Preparation and Performance of Poly(butyl fumarate)-Based Material for Potential Application in LED Encapsulation
Abstract
:1. Introduction
2. Experiments
2.1. Materials
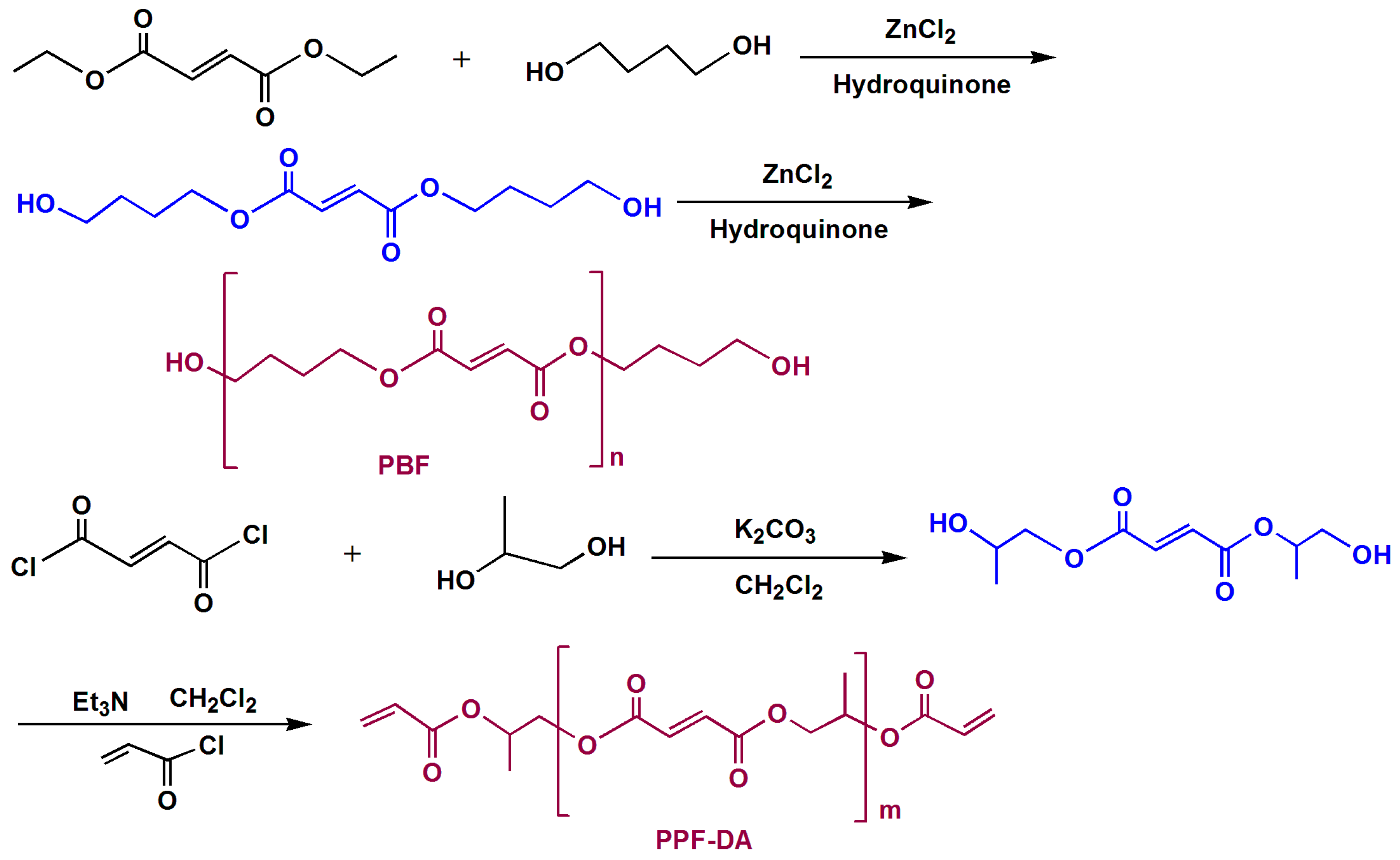
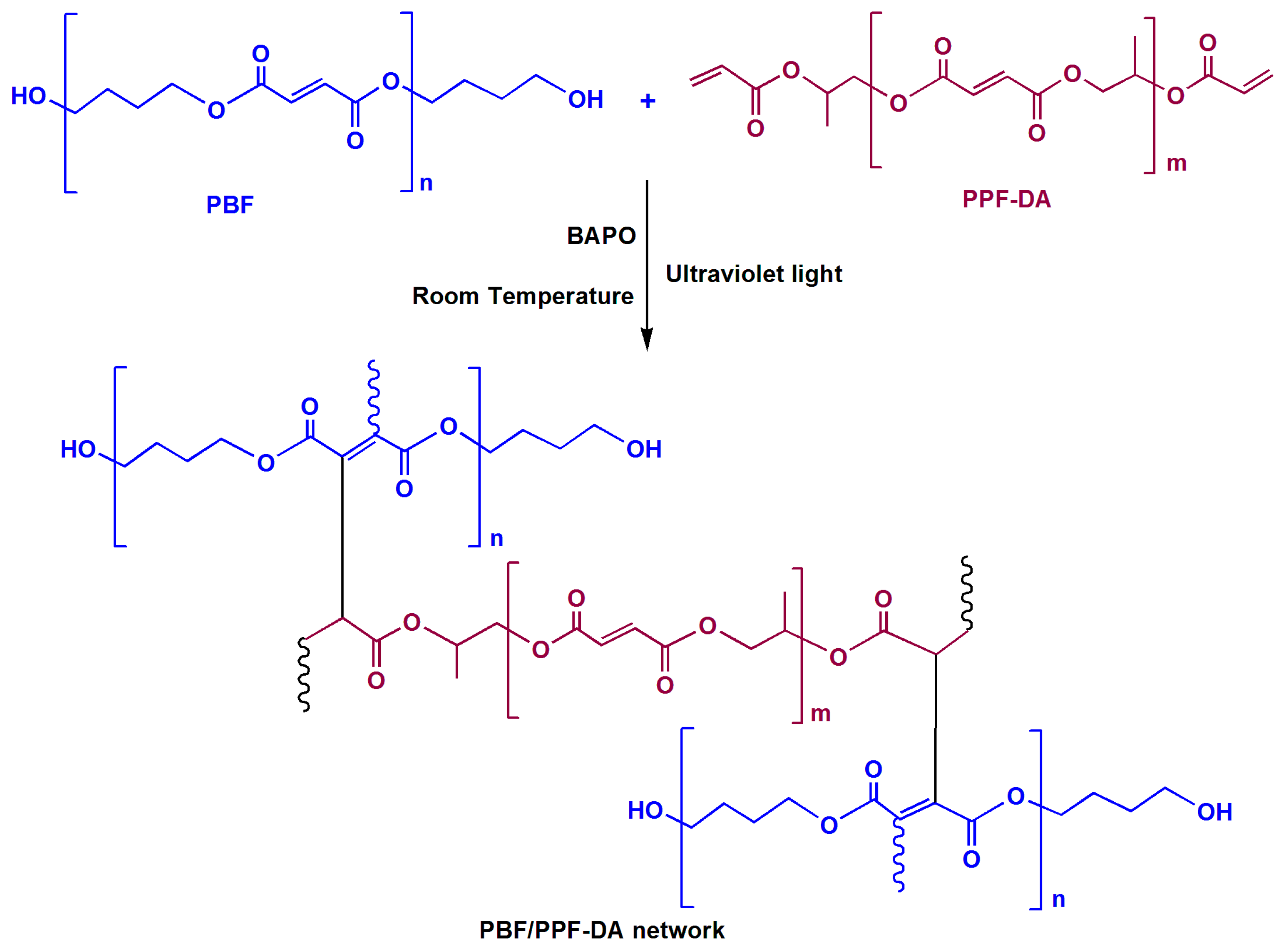
2.2. Preparation of LED Encapsulation Material
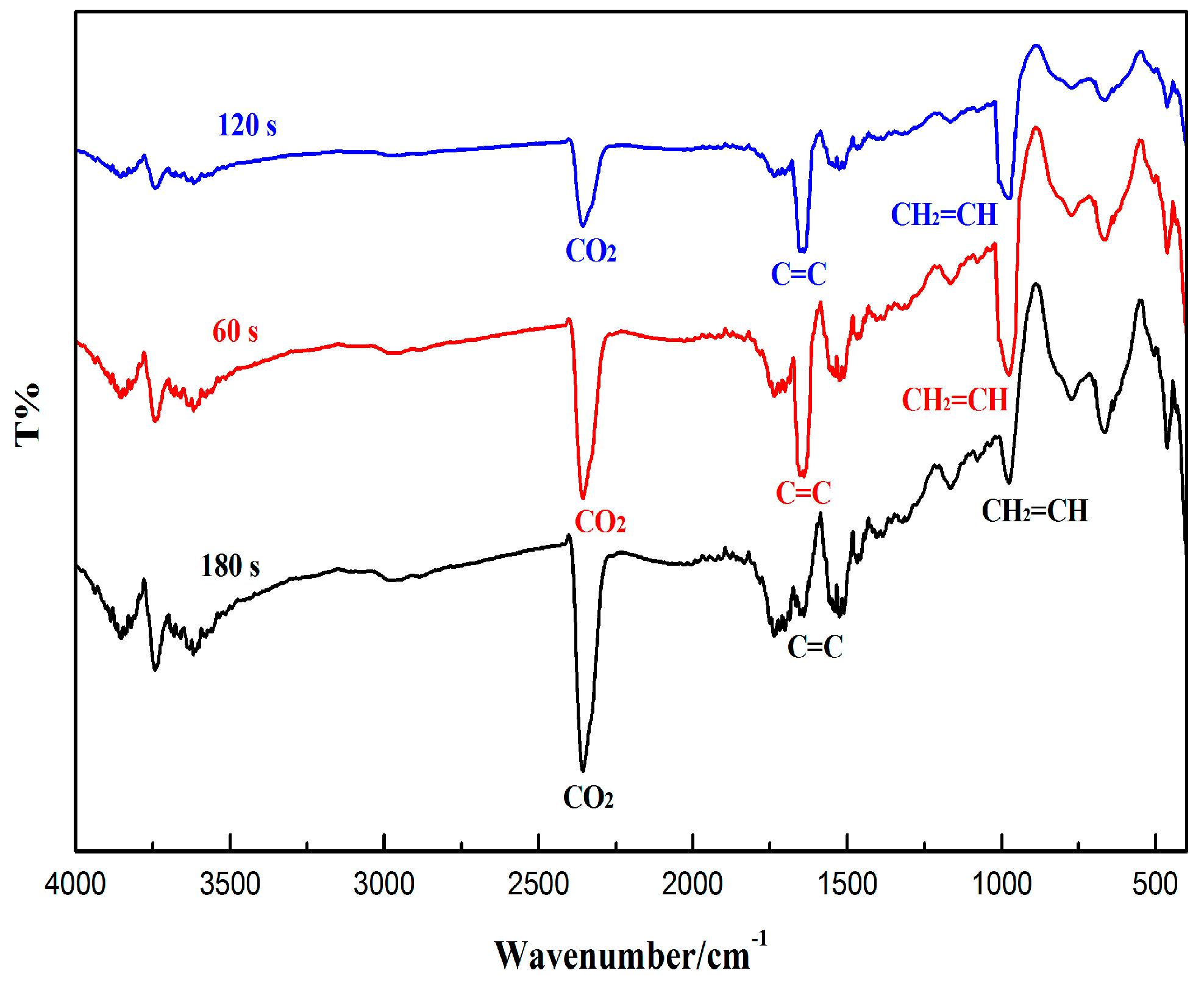
2.3. Degree of Conversion
2.4. Measurements and Characterization
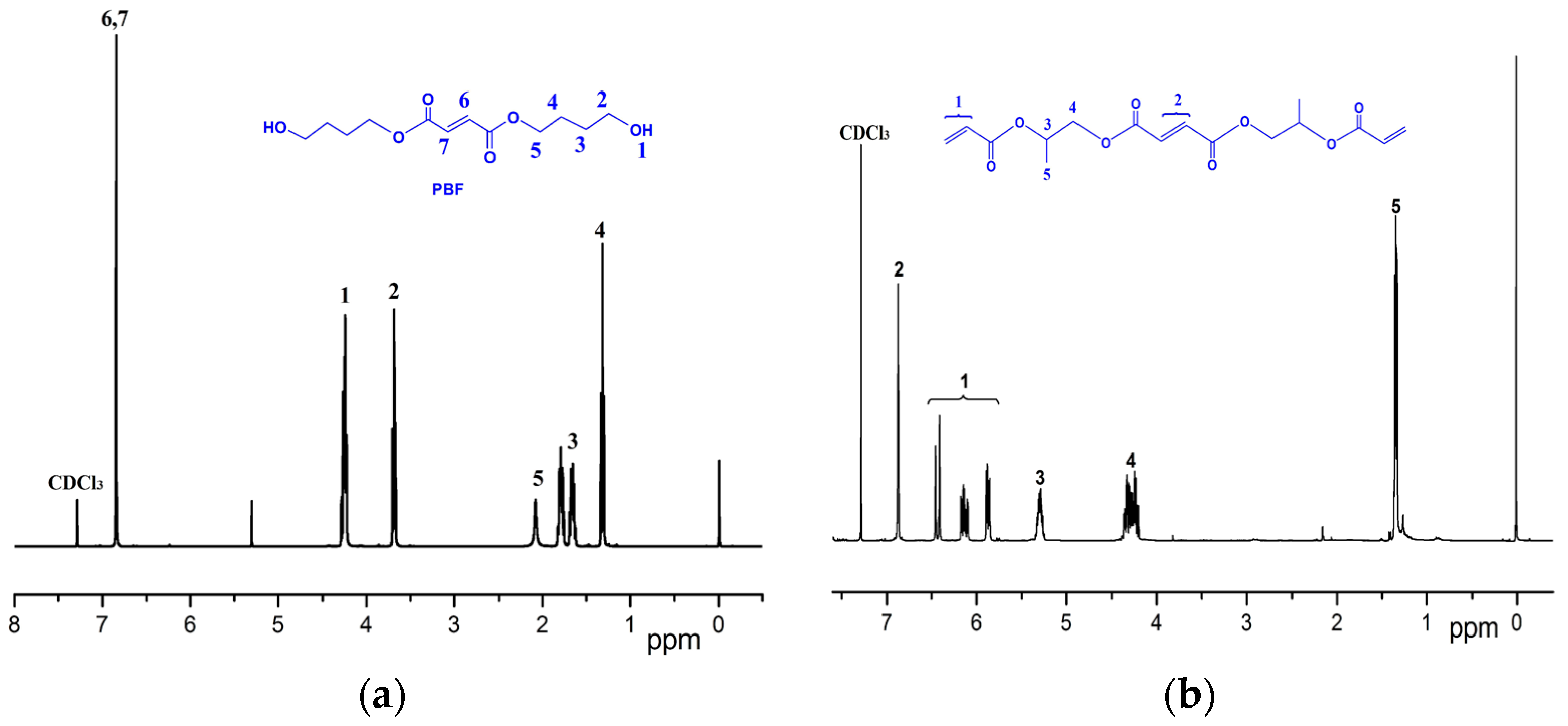
2.4.1. FT-IR and 1H NMR
2.4.2. TGA and DSC
2.4.3. Optical Properties
2.4.4. Water Absorption Test
2.4.5. Mechanical Properties
3. Results and Discussion
3.1. Characterization of Chemical Modifications
3.2 Curing Behavior Monitored by FT-IR
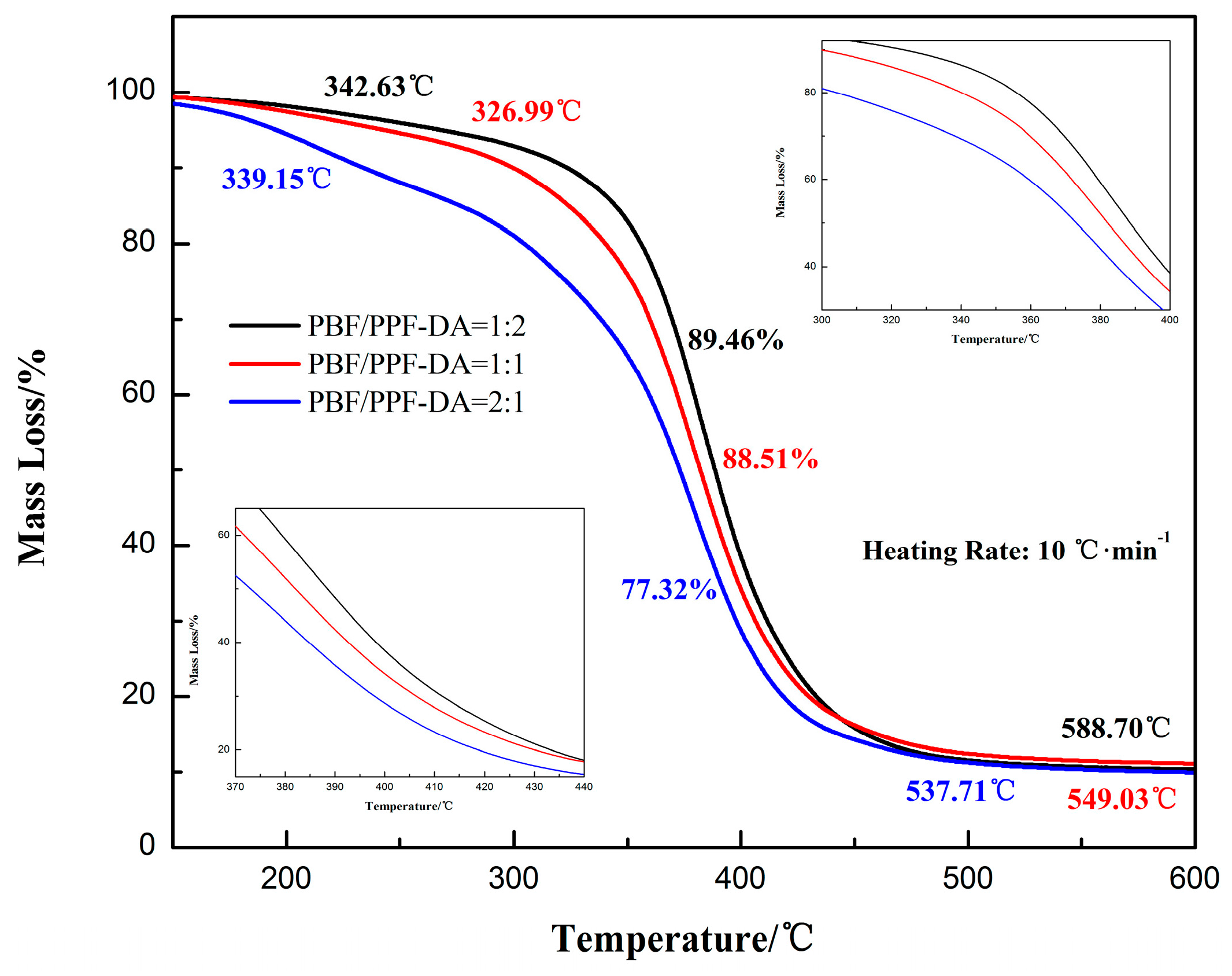
3.3. Thermal Behavior Analysis
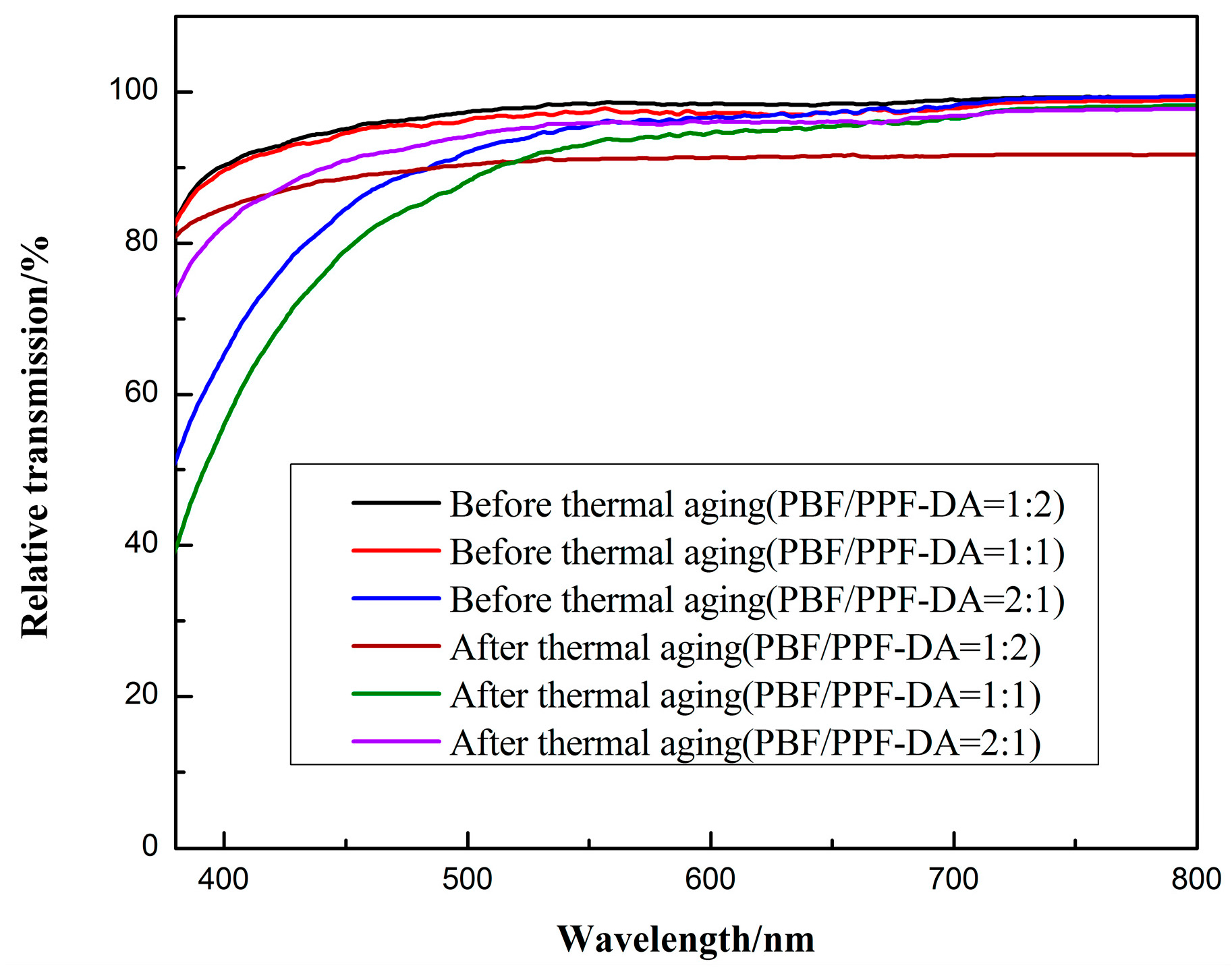
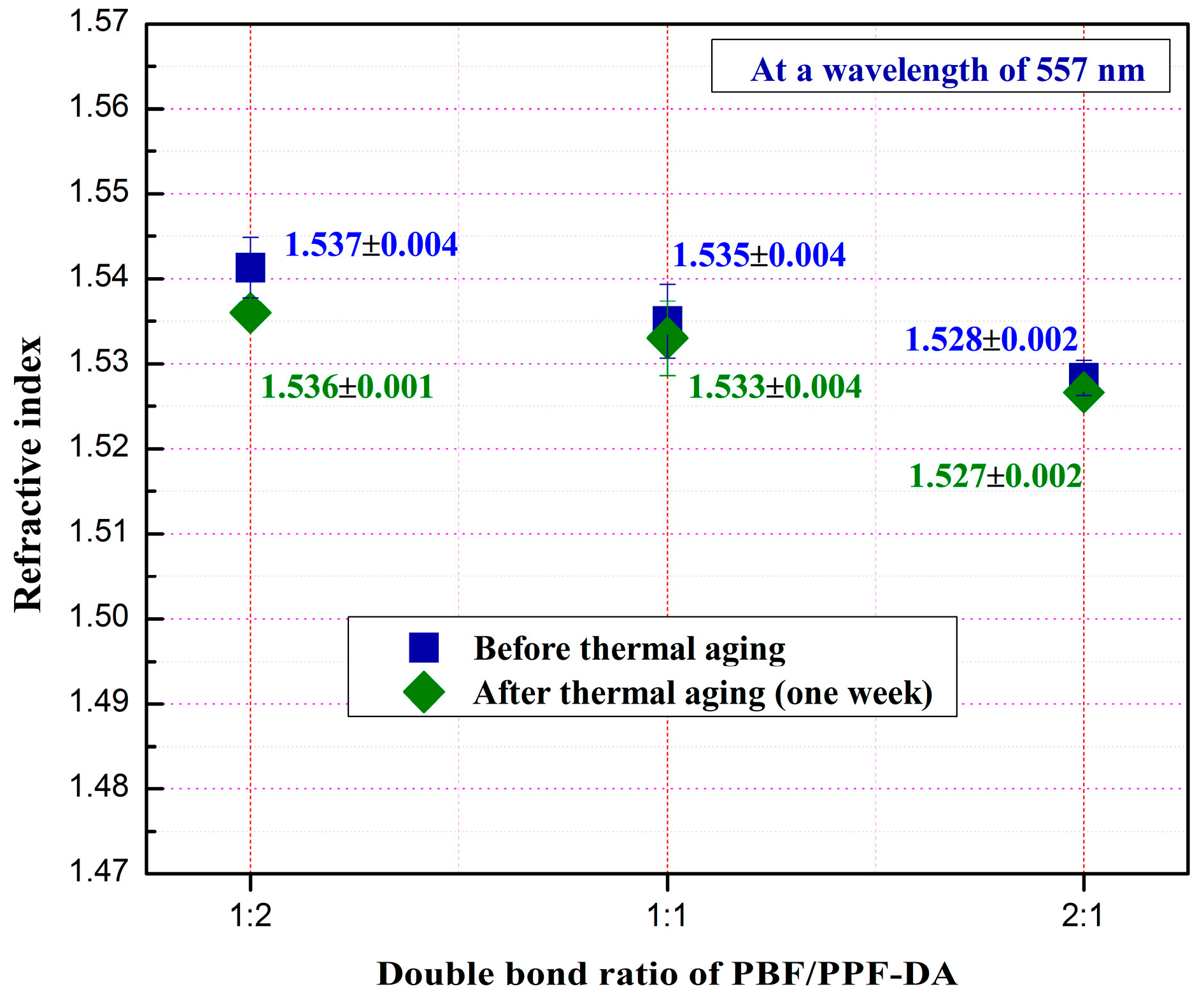
3.4. Optical Properties
3.5. Water Absorption Characterization of the Prepared Encapsulation Material
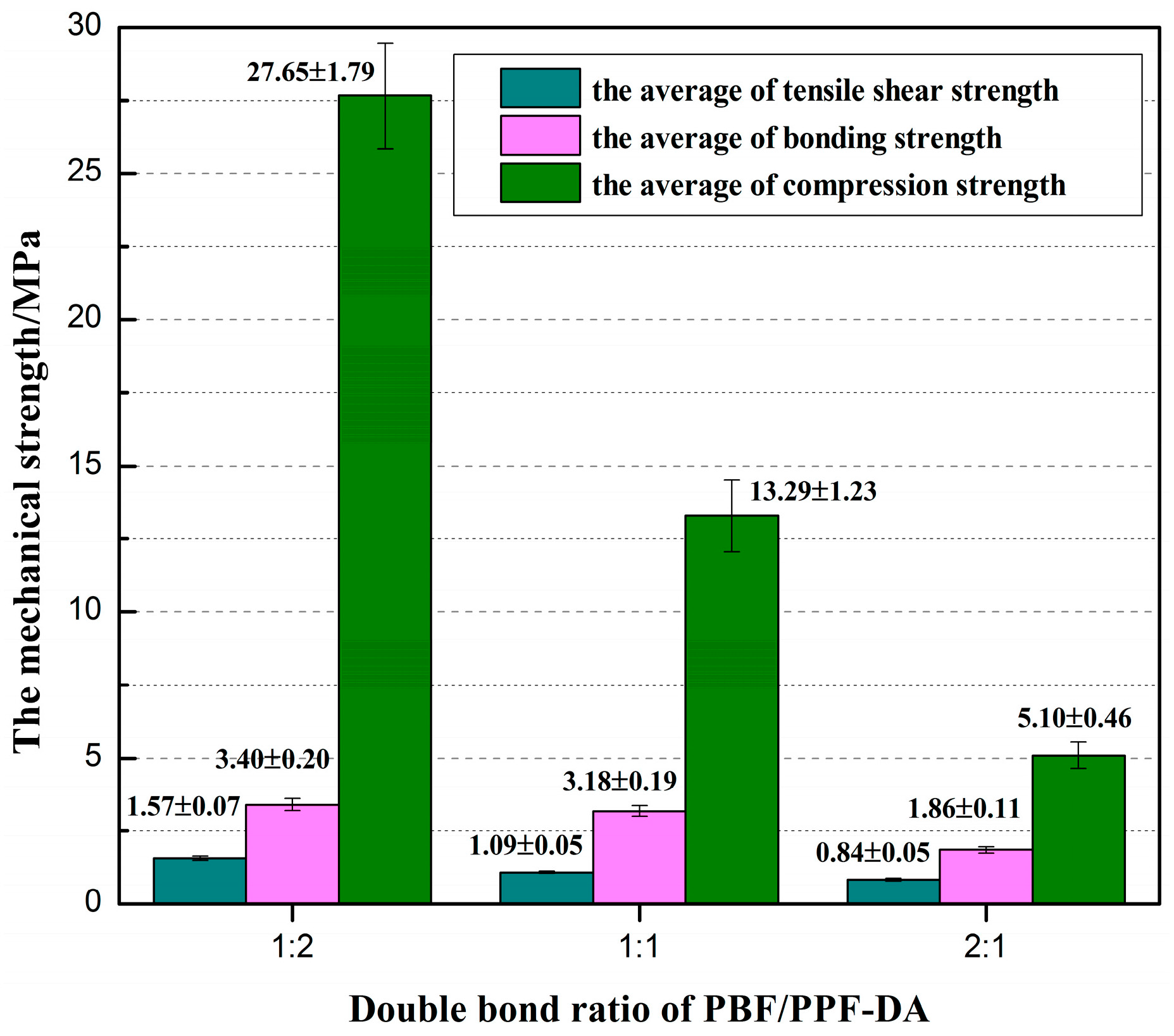
3.6. Mechanical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, M.; Feng, Y.K.; Li, Y.; Li, G.; Wang, Y.L.; Han, Y.; Sun, X.J.; Tan, X.H. Fabrication of siloxane hybrid material with high adhesion and high refractive index for light emitting diodes (LEDs) encapsulation. J. Macromol. Sci. A 2014, 51, 653–658. [Google Scholar] [CrossRef]
- Gao, N.; Liu, W.; Ma, S.; Tang, C.; Yan, Z.J. Cycloaliphatic epoxy resin modified by two kinds of oligo fluorosiloxanes for potential application in light emitting diode (LED) encapsulation. J. Polym. Res. 2012, 19, 9923–9932. [Google Scholar] [CrossRef]
- Bae, J.Y.; Kim, Y.H.; Kim, H.Y.; Lim, Y.W.; Bae, B.S. Sol-gel synthesized linear oligosiloxane-based hybrid material for a thermally-resistant light emitting diode (LED) encapsulant. RSC Adv. 2013, 3, 8871–8877. [Google Scholar] [CrossRef]
- Jung, K.H.; Bae, J.Y.; Park, S.J.; Yoo, S.; Bae, B.S. High performance organic-inorganichybrid barrier coating for encapsulation of OLEDs. J. Mater. Chem. 2010, 21, 1977–1983. [Google Scholar] [CrossRef]
- Yang, S.; Kim, J.S.; Jin, J.H.; Kwak, S.Y.; Bae, B.S. Thermal resistance of cycloaliphatic epoxy hybrimer based on sol-gel derived oligosiloxane for LED encapsulation. J. Appl. Polym. Sci. 2010, 117, 2140–2145. [Google Scholar] [CrossRef]
- Kim, J.S.; Yang, S.C.; Bae, B.S. Thermally stable transparent sol-gel based siloxane hybrid material with high refractive index for light emitting diode (LED) encapsulation. Chem. Mater. 2010, 22, 3549–3555. [Google Scholar] [CrossRef]
- Yam, F.K.; Hassan, Z. Innovative advances in LED technology. Microelectron. J. 2005, 36, 129–137. [Google Scholar] [CrossRef]
- Liu, J.G.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Hsu, C.W.; Maa, C.C.M.; Tan, C.S.; Li, H.T.; Huang, S.C.; Lee, T.M.; Tai, H. Effect of thermal aging on the optical, dynamic mechanical, and morphological properties of phenylmethylsiloxane-modified epoxy for use as an LED encapsulant. Mater. Chem. Phys. 2012, 134, 789–796. [Google Scholar] [CrossRef]
- Chikhi, N.; Fellahi, S.; Bakar, M. Modification of epoxy resin using reactive liquid (ATBN) rubber. Eur. Polym. J. 2002, 38, 251–264. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Yu, Y.Z.; Yuan, Y.X. Studies on UV-stable silicone-epoxy resins. J. Appl. Polym. Sci. 2007, 104, 3954–3959. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Chiou, J.Y.; Liao, C.Y.; Chen, P.Y.; Tung, S.H.; Lin, J.J. Organically modified clays as rheology modifiers and dispersing agents for epoxy packing of white LED. Compos. Sci. Technol. 2016, 132, 9–15. [Google Scholar] [CrossRef]
- Tummala, R.R.; Rymaszewski, E.J.; Klopfenstein, A.G. Microelectronic Packaging Handbook, Part III; Chapman & Hall: New York, NY, USA, 1997. [Google Scholar]
- Lau, J.H. Handbook of Fine Pitch Surface Mount Technology; Van Nostrand Reinhold: New York, NY, USA, 1994. [Google Scholar]
- Yang, Y.; Li, W.N.; Luo, Y.S.; Xiao, H.M.; Fu, S.Y.; Mai, Y.W. Novel ultraviolet-opaque, visible transparent and light emitting ZnO-QD/silicone composites with tunable luminescence colors. Polymer 2010, 51, 2755–2762. [Google Scholar] [CrossRef]
- Ji, L.F.; Gu, A.J.; Liang, G.Z.; Yuan, L. Novel modification of bismaleimide-triazine resin by reactive hyperbranched polysiloxane. J. Mater. Sci. 2010, 45, 1859–1865. [Google Scholar] [CrossRef]
- Yang, S.C.; Kwak, S.Y.; Jin, J.H.; Kim, J.S.; Choi, Y.W.; Paik, K.W.; Bae, B.S. Thermally resistant UV-curable epoxy-siloxane hybrid materials for light emitting diode (LED) encapsulation. J. Mater. Chem. 2012, 22, 8874–8880. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Xiao, P.; Zhang, J.; Graff, B.; Fabrice, M.S.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Photoinitiators based on a phenazine scaffold: High performance systems upon near-UV or visible LED (385, 395 and 405 nm) irradiations. Polymer 2014, 55, 2285–2293. [Google Scholar] [CrossRef]
- Shih, H.M.; Wu, R.C.; Shih, P.I.; Wang, C.L.; Hsu, C.S. Synthesis of fluorene-based hyperbranched polymers for solution-processable blue, green, red, and white light-emitting devices. J. Polym. Sci. Pol. Chem. 2012, 50, 696–710. [Google Scholar] [CrossRef]
- Gresser, J.D.; Hsu, S.H.; Nagaoka, H.; Lyons, C.M.; Nieratko, D.P.; Wise, D.L.; Barabino, G.A.; Trantolo, D.J. Analysis of a vinyl pyrrolidone poly(propylene fumarate) resorbable bone-cement. J. Biomed. Mater. Res. 1995, 29, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Domb, A.J.; Manor, N.; Elmalak, O. Biodegradable bone cement compositions based on acrylate and epoxide terminated poly(propylene fumarate) oligomers and calcium salt compositions. Biomaterials 1996, 17, 411–417. [Google Scholar] [CrossRef]
- Timmer, M.D.; Ambrose, C.G.; Mikos, A.G. In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)diacrylate. Biomaterials 2003, 24, 571–577. [Google Scholar] [CrossRef]
- Wang, L.; Guo, D.G.; Zhu, H.; Xie, L. Light emitting diodes (LEDs) encapsulation of polymer composites based on poly(propylene fumarate) crosslinked with poly(propylene fumarate)-diacrylate. RSC Adv. 2015, 5, 52888–52895. [Google Scholar] [CrossRef]
- Otsu, T.; Matsumoto, A.; Shiraishi, K.; Amaya, N.; Koinume, Y. Effect of the substituents on radical copolymerization of dialkyl fumarates with some vinyl monomers. J. Polym. Sci. Part A Polym. Chem. 2003, 30, 1559–1565. [Google Scholar] [CrossRef]
- He, S.; Timmer, M.D.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer 2001, 42, 1251–1260. [Google Scholar] [CrossRef]
- Wasey, J.A.E.; Barnes, W.L. Birefringence and light emission from the polymer LED. Synth. Met. 2000, 111–112, 213–215. [Google Scholar] [CrossRef]
- Osaki, S.; Uranishi, K. Determination of the refractive index and birefringence for biaxially stretched poly(ethylene terephthalate) at microwave frequencies. Polymer 1990, 31, 33–35. [Google Scholar] [CrossRef]
- Ghosh, G. Dispersion-equation coefficients for the refractive index and birefringence of calcite and quartz crystals. Opt. Commun. 1999, 163, 95–102. [Google Scholar] [CrossRef]
- Fisher, J.P.; Timmer, M.D.; Holland, T.A.; Dean, D.; Engel, P.S.; Mikos, A.G. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part I. Determination of network structure. Biomacromolecules 2003, 4, 1327–1334. [Google Scholar] [PubMed]
- Shung, A.K.; Timmer, M.D.; Jo, S.; Engel, P.S.; Mikos, A.G. Kinetics of poly(propylene fumarate) synthesis by step polymerization of diethyl fumarate and propylene glycol using zinc chloride as a catalyst. J. Biomater. Sci. Polym. Ed. 2002, 13, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.J.; Yaszemski, M.J.; Suggs, L.J.; Payne, R.G.; Langer, R.; Hayes, W.C.; Unroe, M.R.; Alemany, L.B.; Engel, P.S.; Mikos, A.G. Characterization of partially saturated poly(propylene fumarate) for orthopaedic application. J. Biomater. Sci. Polym. Ed. 1997, 8, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.J.; Suggs, L.J.; Yaszemski, M.J.; Engel, P.S.; Mikos, A.G. Synthesis of poly (propylene fumarate) by acylation of propylene glycol in the presence of a proton scavenger. J. Biomater. Sci. Polym. Ed. 1999, 10, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kunwong, D.; Sumanochitraporn, N.; Kaewpirom, S. Curing behavior of a UV-curable coating based on urethane acrylate oligomer: The influence of reactive monomers. J. Sci. Technol. 2011, 33, 201–207. [Google Scholar]
- Kaewpirom, S.; Kunwong, D. Curing behavior and cured film performance of easy-to-clean UV-curable coatings based on hybrid urethane acrylate oligomers. J. Polym. Res. 2012, 19, 9995. [Google Scholar] [CrossRef]
- Elrehim, M.A.; Voit, B.; Bruchmann, B.; Eichhorn, K.J.; Grundke, K.; Bellmann, C. Structural and end group effects on bulk and surface properties of hyperbranched poly(urea urethane)s. J. Polym. Sci. Pol. Chem. 2005, 15, 3376–3393. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, T.G.; Park, H.S.; Lee, D.S.; Lee, Y.K.; Yoon, S.C.; Nam, J.D. Thermal and mechanical characteristics of poly(l-lactic acid) nanocomposite scaffold. Biomaterials 2003, 24, 2773–2778. [Google Scholar] [CrossRef]
- Timmer, M.D.; Jo, S.; Wang, C.; Ambrose, C.G.; Mikos, A.G. Characterization of the cross-linked structure of fumarate-based degradable polymer networks. Macromolecules 2002, 35, 4373–4379. [Google Scholar] [CrossRef]
- Kandalam, U.; Bouvier, A.J.; Casas, S.B.; Smith, R.L.; Gallego, A.M.; Rothrock, J.K.; Thompson, J.Y.; Huang, C.-Y.C.; Stelnicki, E.J. Novel bone adhesives: A comparison of bond strengths in vitro. Int. J. Oral Maxillofac. Surg. 2013, 42, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Chu, Y.P.; Wei, M.; Deanin, R.D. Comparison of epoxy resins for applications in light-emitting diodes. Adv. Polym. Technol. 2004, 23, 298–306. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Guo, D.-G. Preparation and Performance of Poly(butyl fumarate)-Based Material for Potential Application in LED Encapsulation. Materials 2017, 10, 149. https://doi.org/10.3390/ma10020149
Wang L, Guo D-G. Preparation and Performance of Poly(butyl fumarate)-Based Material for Potential Application in LED Encapsulation. Materials. 2017; 10(2):149. https://doi.org/10.3390/ma10020149
Chicago/Turabian StyleWang, Liang, and Da-Gang Guo. 2017. "Preparation and Performance of Poly(butyl fumarate)-Based Material for Potential Application in LED Encapsulation" Materials 10, no. 2: 149. https://doi.org/10.3390/ma10020149





