Refining Mechanism of 7075 Al Alloy by In-Situ TiB2 Particles
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
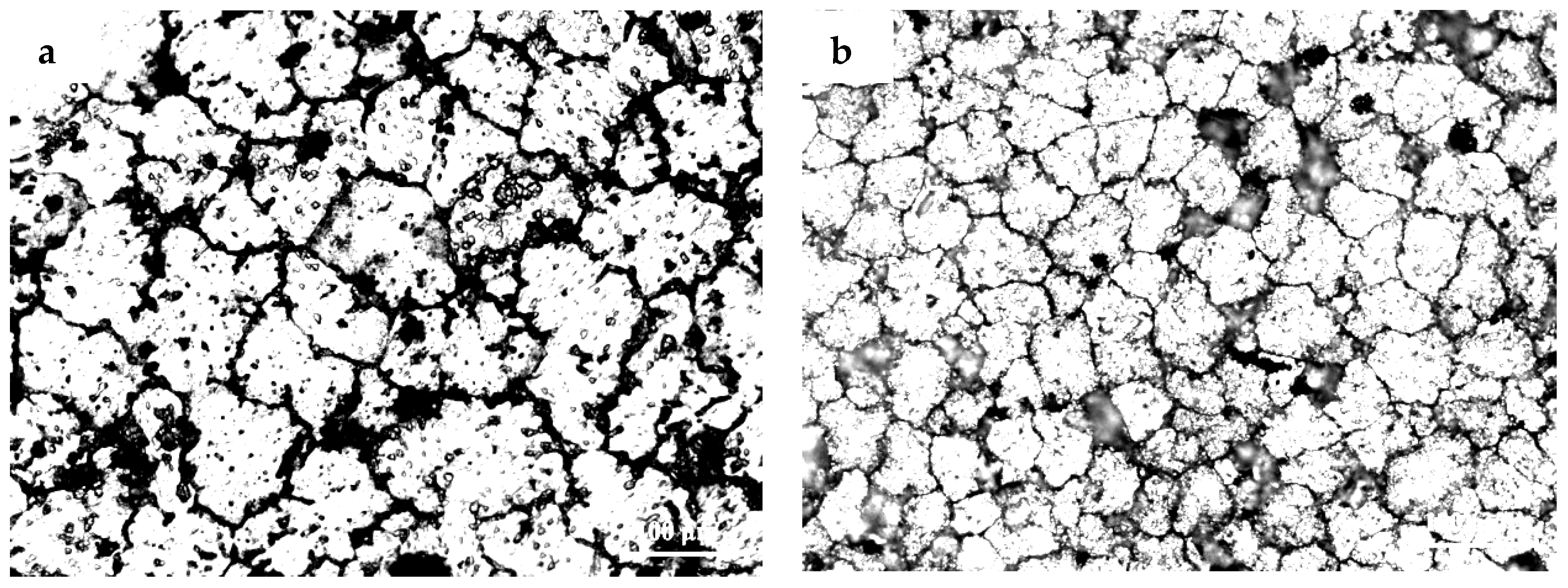
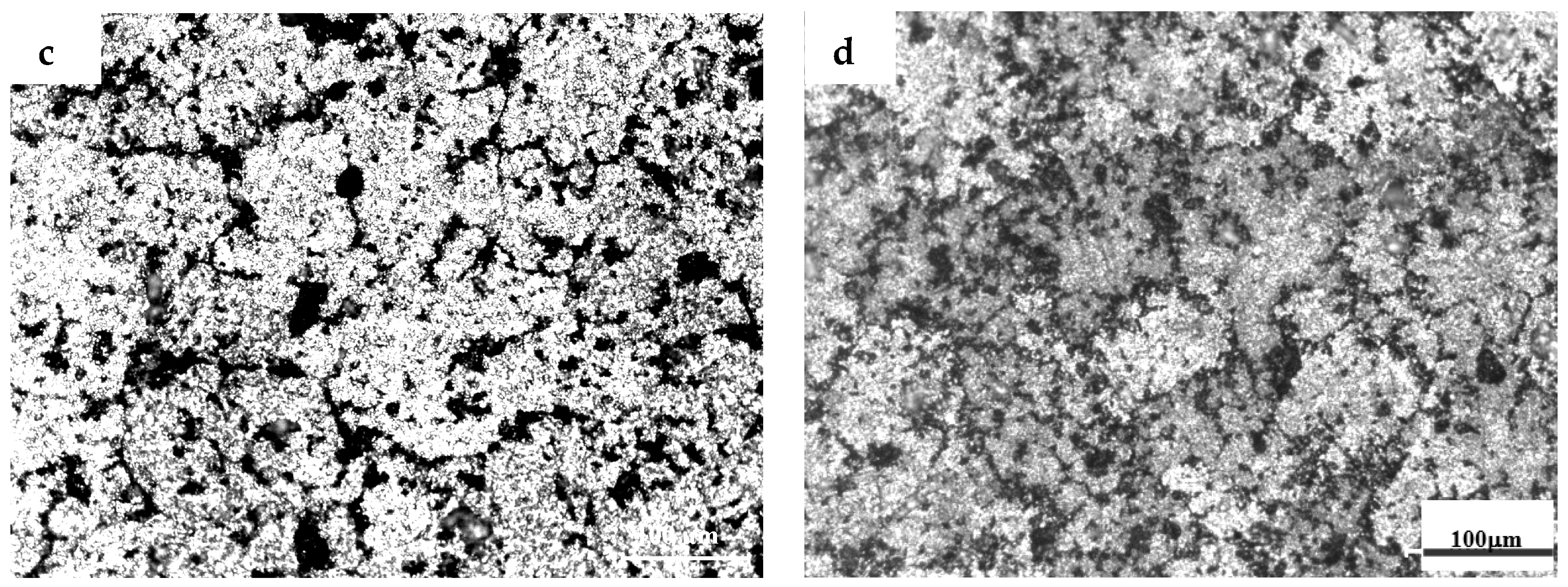
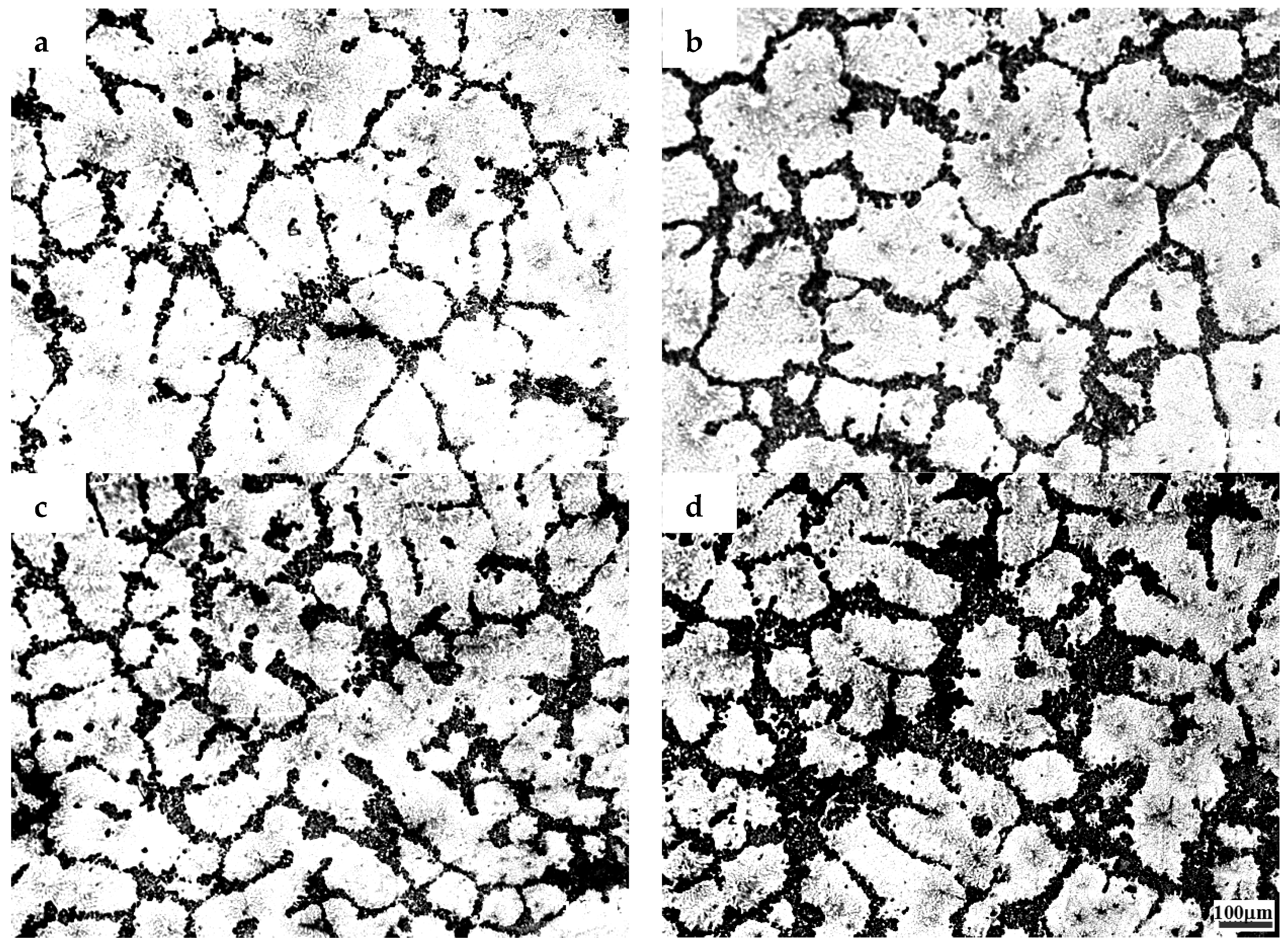
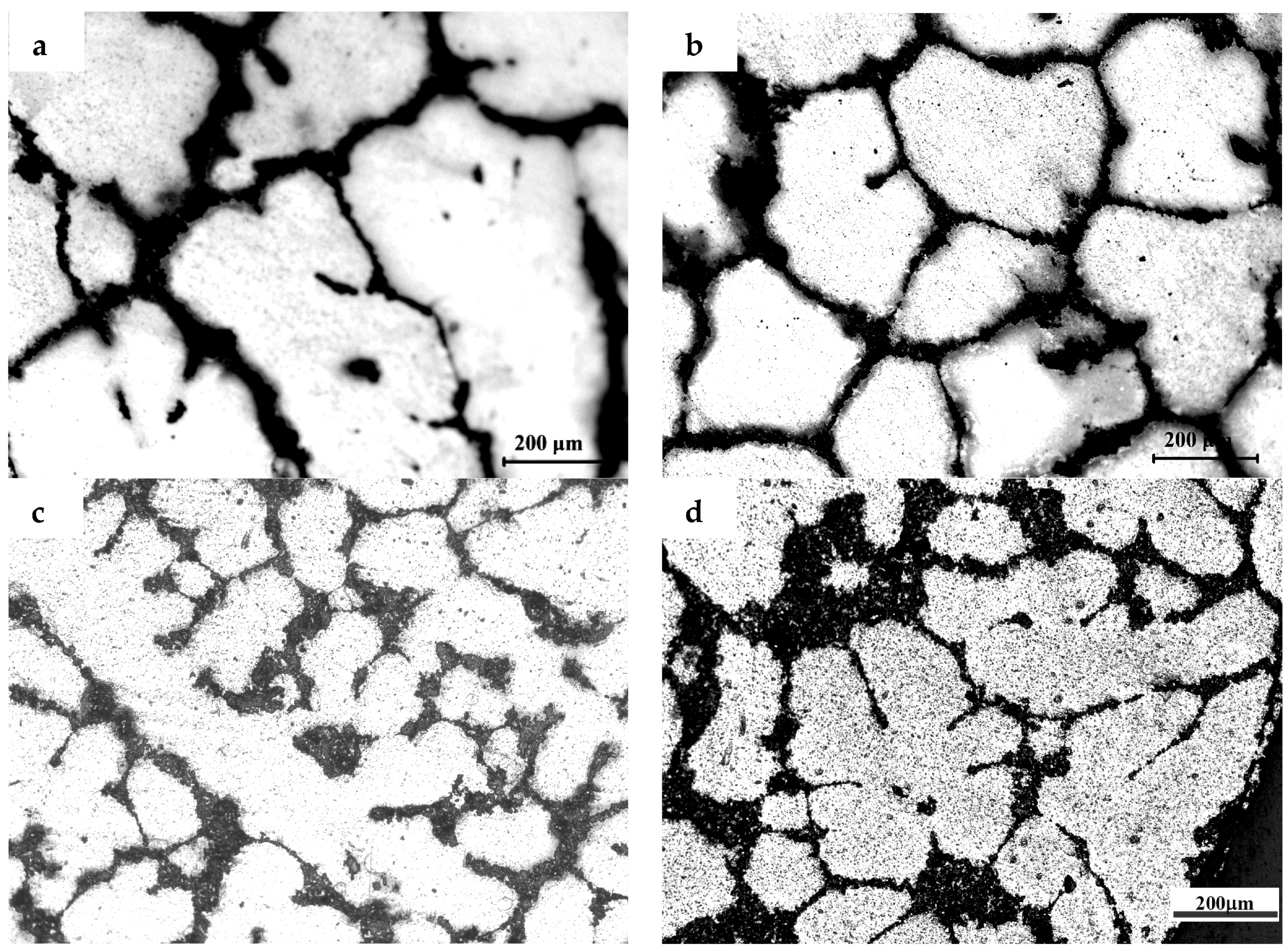
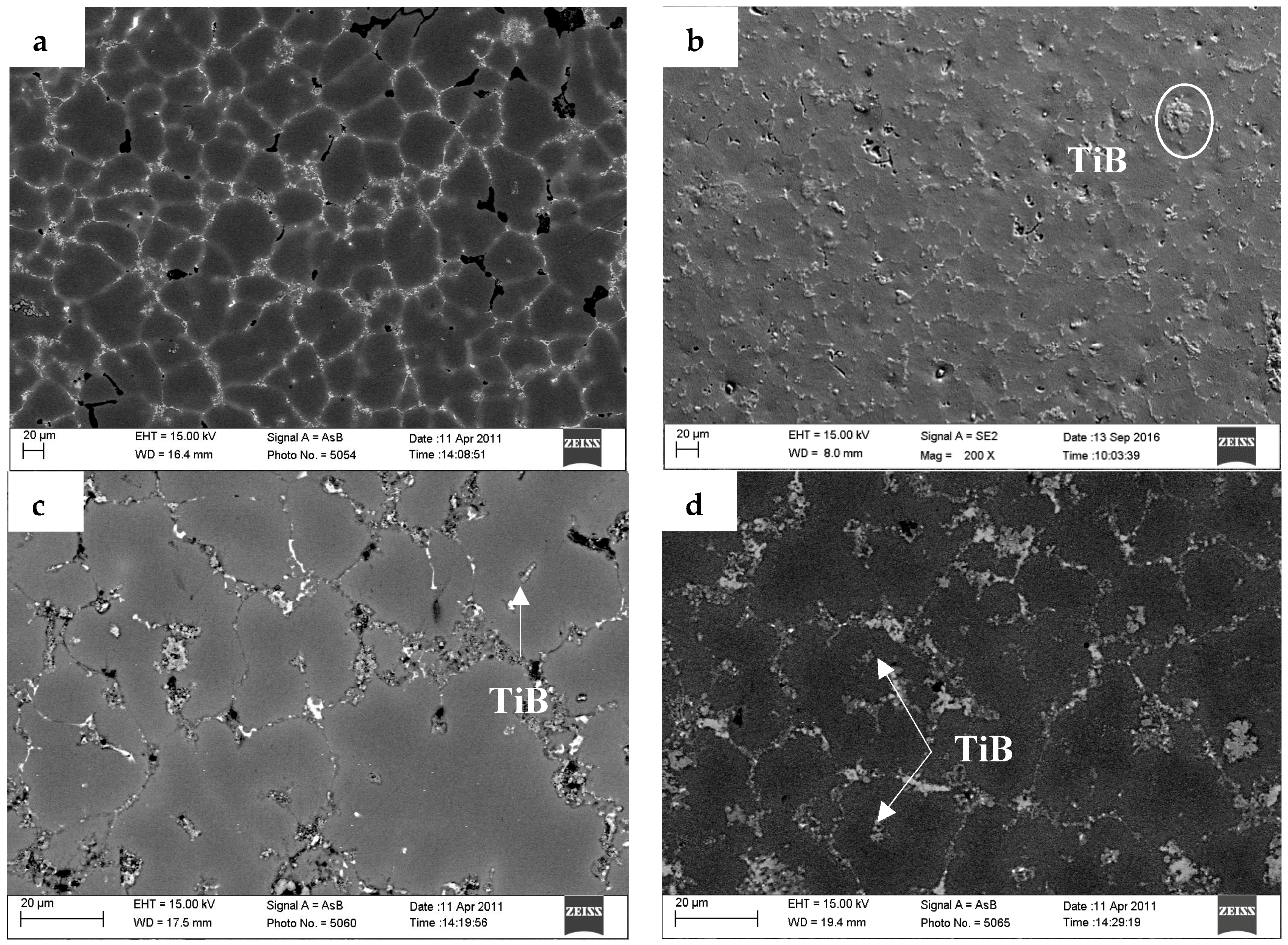
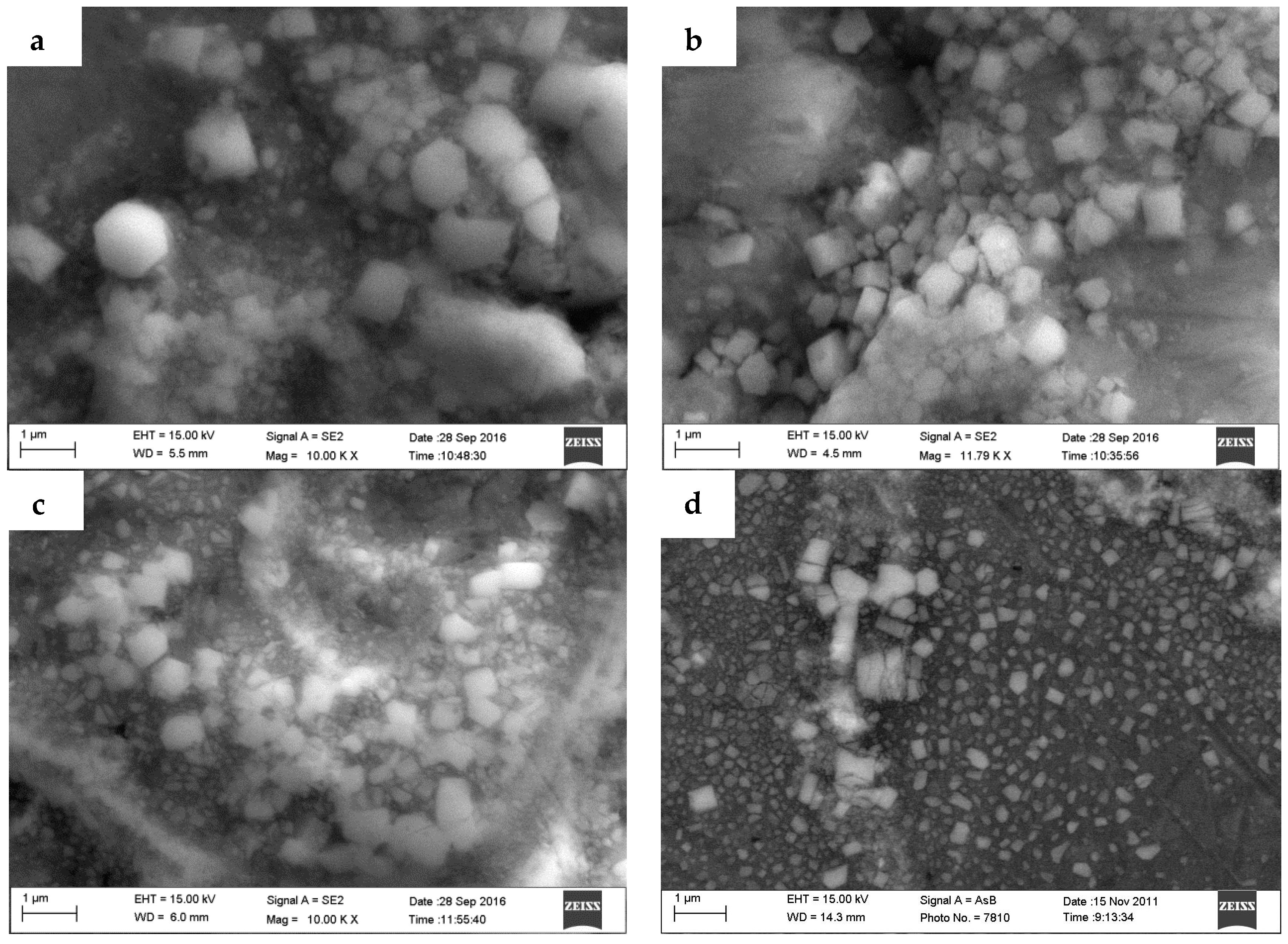
3.1. The Microstructures of TiB2/7075 Al Matrix Composites with Different Cooling Rate
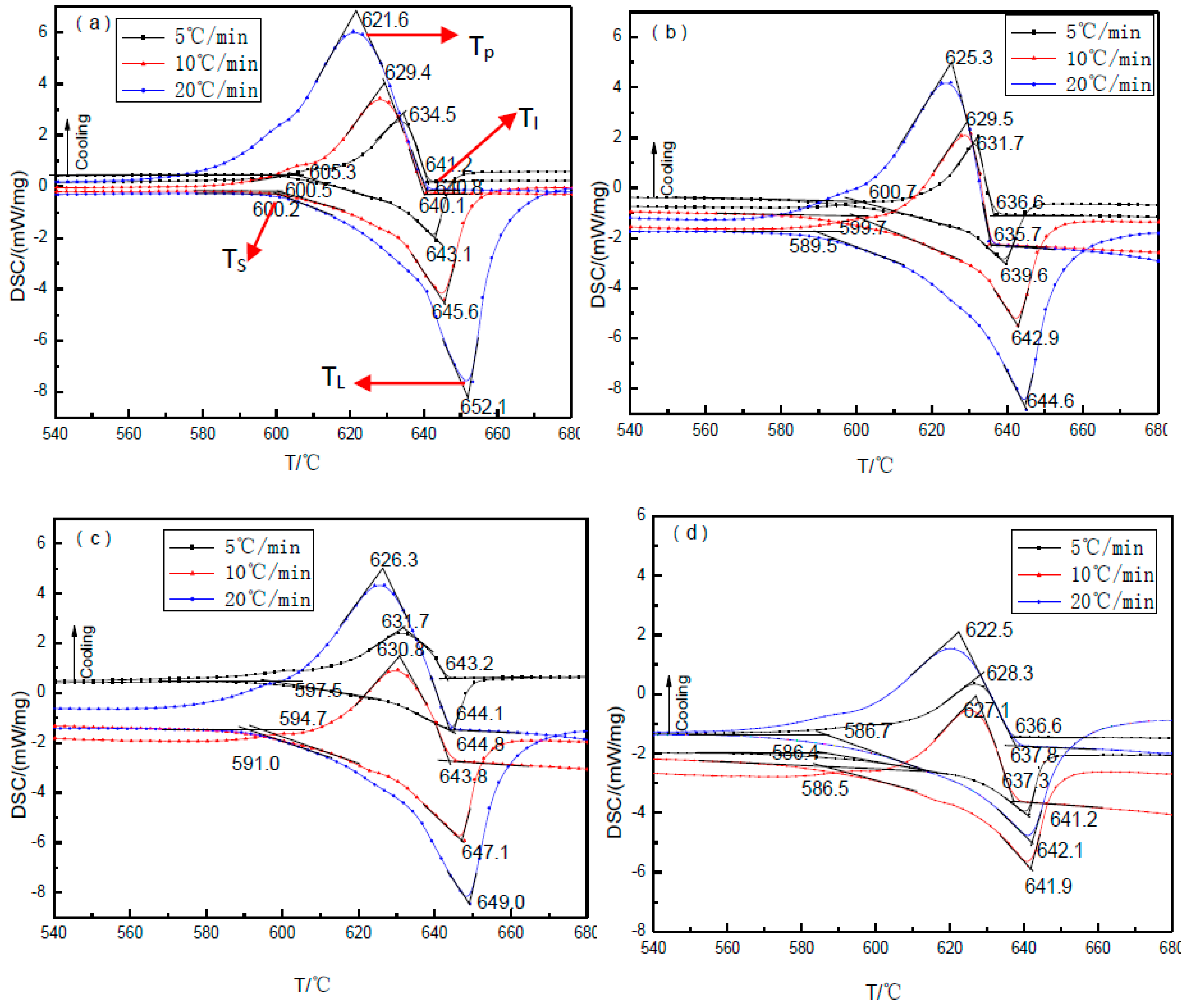
3.2. The Undercooling of TiB2/7075 Al Matrix Composites with Different Cooling Rate
3.3. Effect of TiB2 Content on the Nucleation of 7075 Al Alloy
4. Conclusions
- (1)
- The grain sizes of TiB2/7075 Al matrix composites firstly decreased, then increased, and finally decreased again with the increase of TiB2 content. The grain sizes of TiB2/7075 Al matrix composites increased with decreasing cooling rate, but the changes were not very noticeable from 20 °C/min to 5 °C/min. The mean grain size of 3.0% and 4.5% TiB2/7075 Al matrix composites could reach 236 μm and 165 μm, respectively, at a cooling rate of 1 °C/min.
- (2)
- The liquidus temperatures of TiB2/7075 Al matrix composites firstly decreased, then increased, and finally decreased again with the increase of TiB2 content. The initial solidification temperatures and the nucleation undercooling of TiB2/7075 Al matrix composites firstly increased, then decreased, and finally increased again with the increase of TiB2 content. The melting and solidification process showed no significant change with the decrease of cooling rate in 9.0% TiB2/7075 Al matrix composites. A large change of the nucleation undercooling with cooling rate meant a wide range in particle diameter in TiB2/7075 composites, so a small change meant narrow range.
- (3)
- Most small particles can act as heterogeneous nuclei, which induced the grain growth and were captured into the grain by solid/liquid interface. At the same time, most of larger particles and a minority of small TiB2 particles were pushed into the grain boundary, locating in the grain boundary can hinder the Al atoms from diffusing during the solidification of the 7075 Al alloy and restrain α-Al phase growth. The influence of particles shifted from dominating by locating to dominating by nucleation as the quantity of TiB2 particles increased due to the decrease of the average diameter of TiB2 particles.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.; Liu, Z.; Dai, W.; Han, Q. On the understanding of aluminum grain refinement by Al-5Ti-B type master alloys. Metall. Mater. Trans. B 2015, 46, 1620–1625. [Google Scholar] [CrossRef]
- Ghadimi, H.; Nedjhad, S.H.; Eghbali, B. Enhanced grain refinement of cast aluminum alloy by thermal and mechanical treatment of Al-5Ti-B master alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 1563–1569. [Google Scholar] [CrossRef]
- Wang, M.X.; Pang, J.H.; Liu, Z.Y.; Liu, Z.X.; Song, T.F.; Yang, S. Grain refining action of Ti existing in electrolytic low-titanium aluminum with Al-4B addition for superheated Al melt. Trans. Nonferrous Met. Soc. China 2010, 20, 950–957. [Google Scholar] [CrossRef]
- Wang, E.Z.; Liu, X.F. Grain reinement limit of commercial pure al inoculated by Al-Ti-C (B) master alloys. China Foundry 2015, 12, 99–103. [Google Scholar]
- Chen, Z.; Wang, T.; Zheng, Y.; Zhao, Y.; Kang, H.; Gao, L. Development of TiB2 reinforced Aluminum foundry alloy based in situ composites: Part I: An improved halide salt route to fabricate Al-5 wt.% TiB2 master composite. Mater. Sci. Eng. A 2014, 605, 301–309. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Liu, Z.; Liu, Z.; Song, T.; Zuo, X. Effect of B/Ti mass ratio on grain refining of low-titanium aluminum produced by electrolysis. Mater. Sci. Eng. A 2006, 416, 312–316. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, B.; Mei, H.; Wu, H.C. Microstructure and wear resistance of semisolid TiB2/7050 composites produced by serpentine tube pouring technique. Mater. Manuf. Process. 2016, 31, 1029–1036. [Google Scholar] [CrossRef]
- Wang, C.L.; Wang, M.X.; Yu, B.H.; Chen, D.; Qin, P.; Feng, M.H. The grain refinement behavior of TiB2 particles prepared with in situ technology. Mater. Sci. Eng. A 2007, 459, 238–243. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Bian, X.F.; Wang, Y.; Liu, X.F. Microstructure and grain refining performance of melt-spun Al–5Ti–1B master alloy. Mater. Sci. Eng. A 2003, 352, 8–15. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al–Ti–B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Sritharan, T.; Li, H. Influence of titanium to boron ratio on the ability to grain refine aluminium-silicon alloys. J. Mater. Process. Technol. 1997, 63, 585–589. [Google Scholar] [CrossRef]
- Kori, S.A.; Murty, B.S.; Chakraborty, M. Development of an efficient grain refiner for Al–7Si alloy and its modification with strontium. Mater. Sci. Eng. A 2000, 280, 58–61. [Google Scholar] [CrossRef]
- Arnberg, L.; Bäckerud, L.; Klang, H. Evidence of metastable phase in Al–Ti–(B) system. Metals Technol. 1982, 9, 14–17. [Google Scholar] [CrossRef]
- Vatne, H.E. Efficient grain refinement of ingots of commercial wrought aluminium alloys. Part I: Methods for grain refining. Aluminium 1999, 75, 84–90. [Google Scholar]
- Maxwell, I.; Hellawell, A. A simple model for grain refinement during solidification. Scr. Metall. 1975, 23, 229–237. [Google Scholar] [CrossRef]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Greer, A.L. Grain refinement of alloys by inoculation of melts. Philos. Trans. R. Soc. Lond. Ser. A 2003, 361, 479–495. [Google Scholar] [CrossRef]
- Tronche, A.; Greer, A.L. Design of grain refiners for aluminium alloys. In Essential Readings in Light Metals; Springer International Publishing: Cham, Switzerland, 2000; pp. 827–832. [Google Scholar]
- Quested, T.; Greer, A.L.; Cooper, P.S. The Variable Potency of TiB2 Nucleant Particles in the Grain Refinement of Aluminium by Al-Ti-B Additions. Mater. Sci. Forum 2002, 396–402, 53–58. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Jung, H.; Mangelinck-Noël, N.; Bergman, C.; Billia, B. Determination of the average nucleation undercooling of primary Al-phase on refining particles from Al–5.0 wt % Ti–1.0 wt % B in Al-based alloys using DSC. J. Alloys Compd. 2009, 477, 622–627. [Google Scholar] [CrossRef]
- Ning, J.; Li, X.; Jiang, R.; Huang, M. Effect of Al-5Ti-0.2C refiner on nucleation and undercooling degree of 7085 aluminum alloy. Zhongnan Daxue Xuebao 2015, 46, 2837–2842. (In Chinese) [Google Scholar]
- Li, Y.L.; Feng, H.K.; Cao, F.R. Effect of high density ultrasonic on the microstructure and refining property of Al-5Ti-0.25C grain refiner alloy. Mater. Sci. Eng. A 2008, 487, 518–523. [Google Scholar] [CrossRef]
- Gan, G.S.; Yang, B.; Wu, H.C.; Han, J.; Gao, Q.; Du, C.H. The Effect of TiB2 particles on the microstructure of semi-solid 7075 Alloy slurry. Mater. Trans. 2012, 53, 1178–1183. [Google Scholar] [CrossRef]
- Pysz, S.; Czekaj, E.; Żuczek, R.; Maj, M.; Piekło, J. Low cycle mechanical and fatigue properties of AlZnMgCu alloy. Arch. Foundry Eng. 2016, 16, 55–60. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. Grain refinement of al alloys: Mechanisms determining as-cast grain size in directional solidification. Acta Mater. 2005, 53, 4643–4653. [Google Scholar] [CrossRef]
- Lakshmi, S.; Lu, L.; Gupta, M. In situ preparation of TiB2, reinforced al based composites. J. Mater. Process. Technol. 1998, 73, 160–166. [Google Scholar] [CrossRef]



| Material | 3% TiB2/7075 | 4.5% TiB2/7075 | 6% TiB2/7075 | 9% TiB2/7075 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heating rate (°C·min−1) | 5 | 10 | 20 | 5 | 10 | 20 | 5 | 10 | 20 | 5 | 10 | 20 |
| Solidus temperature (°C) | 605.3 | 600.5 | 600.2 | 600.7 | 599.7 | 589.5 | 597.5 | 594.7 | 591.0 | 586.4 | 586.5 | 586.7 |
| Liquidus temperature(°C) | 643.1 | 645.6 | 652.1 | 639.6 | 642.9 | 644.6 | 644.8 | 647.1 | 649.0 | 641.2 | 641.9 | 642.1 |
| Temperature differences (°C) | 37.8 | 44.9 | 51.9 | 38.9 | 43.2 | 55.1 | 47.3 | 52.4 | 58.0 | 54.8 | 54.4 | 55.6 |
| Initial solidification temperature (°C) | 641.2 | 640.1 | 640.8 | 636.6 | 635.6 | 635.6 | 643.2 | 643.8 | 644.1 | 636.6 | 637.3 | 637.8 |
| Peak temperatures of solidification (°C) | 634.5 | 629.4 | 621.6 | 631.7 | 629.5 | 625.3 | 631.7 | 630.8 | 626.3 | 628.3 | 627.1 | 622.5 |
| Nucleation undercooling (°C) | 1.9 | 5.5 | 11.4 | 3.0 | 7.3 | 9.0 | 1.6 | 3.3 | 4.9 | 4.6 | 4.6 | 4.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, G.; Yang, B.; Zhang, B.; Jiang, X.; Shi, Y.; Wu, Y. Refining Mechanism of 7075 Al Alloy by In-Situ TiB2 Particles. Materials 2017, 10, 132. https://doi.org/10.3390/ma10020132
Gan G, Yang B, Zhang B, Jiang X, Shi Y, Wu Y. Refining Mechanism of 7075 Al Alloy by In-Situ TiB2 Particles. Materials. 2017; 10(2):132. https://doi.org/10.3390/ma10020132
Chicago/Turabian StyleGan, Guisheng, Bin Yang, Bo Zhang, Xin Jiang, Yunlong Shi, and Yiping Wu. 2017. "Refining Mechanism of 7075 Al Alloy by In-Situ TiB2 Particles" Materials 10, no. 2: 132. https://doi.org/10.3390/ma10020132





