B.; Gourier, D. ZnGa2O4:Cr3+: A new red long-lasting phosphor with high brightness. Opt. Express 2011, 19, 10131–10137. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lu, Y.-Y.; Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 2012, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, S.; Li, Y.; Sharafudeen, K.; Ma, Z.; Dong, G.; Peng, M.; Qiu, J. Long persistent and photo-stimulated luminescence in Cr3+-doped Zn–Ga–Sn–O phosphors for deep and reproducible tissue imaging. J. Mater. Chem. C 2014, 2, 2657–2663. [Google Scholar] [CrossRef]
- Liu, F.; Yan, W.; Chuang, Y.-J.; Zhen, Z.; Xie, J.; Pan, Z. Photostimulated near-infrared persistent luminescence as a new optical read-out from Cr3+-doped LiGa5O8. Sci. Rep. 2013, 3, 1554. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, O.Q.; Martin, L.I.; Korthout, K.; Kusakovskij, J.; Vrielinck, H.; Poelman, D. Probing the local structure of the near-infrared emitting persistent phosphor LiGa5O8:Cr3+. J. Mater. Chem. C 2017, 5, 10861. [Google Scholar] [CrossRef]
- De Clercq, O.Q.; Poelman, D. Local, temperature-dependent trapping and detrapping in the LiGa5O8:Cr infrared emitting persistent phosphor. ECS J. Solid State Sci. Technol. 2017, 7, R3171–R3175. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.-Y.; Sharafudeen, K.; Dong, G.-P.; Zhou, S.-F.; Ma, Z.-J.; Peng, M.-Y.; Qiu, J.-R. A strategy for developing near infrared long-persistent phosphors: Taking MAlO3:Mn4+, Ge4+ (M = La, Gd) as an example. J. Mater. Chem. C 2014, 2, 2019–2027. [Google Scholar] [CrossRef]
- Sijbom, H.F.; Joos, J.J.; Martin, L.I.; Van den Eeckhout, K.; Poelman, D.; Smet, P.F. Luminescent behavior of the K2SiF6:Mn4+ red phosphor at high fluxes and at the microscopic level. ECS J. Solid State Sci. Technol. 2016, 5, R3040–R3048. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Lin, C.C.; Luo, W.; Shu, S.; Liu, Z.; Liu, Y.; Kong, J.; Ma, E.; Cao, Y.; Liu, R.-S. Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Commun. 2014, 5, 4312. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-M.; Liu, Y.-Y.; Zhang, D.-D.; Fang, G.-Z.; Wang, S. Synthesis of GdAlO3:Mn4+, Ge4+@Au core-shell nanoprobes with plasmon-enhanced near-infrared persistent luminescence for in vivo trimodality bioimaging. ACS Appl. Mater. Interfaces 2016, 8, 29939–29949. [Google Scholar] [CrossRef] [PubMed]
- Sijbom, H.F.; Verstraete, R.; Joos, J.J.; Poelman, D.; Smet, P.F. K2SiF6:Mn4+ as a red phosphor for displays and warm-white LEDs: A review of properties and perspectives. Opt. Mater. Express 2017, 7, 3332–3365. [Google Scholar] [CrossRef]
- Katayama, Y.; Kobayashi, H.; Tanabe, S. Deep-red persistent luminescence in Cr3+-doped LaAlO3 perovskite phosphor for in vivo imaging. Appl. Phys. Express 2014, 8, 012102. [Google Scholar] [CrossRef]
- Xu, J.; Murata, D.; Katayama, Y.; Ueda, J.; Tanabe, S. Cr3+/Er3+ co-doped LaAlO3 perovskite phosphor: A near-infrared persistent luminescence probe covering the first and third biological windows. J. Mater. Chem. B 2017, 5, 6385–6393. [Google Scholar] [CrossRef]
- Hayward, S.; Morrison, F.; Redfern, S.; Salje, E.; Scott, J.; Knight, K.; Tarantino, S.; Glazer, A.; Shuvaeva, V.; Daniel, P. Transformation processes in LaAlO3: Neutron diffraction, dielectric, thermal, optical and Raman studies. Phys. Rev. B 2005, 72, 054110. [Google Scholar] [CrossRef]
- Sanz-Ortiz, M.N.; Rodríguez, F.; Rodríguez, J.; Demazeau, G. Optical and magnetic characterisation of Co3+ and Ni3+ in LaAlO3: Interplay between the spin state and Jahn—Teller effect. J. Phys. Condens. Matter 2011, 23, 415501. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Brik, M. Crystal field studies of the Mn4+ energy levels in the perovskite, LaAlO3. Opt. Mater. 2013, 35, 1544–1548. [Google Scholar] [CrossRef]
- Faucher, M.; Caro, P. Optical study of LaAlO3:Eu at temperatures approaching the rhombohedric→cubic transition. J. Chem. Phys. 1975, 63, 446–454. [Google Scholar] [CrossRef]
- Wen-Chen, Z. Theoretical studies of the electron paramagnetic resonance and optical spectra of Cr3+ ions in the rhombohedral phase of a LaAlO3 crystal. J. Phys. Condens. Matter 1995, 7, 4499. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Cao, R.; Ceng, D.; Liu, P.; Yu, X.; Guo, S.; Zheng, G. Synthesis and photoluminescence properties of LaAlO3:Mn4+, Na+ deep red-emitting phosphor. Appl. Phys. A 2016, 122, 299. [Google Scholar] [CrossRef]
- Van Ipenburg, M.; Dirksen, G.; Blasse, G. Charge-transfer excitation of transition-metal-ion luminescence. Mater. Chem. Phys. 1995, 39, 236–238. [Google Scholar] [CrossRef]
- Brik, M.; Srivastava, A. On the optical properties of the Mn4+ ion in solids. J. Lumin. 2013, 133, 69–72. [Google Scholar] [CrossRef]
- Brik, M.; Camardello, S.; Srivastava, A. Influence of covalency on the Mn4+ 2Eg→4A2g emission energy in crystals. ECS J. Solid State Sci. Technol. 2015, 4, R39–R43. [Google Scholar] [CrossRef]
- Du, M.-H. Chemical trends of Mn4+ emission in solids. J. Mater. Chem. C 2014, 2, 2475–2481. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, F.; Cao, C.; Yu, X.; Liang, A.; Guo, S.; Xue, H. Synthesis and luminescence properties of CaAl2O4:Mn4+ phosphor. Opt. Mater. 2014, 38, 53–56. [Google Scholar] [CrossRef]
- Seki, K.; Uematsu, K.; Toda, K.; Sato, M. Novel deep red emitting phosphors Ca14Zn6M10O35:Mn4+ (M = Al3+ and Ga3+). Chem. Lett. 2014, 43, 1213–1215. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sugano, S. On the absorption spectra of complex ions II. J. Phys. Soc. Jpn. 1954, 9, 766–779. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Brik, M.G.; Camardello, S.J.; Comanzo, H.A.; Garcia-Santamaria, F. Optical spectroscopy and crystal field studies of the Mn4+ ion (3d3) in the double perovskite NaLaMgTeO6. Z. Naturforschung B 2014, 69, 141–149. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, N.; Xia, M.; Yokoyama, M.; Hintzen, H.B. Research progress and application prospects of transition metal Mn4+-activated luminescent materials. J. Mater. Chem. C 2016, 4, 9143–9161. [Google Scholar] [CrossRef]
- Pauling, L. The principles determining the structure of complex ionic crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Shu, W.; Jiang, L.; Xiao, S.; Yang, X.; Ding, J. GeO2 dopant induced enhancement of red emission in CaAl12O19:Mn4+ phosphor. Mater. Sci. Eng. B 2012, 177, 274–277. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Wang, L.; Shi, M.; Liu, L.; Chen, Y. Red emission enhancement for CaAl12O19:Cr3+ and CaAl12O19:Mn4+ phosphors. J. Mater. Sci. Mater. Electron. 2017, 28, 12032–12038. [Google Scholar] [CrossRef]
- Meng, L.; Liang, L.; Wen, Y. Deep red phosphors SrMgAl10O17:Mn4+, M (M = Li+, Na+, K+, Cl−) for warm white light emitting diodes. J. Mater. Sci. Mater. Electron. 2014, 25, 2676–2681. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, G. Enhancement of phosphor efficiency via composition modification. Opt. Lett. 2008, 33, 1816–1818. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; De Clercq, O.Q.; Korthout, K.; Poelman, D. LaAlO3:Mn4+ as Near-Infrared Emitting Persistent Luminescence Phosphor for Medical Imaging: A Charge Compensation Study. Materials 2017, 10, 1422. https://doi.org/10.3390/ma10121422
Du J, De Clercq OQ, Korthout K, Poelman D. LaAlO3:Mn4+ as Near-Infrared Emitting Persistent Luminescence Phosphor for Medical Imaging: A Charge Compensation Study. Materials. 2017; 10(12):1422. https://doi.org/10.3390/ma10121422
Chicago/Turabian StyleDu, Jiaren, Olivier Q. De Clercq, Katleen Korthout, and Dirk Poelman. 2017. "LaAlO3:Mn4+ as Near-Infrared Emitting Persistent Luminescence Phosphor for Medical Imaging: A Charge Compensation Study" Materials 10, no. 12: 1422. https://doi.org/10.3390/ma10121422