Development and Assessment of a 3D-Printed Scaffold with rhBMP-2 for an Implant Surgical Guide Stent and Bone Graft Material: A Pilot Animal Study
Abstract
:1. Introduction
2. Results
2.1. In Vitro Results
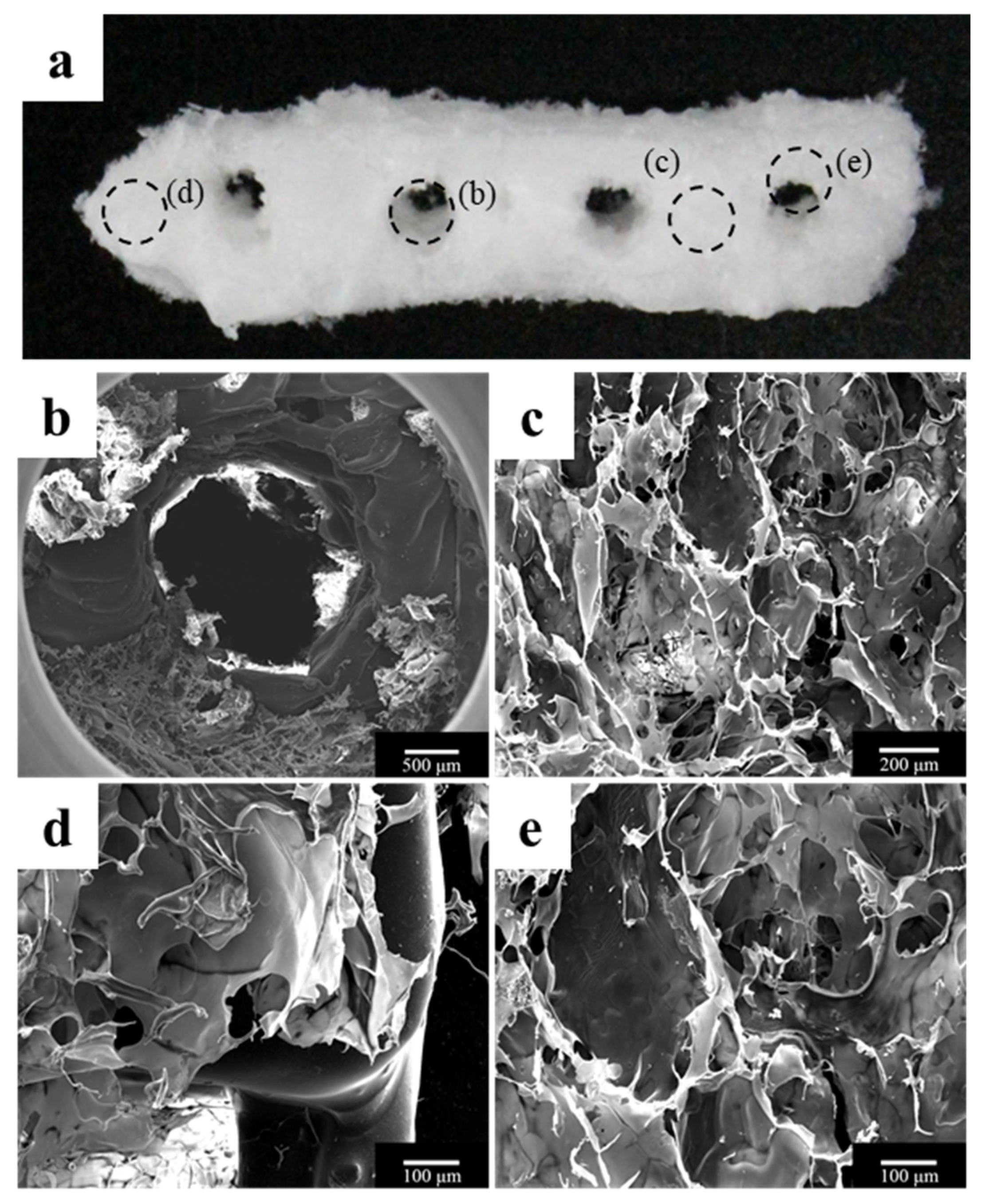
2.1.1. Morphology of a Fabricated Implant Guide Scaffold Using an In-House System
2.1.2. Compressive Strength Comparison of PCL/β-TCP and PCL/β-TCP/bdECM Scaffolds
2.1.3. In Vitro Release Kinetics of Loaded rhBMP-2
2.1.4. In Vitro Analysis of the Cell Bioactivity
2.2. In Vivo Results
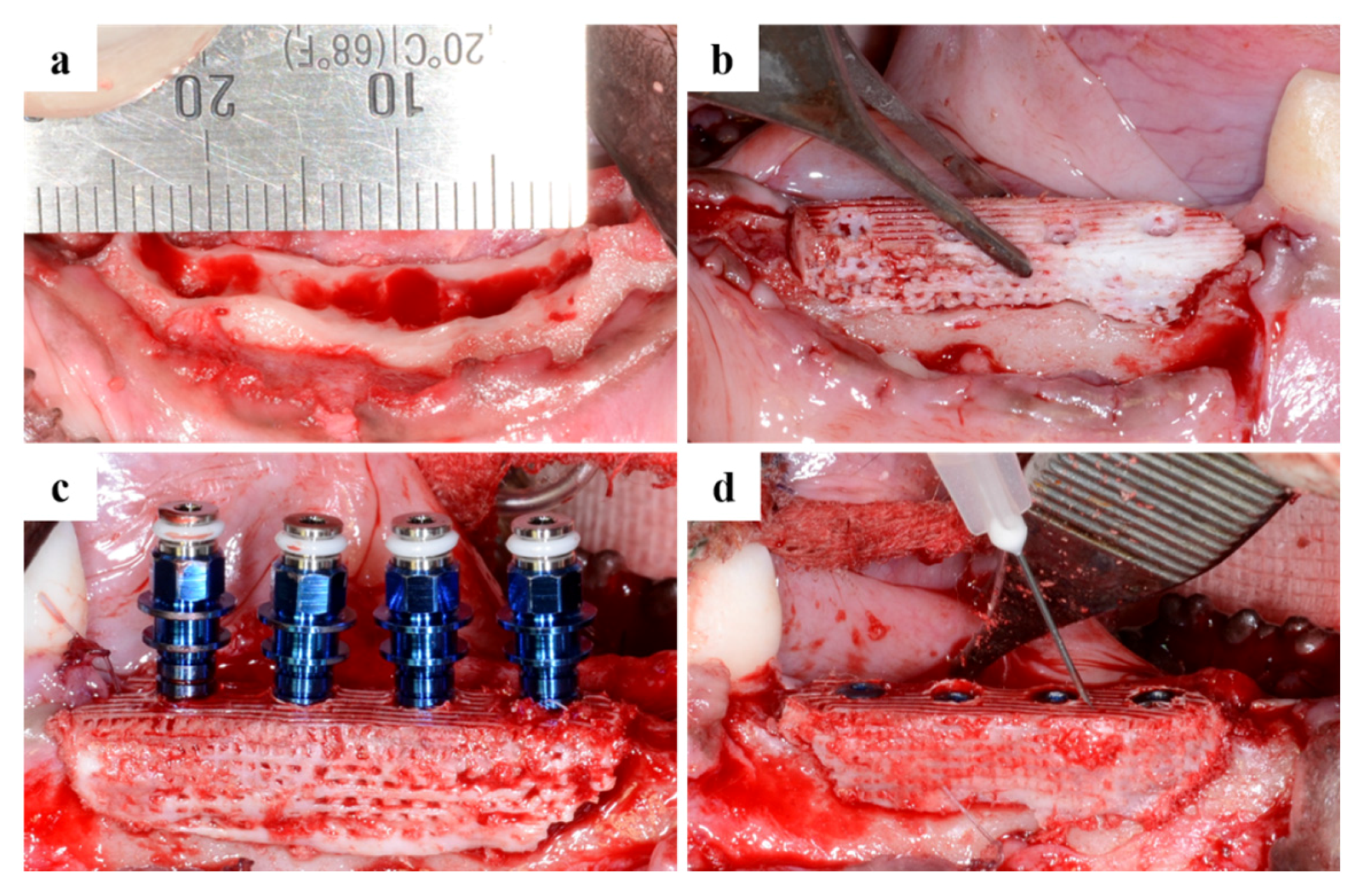
2.2.1. Clinical Findings
2.2.2. Findings Using Micro-Computed Tomography (Micro-CT)
2.2.3. Histologic Findings
2.2.4. Histometric Findings
3. Discussion
4. Materials and Methods
4.1. Fabrication of the PCL/β-TCP Scaffold
4.1.1. Preparation of Blended PCL/β-TCP
4.1.2. Preparation of the Blended Bone-Derived Extracellular Matrix
4.1.3. Development of a Micro Extrusion-Based 3D Printer
4.1.4. Construction of a 3D Implant Model Using CAD Software
4.1.5. Fabrication of the PCL/β-TCP Scaffold for In Vitro and In Vivo Use Using an In-House System
4.1.6. Preparation of bdECM with rhBMP-2
4.1.7. The Coating Process of the in Vitro and in Vivo Scaffolds Using bdECM
4.1.8. Scanning Electron Microscope Analysis of the Scaffolds
4.2. In Vitro Test with Scaffold
4.2.1. Compressive Strength Test Using PCL/β-TCP and PCL/β-TCP having bdECM
4.2.2. In Vitro Release Kinetics of rhBMP-2
4.2.3. Cell Culture and Seeding of MC3T3-E1
4.2.4. Analysis of Proliferation and Osteogenic Differentiation of MC3T3-E1
4.3. In Vivo Study
Experimental Animals and Design
- Control group (Defect: 2 (fixture: 8): PCL/β-TCP/bdECM scaffold.
- BMP group (Defect: 2 (fixture: 8): PCL/β-TCP/bdECM scaffold + rhBMP-2 (400 μg/mL, total volume/defect 200 uL).
4.4. Design of Surgical Surgery
4.4.1. Tooth Extraction
4.4.2. Produce Scaffold for Defect Size
4.4.3. Sacrifice after Implant Placement
4.4.4. Micro-Computed Tomography (µCT) Analysis
4.4.5. Histological Analysis
- New bone area (NBA, %) = New bone Area/Area of interest × 100.
- Bone-to-implant contact (BIC, %) = length of bone-to-implant contact/length from the top of the implant to the bottom of new bone (NB) × 100.
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Hultin, M.; Svensson, K.G.; Trulsson, M. Clinical advantages of computer-guided implant placement: A systematic review. Clin. Oral. Implant. Res. 2012, 23, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Rocci, A.; Martignoni, M.; Gottlow, J. Immediate loading in the maxilla using flapless surgery, implants placed in predetermined positions, and prefabricated provisional restorations: A retrospective 3-year clinical study. Clin. Implant. Dent. Rel. Res. 2003, 5, 29–36. [Google Scholar] [CrossRef]
- Rocci, A.; Martignoni, M.; Gottlow, J. Immediate loading of Branemark system TiUnite and machined-surface implants in the posterior mandible: A randomized open-ended clinical trial. Clin. Implant. Dent. Rel. Res. 2003, 5, 57–63. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Dula, K.; Hirt, H.P.; Schenk, R.K. Lateral ridge augmentation using autografts and barrier membranes: A clinical study with 40 partially edentulous patients. J. Oral. Maxillofac. Surg. 1996, 54, 420–432. [Google Scholar] [CrossRef]
- Al Ruhaimi, K.A. Bone graft substitutes: A comparative qualitative histologic review of current osteoconductive grafting materials. Int. J. Oral. Maxillofac. Implants. 2001, 16, 105–114. [Google Scholar] [PubMed]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Urist, M.R. Bone formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Alves, C.M.; Kasper, F.K.; Mikos, A.G.; Reis, R.L. Responsive and in situ-forming chitosan scaffolds for bone tissue engineering applications: An overview of the last decade. J. Mater. Chem. 2010, 20, 1638–1645. [Google Scholar] [CrossRef]
- Zorlutuna, P.; Annabi, N.; Camci-Unal, G.; Nikkhah, M.; Cha, J.M.; Nichol, J.W.; Manbachi, A.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabricated biomaterials for engineering 3D tissues. Adv. Mater. 2012, 24, 1782–1804. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Guillemin, G. Tissue-engineered bone regeneration. Nature Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Harder, Y.; Amon, M.; Martin, I.; Farhadi, J.; Ring, A.; Häufel, J.M. Angiogenesis in tissue engineering: Breathing life into constructed tissue substitutes. Tissue Eng. 2006, 12, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Moon, T.S.; Yun, M.J.; Jeon, Y.C.; Jeong, C.M.; Cho, D.W.; Huh, J.B. Stimulation of healing within a rabbit calvarial defect by a PCL/PLGA scaffold blended with TCP using solid freeform fabrication technology. J. Mater. Sci. Mater. Med. 2012, 23, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cen, L.; Yin, S.; Chen, L.; Liu, G.; Chang, J.; Cui, L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and beta-TCP ceramics. Biomaterials 2008, 29, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Biomaterials 2005, 26, 3587–3596. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Wozney, J.M. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001, 19, 255–265. [Google Scholar] [CrossRef]
- Park, J.K.; Shim, J.H.; Kang, K.S.; Yeom, J.; Jung, H.S.; Kim, J.Y.; Lee, K.H.; Kim, T.H.; Kim, S.Y.; Cho, D.W.; et al. Solid free-form fabrication of tissue-engineering scaffolds with a poly(lactic-co-glycolic acid) grafted hyaluronic acid conjugate encapsulating an intact bone morphogenetic protein-2/poly(ethylene glycol) complex. Adv. Funct. Mater. 2011, 21, 2906–2912. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, K.S.; Lee, S.H.; Kim, J.Y.; Lee, B.K.; Cho, D.W. Bone regeneration using a microstereolithography-produced customized poly(propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP2 loaded PLGA microspheres. Biomaterials 2011, 32, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.A.; Song, S.J.; Susanto, E.; Chuan, P.; Lam, C.X.; Woodruff, M.A.; Hutmacher, D.W.; Cool, S.M. The stimulation of healing within a rat calvarial defect by mPCL-TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials 2009, 30, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kang, H.W.; Kang, T.Y.; Park, H.; Park, J.S.; Cho, D.W. Projection image-generation algorithm for fabrication of a complex structure using projection-based microstereolithography. Int. J. Precis. Eng. Manuf. 2012, 13, 445–459. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.J.; Alexandra, L.R.; Ramille, N.S. Advancing the field of 3D biomaterial printing. Biomed. Matter. 2016, 11. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- La, W.G.; Jang, J.; Kim, B.S.; Lee, M.S.; Cho, D.W.; Yang, H.S. Systemically replicated organic and inorganic bony microenvironment for new bone formation generated by a 3D printing technology. RSC Adv. 2016, 6, 11546–11553. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011, 15, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Huh, J.Y.; Choi, B.H.; Kim, B.Y.; Lee, S.H.; Zhu, S.J.; Jung, J.H. Critical size defect in the canine mandible. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2005, 100, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Solofomalala, G.D.; Guery, M.; Lesiourd, A.; Huec, J.C.; Chauveaux, D.; Laffentre, O. Bone morphogenetic proteins: From their discoveries till their clinical application. Eur. J. Orthop. Surg. Trauamtol. 2007, 17, 609–615. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, N.S.; Lee, J.Y.; Kim, U.C. Successful strategy of treatment using rhBMP-2 for maxillary sinus graft. J. Kor. Dent. Assoc. 2015, 53, 14–27. [Google Scholar]
- Li, G.; Cui, Y.; McIlmurray, L.; Allen, W.E.; Wang, H. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J. Orthop. Res. 2005, 23, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Takaoka, K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 2003, 24, 2287–2293. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Schaner, P.J.; Martin, N.D.; Tulenko, T.N.; Shapiro, I.M.; Tarola, N.A.; Leichter, R.F.; DiMuzio, P.J. Decellularized vein as a potential scaffold for vascular tissue engineering. J. Vasc. Surg. 2004, 40, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Trans. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.S. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shim, J.H.; Choi, S.A.; Jang, J.; Kim, M.; Lee, S.H.; Huh, J.B. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J. Mater. Chem. B 2015, 3, 5415–5425. [Google Scholar] [CrossRef]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Chiron, P. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone. Jt. Surg. Am. 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Jung, R.E.; Windisch, S.I.; Eggenschwiler, A.M.; Thoma, D.S.; Weber, F.E.; Hämmerle, C.H. A randomizedcontrolled clinical trial evaluating clinical and radiological outcomes after 3 and 5 years of dental implants placed in bone regenerated by means of GBR techniques with or without the addition of BMP-2. Clin. Oral. Implant. Res. 2009, 20, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herford, A.S.; Boyne, P.J.; Rawson, R.; Williams, R.P. Bone morphogenetic protein induced repair of the premaxillary cleft. J. Oral. Maxillofac. Surg. 2007, 65, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.P.; Ashley, R.K.; Wasson, K.L.; O’Hara, C.; Gabbay, J.; Heller, J.B.; Bradley, J.P. Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast. Reconstr. Surg. 2008, 121, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Talley, A.D.; Kalpakci, K.N.; Shimko, D.A.; Zienkiewicz, K.J.; Cochran, D.L.; Guelcher, S.A. Effects of Recombinant Human Bone Morphogenetic Protein-2 Dose and Ceramic Composition on New Bone Formation and Space Maintenance in a Canine Mandibular Ridge Saddle Defect Model. Tissue Eng. Part A 2016, 22, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, N.; Oda, S.; Kinoshita, A.; Kikuchi, S.; Tsuchioka, H.; Akizuki, T.; Hayashi, C.; Kokubo, S.; Ishikawa, I.; Izumi, Y. Effect of rhBMP-2 with PLGA/gelatin sponge type (PGS) carrier on alveolar ridge augmentation in dogs. J. Oral. Rehabil. 2008, 35, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Ng, T.; Tom, W.K.; Carstens, M. Repair of alveolar clefts with recombinant human bone morphogenetic protein (rhBMP-2) in patients with clefts. J. Craniofac. Surg. 2005, 16, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Kuipers, E.; Gawlitta, D.; Dhert, W.J.; Alblas, J. Scaffold porosity and oxygenation of printed hydrogel constructs affect functionality of embedded osteogenic progenitors. Tissue Eng. Part A 2011, 17, 2473–2486. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Guo, H.; Lu, J.; Shi, J.; Wei, J.; Liu, C. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP-2 by synchrotron radiation-based μCT. Biomaterials 2011, 32, 8506–8517. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.G.; Lee, H.; Cho, D.W. 3D Printing of Organs-On-Chips. Bioengineering 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, C.; Deng, Y.; Li, T.; Li, N.; Peng, M.; Wang, J. RhBMP-2 loaded 3D-printed mesoporous silica/calcium phosphate cement porous scaffolds with enhanced vascularization and osteogenesis properties. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, B.; Meng, Q.; Liu, S.; Zheng, X.; Zhang, C.; Wang, H.; Li, H.; Wang, N.; Dai, J. 3D bioprinting of bmsc-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. Biofabrication 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Kim, S.W.; Rhie, J.W.; Shim, J.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nature Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lee, J.S.; Kim, J.Y.; Cho, D.W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Barralet, J.E.; Gaunt, T.; Wright, A.J.; Gibson, I.R.; Knowles, J.C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J. Biom. Mater. Rese. Part A 2002, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biom. Mater. Res. Part A 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin, Y.C.; Lee, J.J.; Bae, E.B.; Jeon, Y.C.; Jeong, C.M.; Yun, M.J.; Lee, S.H.; Han, D.W.; Huh, J.B. The effect of reduced graphene oxide-coated biphasic calcium phosphate bone graft material on osteogenesis. Int. J. Mol. Sci. 2017, 18, 1725. [Google Scholar] [CrossRef] [PubMed]














| Group | Mean ± SD | Median | p-Value |
|---|---|---|---|
| Control | 6.30 ± 2.90 | 6.26 | <0.01 |
| BMP | 10.08 ± 2.48 | 9.93 |
| Variable | Group | Mean ± SD | Median | p-Value |
|---|---|---|---|---|
| BIC (%) | Control | 22.61 ± 6.92 | 20.78 | <0.001 *** |
| BMP | 51.29 ± 14.64 | 47.41 | ||
| NBA (%) | Control | 38.84 ± 7.61 | 37.63 | 0.073 |
| BMP | 48.95 ± 12.35 | 44.57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.C.; Lee, J.-J.; Shim, J.-H.; Park, K.-H.; Lee, J.-S.; Bae, E.-B.; Choi, J.-W.; Huh, J.-B. Development and Assessment of a 3D-Printed Scaffold with rhBMP-2 for an Implant Surgical Guide Stent and Bone Graft Material: A Pilot Animal Study. Materials 2017, 10, 1434. https://doi.org/10.3390/ma10121434
Bae JC, Lee J-J, Shim J-H, Park K-H, Lee J-S, Bae E-B, Choi J-W, Huh J-B. Development and Assessment of a 3D-Printed Scaffold with rhBMP-2 for an Implant Surgical Guide Stent and Bone Graft Material: A Pilot Animal Study. Materials. 2017; 10(12):1434. https://doi.org/10.3390/ma10121434
Chicago/Turabian StyleBae, Ji Cheol, Jin-Ju Lee, Jin-Hyung Shim, Keun-Ho Park, Jeong-Seok Lee, Eun-Bin Bae, Jae-Won Choi, and Jung-Bo Huh. 2017. "Development and Assessment of a 3D-Printed Scaffold with rhBMP-2 for an Implant Surgical Guide Stent and Bone Graft Material: A Pilot Animal Study" Materials 10, no. 12: 1434. https://doi.org/10.3390/ma10121434





