Exploring the Mechanical Anisotropy and Ideal Strengths of Tetragonal B4CO4
Abstract
:1. Introduction
2. Computational Methods
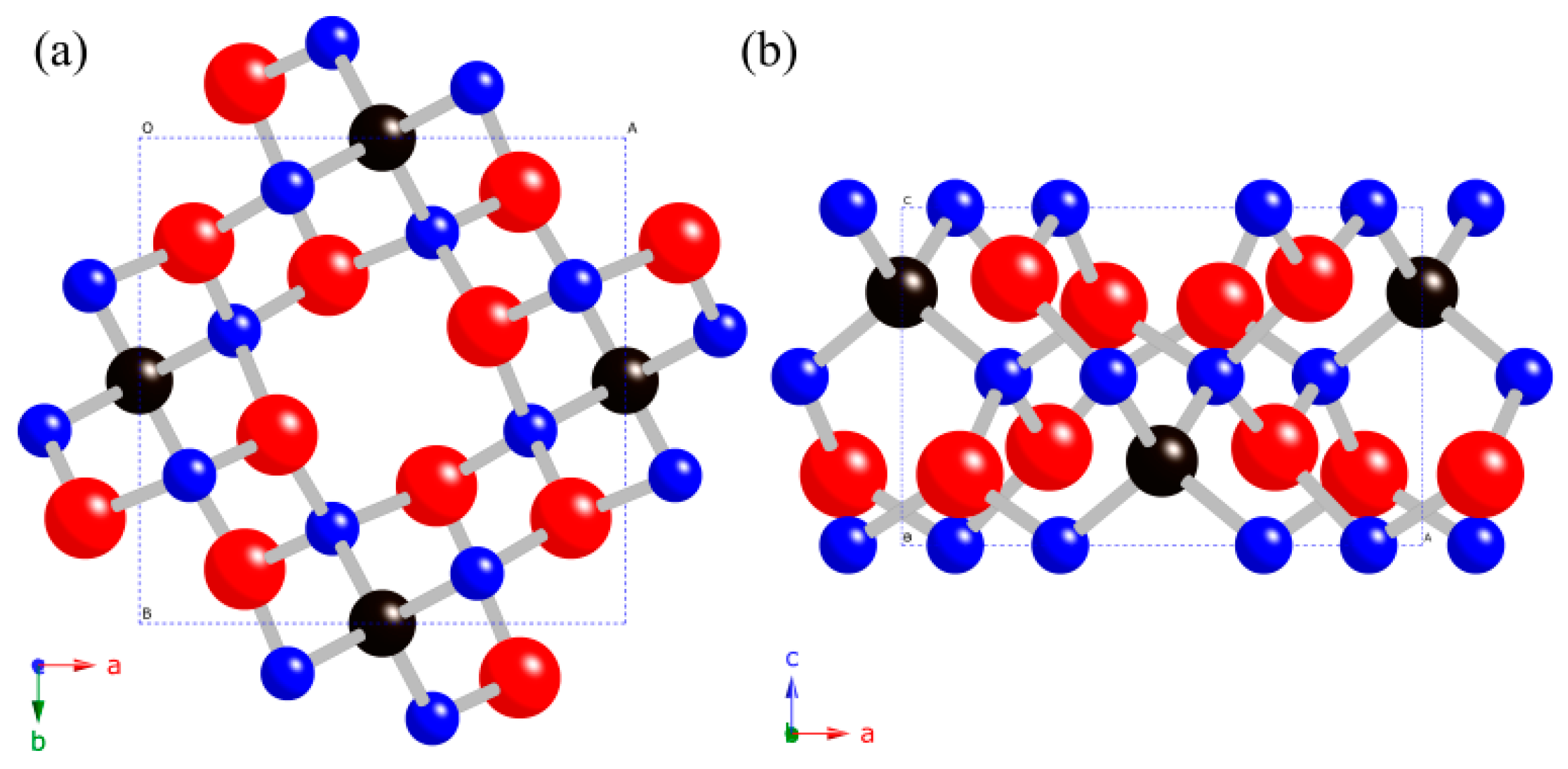
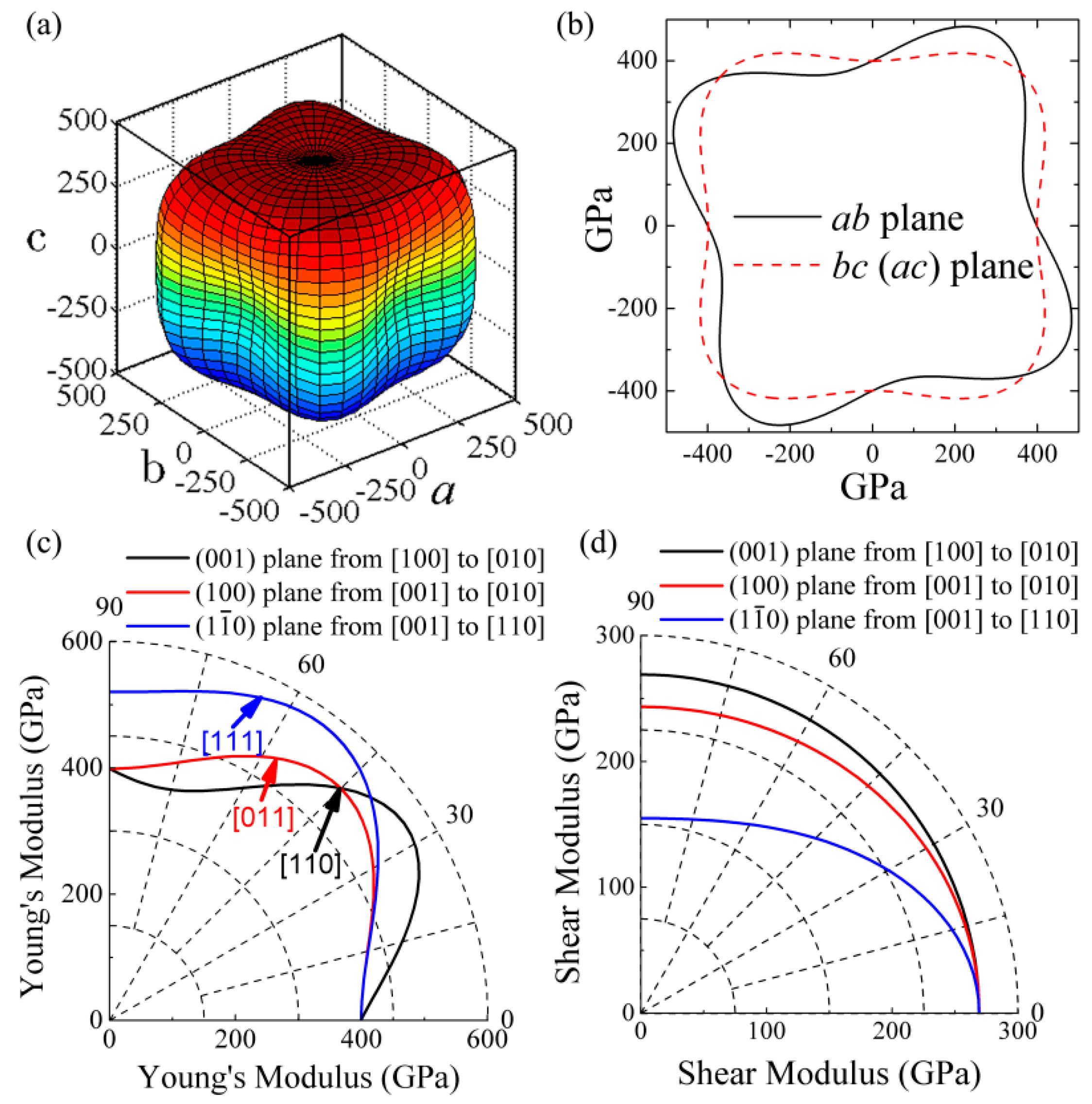
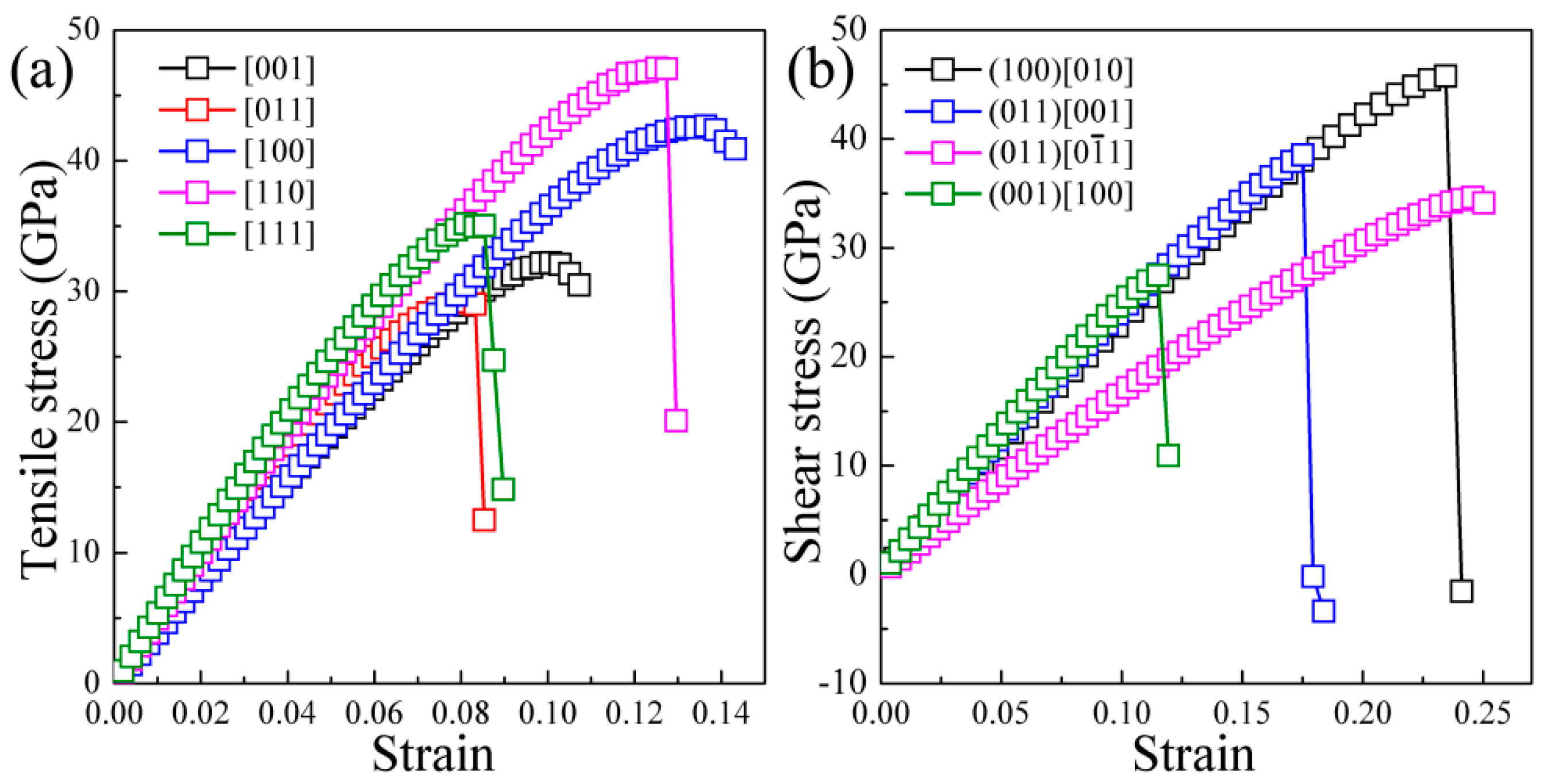
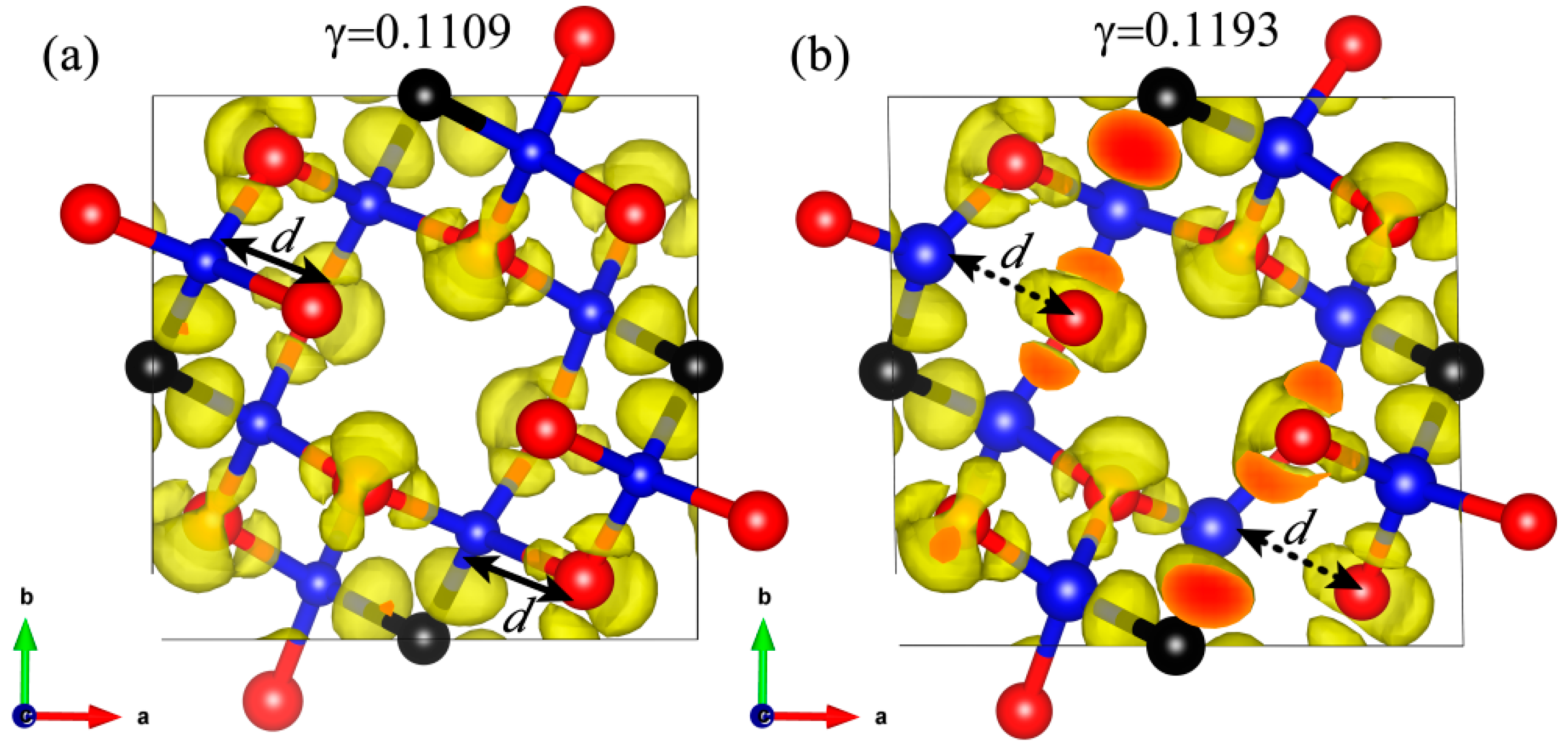
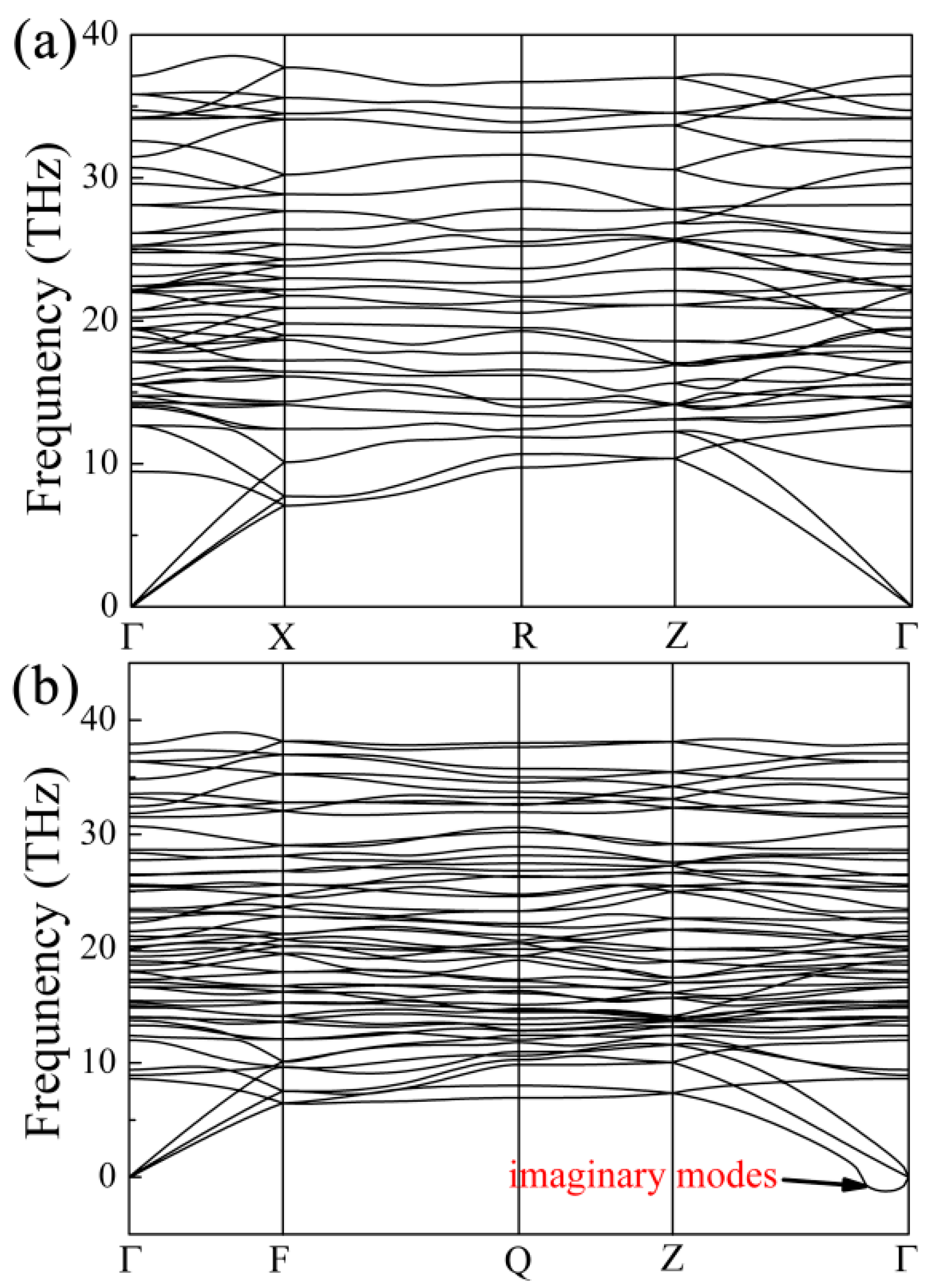
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, Y.; Xu, B.; Zhao, Z. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 2012, 33, 93–106. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Weinberger, M.B.; Levine, J.B.; Kavner, A.; Yang, J.-M.; Tolbert, S.H.; Kaner, R.B. Synthesis of Ultra-Incompressible Superhard Rhenium Diboride at Ambient Pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Cumberland, R.W.; Weinberger, M.B.; Gilman, J.J.; Clark, S.M.; Tolbert, S.H.; Kaner, R.B. Osmium Diboride, An Ultra-Incompressible, Hard Material. J. Am. Chem. Soc. 2005, 127, 7264–7265. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Lech, A.T.; Xie, M.; Weaver, B.E.; Yeung, M.T.; Tolbert, S.H.; Kaner, R.B. Tungsten tetraboride, an inexpensive superhard material. Proc. Natl. Acad. Sci. USA 2011, 108, 10958–10962. [Google Scholar] [CrossRef] [PubMed]
- Lech, A.T.; Turner, C.L.; Mohammadi, R.; Tolbert, S.H.; Kaner, R.B. Structure of superhard tungsten tetraboride: A missing link between MB2 and MB12 higher borides. Proc. Natl. Acad. Sci. USA 2015, 112, 3223–3228. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Dubrovinskaia, N.; Bykova, E.; Tsirlin, A.A.; Kasinathan, D.; Schnelle, W.; Richter, A.; Merlini, M.; Hanfland, M.; Abakumov, A.M.; et al. Discovery of a Superhard Iron Tetraboride Superconductor. Phys. Rev. Lett. 2013, 111, 157002. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.R.S.; Sharat, C.; Amirthapandian, S.; Shekar, N.V.C.; Sahu, P.C. Investigations of the high pressure synthesized osmium carbide by experimental and computational techniques. Mater. Res. Express 2015, 2, 016503. [Google Scholar] [CrossRef]
- Crowhurst, J.C.; Goncharov, A.F.; Sadigh, B.; Evans, C.L.; Morrall, P.G.; Ferreira, J.L.; Nelson, A.J. Synthesis and Characterization of the Nitrides of Platinum and Iridium. Science 2006, 311, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.-K. Synthesis of Novel Transition Metal Nitrides IrN2 and OsN2. Phys. Rev. Lett. 2006, 96, 155501. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; He, D.; Wang, J.; Fang, L.; Lei, L.; Li, Y.; Hu, J.; Kou, Z.; Bi, Y. Is Rhenium Diboride a Superhard Material? Adv. Mater. 2008, 20, 4780–4783. [Google Scholar] [CrossRef]
- Yang, J.; Sun, H.; Chen, C. Is Osmium Diboride An Ultra-Hard Material? J. Am. Chem. Soc. 2008, 130, 7200–7201. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, H.; Chen, C. First-principles calculation of the indentation strength of FeB4. Phys. Rev. B 2014, 90, 014106. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, M.; Du, Y.; Gao, L.; Lu, C.; Liu, H. Hardness of FeB4: Density functional theory investigation. J. Chem. Phys. 2014, 140, 174505. [Google Scholar] [CrossRef] [PubMed]
- Zinin, P.V.; Ming, L.C.; Ishii, H.A.; Jia, R.; Acosta, T.; Hellebrand, E. Phase transition in BCx system under high-pressure and high-temperature: Synthesis of cubic dense BC3 nanostructured phase. J. Appl. Phys. 2012, 111, 114905. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Kurakevych, O.O.; Andrault, D.; Le Godec, Y.; Mezouar, M. Ultimate Metastable Solubility of Boron in Diamond: Synthesis of Superhard Diamondlike BC5. Phys. Rev. Lett. 2009, 102, 015506. [Google Scholar] [CrossRef] [PubMed]
- Solozhenko, V.L.; Andrault, D.; Fiquet, G.; Mezouar, M.; Rubie, D.C. Synthesis of superhard cubic BC2N. Appl. Phys. Lett. 2001, 78, 1385–1387. [Google Scholar] [CrossRef]
- Badzian, A.R. Superhard material comparable in hardness to diamond. Appl. Phys. Lett. 1988, 53, 2495–2497. [Google Scholar] [CrossRef]
- Garvie, L.A.J.; Hubert, H.; Petuskey, W.T.; McMillan, P.F.; Buseck, P.R. High-Pressure, High-Temperature syntheses in the B–C–N–O system. J. Solid State Chem. 1997, 133, 365–371. [Google Scholar] [CrossRef]
- Bolotina, N.B.; Dyuzheva, T.I.; Bendeliani, N.A. Atomic structure of boron suboxycarbide B(C,O)0.155. Crystallogr. Rep. 2001, 46, 734–740. [Google Scholar] [CrossRef]
- Gladkaya, I.S.; Dyuzheva, T.I.; Ekimov, E.A.; Nikolaev, N.A.; Bendeliani, N.A. Crystal growth at high pressure and the problem of characterization of the interstitial phases in the B–C–O system. J. Alloys Compd. 2001, 329, 153–156. [Google Scholar] [CrossRef]
- Broitman, E.; Gueorguiev, G.K.; Furlan, A.; Son, N.T.; Gellman, A.J.; Stafström, S.; Hultman, L. Water adsorption on fullerene-like carbon nitride overcoats. Thin Solid Film. 2008, 517, 1106–1110. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Czigány, Z.; Furlan, A.; Stafström, S.; Hultman, L. Intercalation of P atoms in Fullerene-like CPx. Chem. Phys. Lett. 2011, 501, 400–403. [Google Scholar] [CrossRef]
- Hellgren, N.; Berlind, T.; Gueorguiev, G.K.; Johansson, M.P.; Stafström, S.; Hultman, L. Fullerene-like BCN thin films: A computational and experimental study. Mater. Sci. Eng. B 2004, 113, 242–247. [Google Scholar] [CrossRef]
- Yinwei, L.; Quan, L.; Yanming, M. B2CO: A potential superhard material in the B-C-O system. Europhys. Lett. 2011, 95, 66006. [Google Scholar]
- Liu, C.; Zhao, Z.; Luo, K.; Hu, M.; Ma, M.; He, J. Superhard orthorhombic phase of B2CO compound. Diam. Relat. Mater. 2016. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Zheng, B.; Wei, Q. Influences of carbon concentration on crystal structures and ideal strengths of B2CxO compounds in the B-C-O system. Sci. Rep. 2015, 5, 15481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Oganov, A.R.; Qian, G.; Zhu, Q.; Dong, H.; Dong, X.; Davari Esfahani, M.M. Novel superhard B-C-O phases predicted from first principles. Phys. Chem. Chem. Phys. 2016, 18, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Lond. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Roundy, D.; Krenn, C.R.; Cohen, M.L.; Morris, J.W. Ideal Shear Strengths of fcc Aluminum and Copper. Phys. Rev. Lett. 1999, 82, 2713–2716. [Google Scholar] [CrossRef]
- Zhang, R.F.; Sheng, S.H.; Veprek, S. First principles studies of ideal strength and bonding nature of AlN polymorphs in comparison to TiN. Appl. Phys. Lett. 2007, 91, 031906. [Google Scholar] [CrossRef]
- Zhang, R.F.; Veprek, S.; Argon, A.S. Anisotropic ideal strengths and chemical bonding of wurtzite BN in comparison to zincblende BN. Phys. Rev. B 2008, 77, 172103. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, C.-Y. Mechanical properties and electronic structure of superhard diamondlike BC5: A first-principles study. J. Appl. Phys. 2009, 106, 043513. [Google Scholar] [CrossRef]
- Zhang, R.F.; Lin, Z.J.; Veprek, S. Anisotropic ideal strengths of superhard monoclinic and tetragonal carbon and their electronic origin. Phys. Rev. B 2011, 83, 155452. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.-X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Kelly, A.; MacMillan, N.H. Strong Solids, 3rd ed.; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- Lyakhov, A.O.; Oganov, A.R. Evolutionary search for superhard materials: Methodology and applications to forms of carbon and TiO2. Phys. Rev. B 2011, 84, 092103. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Veprek, S.; Zhang, R.F.; Argon, A.S. Mechanical properties and hardness of boron and boron-rich solids. J. Superhard Mater. 2011, 33, 409–420. [Google Scholar] [CrossRef]
| Compounds | Source | C11 | C33 | C44 | C66 | C12 | C13 | C16 | B | G | E | σmin | τmin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t-B4CO4 | This work | 481 | 452 | 269 | 260 | 150 | 129 | −52 | 248 | 218 | 505 | = 29.0 | = 27.5 |
| Theory 1 | 480 | 449 | 268 | 259 | 152 | 131 | 248 | 220 | 509 | ||||
| tI16-B2CO | Theory 2 | 600 | 646 | 304 | 283 | 182 | 144 | 310 | 265 | ||||
| tP4-B2CO | Theory 3 | 736 | 591 | 240 | 254 | 53 | 157 | 311 | 254 | = 6.1 | = 2.6 | ||
| oP8-B2CO | Theory 4 | 732 | 675 | 238 | 260 | 112 | 69 | 298 | 270 | ||||
| B2C2O | Theory 5 | 763 | 590 | 229 | 174 | 15 | 135 | 299 | 264 | 611 | = 21.3 | = 11.1 | |
| B2C3O | Theory 5 | 808 | 664 | 283 | 299 | 32 | 183 | 322 | 302 | 690 | = 22.4 | = 11.4 | |
| B2C5O | Theory 5 | 889 | 740 | 346 | 335 | 30 | 135 | 345 | 351 | 787 | = 25.1 | = 16.3 | |
| B2O | Theory 2 | 327 | 497 | 232 | 207 | 230 | 144 | 242 | 124 | ||||
| c-BN | Theory 6 | 786 | 445 | 172 | 376 | 390 | = 55.3 | = 58.3 | |||||
| Diamond | Theory 7 | 1052 | 555 | 122 | 432 | 517 | 1109 | = 82.3 | = 86.8 |
| Tensile Plane | Orientation Angle θ | |
|---|---|---|
| (001) | between [hk0] and [100] | |
| (100) | between [0kl] and [001] | |
| between [hkl] and [001] |
| Shear Plane | Orientation Angle θ | |
|---|---|---|
| (001) | between [uvw] and [100] | |
| (100) | between [uvw] and [001] | |
| between [uvw] and [001] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Zhang, M.; Wang, C. Exploring the Mechanical Anisotropy and Ideal Strengths of Tetragonal B4CO4. Materials 2017, 10, 128. https://doi.org/10.3390/ma10020128
Zheng B, Zhang M, Wang C. Exploring the Mechanical Anisotropy and Ideal Strengths of Tetragonal B4CO4. Materials. 2017; 10(2):128. https://doi.org/10.3390/ma10020128
Chicago/Turabian StyleZheng, Baobing, Meiguang Zhang, and Canjun Wang. 2017. "Exploring the Mechanical Anisotropy and Ideal Strengths of Tetragonal B4CO4" Materials 10, no. 2: 128. https://doi.org/10.3390/ma10020128






