Pore Formation Process of Porous Ti3SiC2 Fabricated by Reactive Sintering
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Synthesis
2.2. Characterization of Samples
3. Results and Discussion
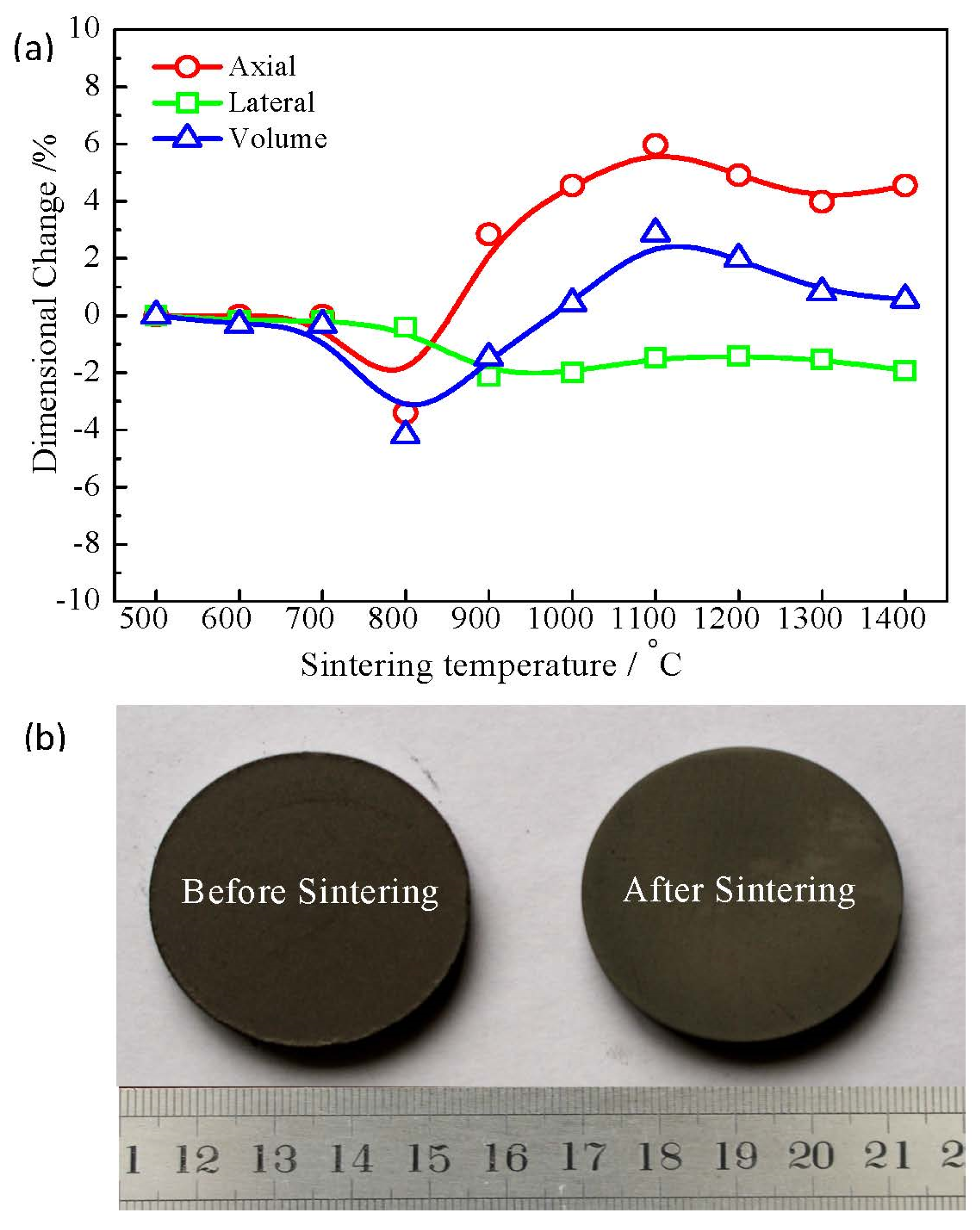
3.1. Thermal Behavior of the Green Compact
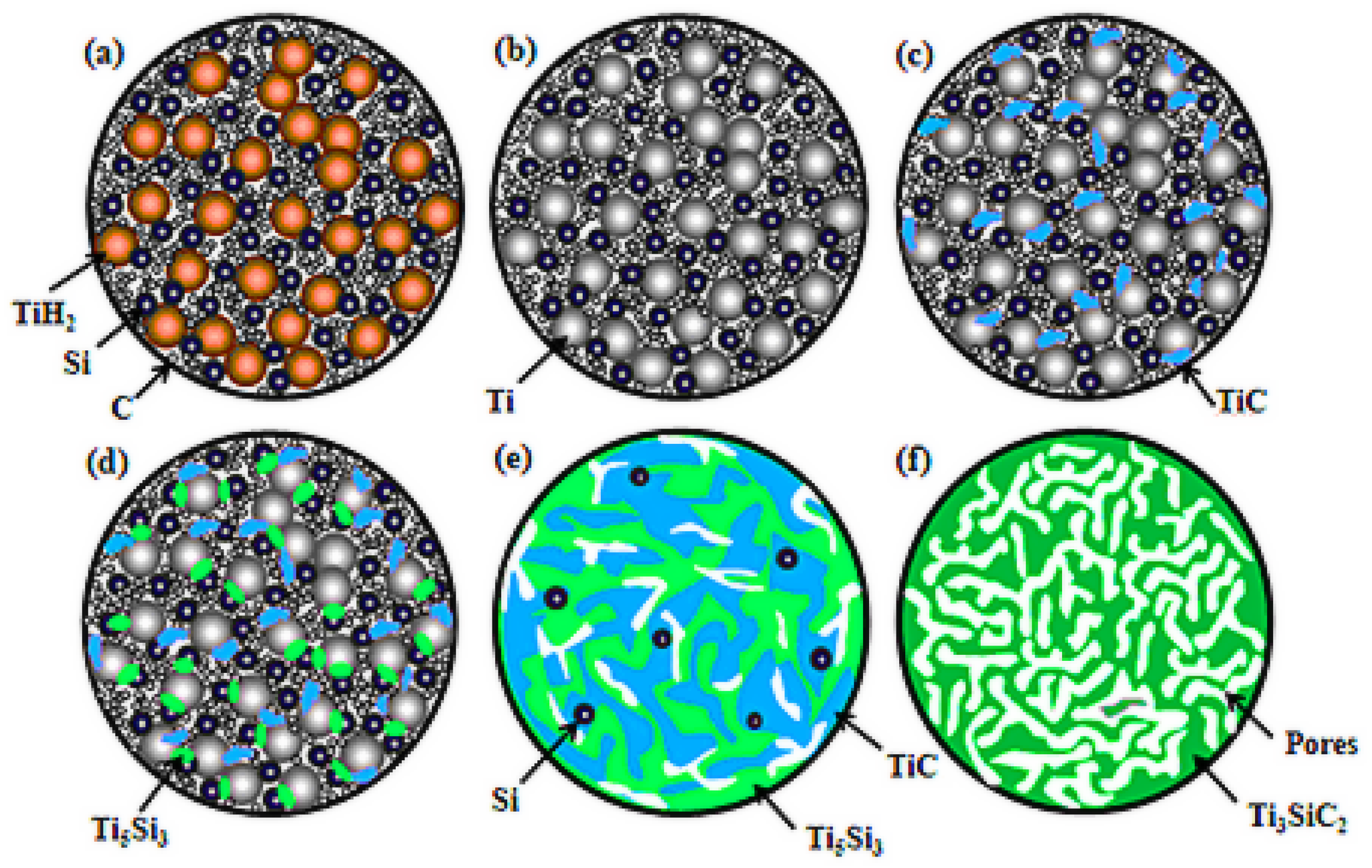
3.2. Reaction Process
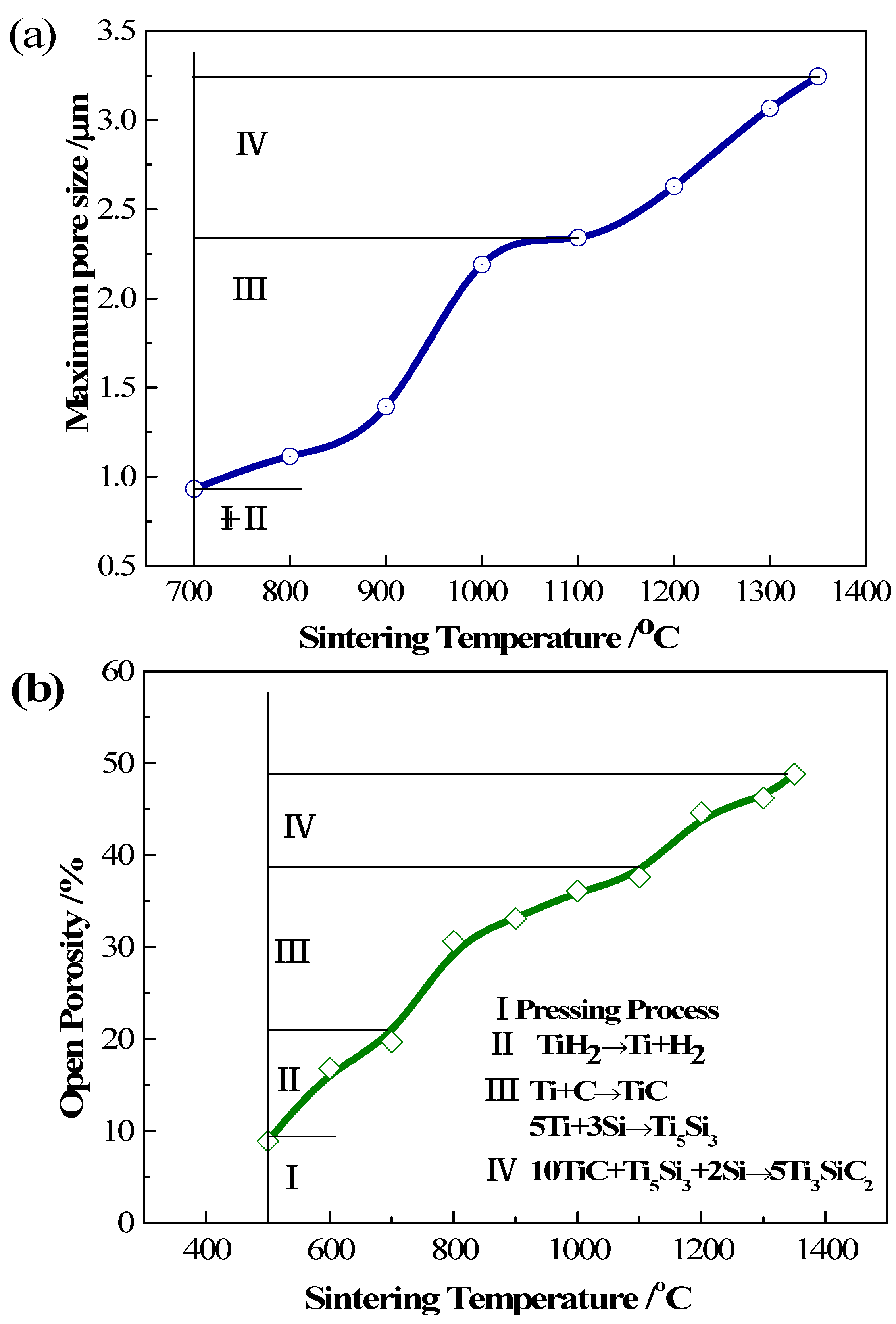
3.3. Pore Evolvement and Formation Procedure
3.4. Microstructure Development of Porous Ti3SiC2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammel, E.C.; Ighodaro, O.L.-R.; Okoli, O.I. Processing and properties of advanced porous ceramics: An application based review. Ceram. Int. 2014, 40, 15351–15370. [Google Scholar] [CrossRef]
- Luyten, J.; Mullens, S.; Thijs, I. Designing with pores—Synthesis and applications. KONA 2010, 28, 131–142. [Google Scholar] [CrossRef]
- Guzman, I.Y. Certain principles of formation of porous ceramic structures, properties and applications (a review). Glass Ceram. 2003, 60, 280–283. [Google Scholar] [CrossRef]
- Liu, R.P.; Xu, C.A.; Wang, T.T. A review of fabrication strategies and applications of porous ceramics prepared by freeze-casting method. Ceram. Int. 2016, 42, 2907–2925. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Hsieh, H.P. Inorganic Membrane for Separation and Reaction; Elsevier Science BV: Amsterdam, the Netherlands, 1996. [Google Scholar]
- Zhang, H.B.; Bao, Y.W.; Zhou, Y.C. Current status in layered ternary carbideTi3SiC2, a review. J. Mater. Sci. Technol. 2009, 25, 1–38. [Google Scholar]
- Zhou, Y.C.; Sun, Z.M. Electronic structure and bonding properties in layered ternary carbide Ti3SiC2. J. Phys. Condens. Matter. 2000, 12, L457–L462. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, H.B.; Jiang, Y.; He, Y.H. Characterization and application of porous Ti3SiC2 ceramic prepared through reactive synthesis. Mater. Des. 2015, 79, 94–98. [Google Scholar] [CrossRef]
- Liu, X.L.; Jiang, Y.; Zhang, H.B.; Yu, L.P.; Kang, J.G.; He, Y.H. Porous Ti3SiC2 fabricated by mixed elemental powders reactive synthesis. J. Eur. Ceram. Soc. 2015, 35, 1349–1353. [Google Scholar] [CrossRef]
- Firstov, S.A.; Pechkovsky, E.P. High-temperature short-term and long hardness of sintered compact and porous titanium-siliceous carbide Ti3SiC2. Sci. Sinter. 2004, 36, 11–20. [Google Scholar] [CrossRef]
- Sun, Z.M.; Murugaiah, A.; Zhen, T.; Zhou, A.; Barsoum, M.W. Microstructure and mechanical properties of porous Ti3SiC2. Acta Mater. 2005, 53, 4359–4366. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, Q.K.; Zhang, H.B.; Jiang, Y.; He, Y.H. Development and characterization of microporous Ti3SiC2 ceramic membranes for filtration of microorganisms. J. Mater. Sci. 2016, 51, 2594–2597. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, H.B.; Jiang, Y.; He, Y.H. Factors affecting the property of porous Ti3SiC2 metal ceramic fabricated through pressureless sintering. J. Porous Mater. 2015, 22, 1285–1290. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Williams, J.; Akinc, M. Synthesis and high-temperature stability of Ti3SiC2. J. Alloys Compd. 1999, 285, 85–88. [Google Scholar] [CrossRef]
- Stepura, G.; Rosenband, V.; Gany, A. A model for the decomposition of titanium hydride and magnesium hydride. J. Alloys Compd. 2012, 513, 159–164. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Review of densification of titanium based powder systems in press and sinter processing. Powder Metall. 2010, 53, 146–162. [Google Scholar] [CrossRef]
- Sadrnezhaad, S.K.; Hosseini, S.A. Fabrication of porous NiTi-shape memory alloy objects by partially hydrided titanium powder for biomedical applications. Mater. Des. 2009, 30, 4483–4487. [Google Scholar] [CrossRef]
- Li, S.B.; Zhai, H.X.; Zhou, Y.C.; Zhang, Z.L. Synthesis of Ti3SiC2 powders by mechanically activated sintering of elemental powders of Ti, Si and C. Mater. Sci. Eng. A 2005, 407, 315–321. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Sun, Z.M.; Hashimoto, H. Rapid synthesis of ternary carbide Ti3SiC2 through pulse-discharge sintering technique from Ti/Si/TiC powders. Met. Mat. Trans. A 2002, 33, 3321–3328. [Google Scholar] [CrossRef]
- El-Raghy, T.; Barsoum, M.W. Processing and mechanical properties of Ti3SiC2: I, reaction path and microstructure evolution. J. Am. Ceram. Soc. 1992, 82, 2849–2854. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Z.M.; Hashimoto, H. Reaction in Ti3SiC2 powder synthesis from a Ti-Si-TiC powder mixture. J. Alloys Compd. 2004, 68, 312–317. [Google Scholar] [CrossRef]
- Sato, F.; Li, J.F.; Watanabe, R. Reaction synthesis of Ti3SiC2 from mixture of elemental powders. J. Am. Ceram. Soc. 2000, 41, 605–608. [Google Scholar]
- Zhang, H.B.; Zhou, Y.C.; Bao, Y.W.; Li, M.S.; Wang, J.Y. Intermediate phases in synthesis of Ti3SiC2 and Ti3Si(Al)C2 solid solutions from elemental powders. J. Eur. Ceram. Soc. 2006, 26, 2373–2380. [Google Scholar] [CrossRef]
- Klemm, H.; Tanihata, K.; Miyamoto, Y. Gas pressure combustion sintering and hot isostatic pressing in the Ti-Si-C system. J. Mater. Sci. 1993, 28, 1557–1562. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhuo, M.J.; Zhou, Y.C.; Li, M.S.; Wang, J.Y. Microstructural relationships between compounds in the Ti-Si-C system. Scr. Mater. 2006, 55, 445–448. [Google Scholar] [CrossRef]



| Raw Material | Atomic Weight | Ratio (wt %) | Density (g/cm3) | Occupied Volume (%) |
|---|---|---|---|---|
| TiH2 | 49.9 | 72.1 | 3.91 | 46.2 |
| Si | 28.1 | 16.3 | 2.34 | 17.4 |
| C | 12.0 | 11.6 | 2.25 | 12.7 |
| Reaction | Resultant | Occupied Volume (%) | Density (g/cm3) | Volume Change (%) | Formed Pore (%) | Contribution Rate (%) |
|---|---|---|---|---|---|---|
| (1) | Ti | 38.2 | 4.54 | −17.3 | 8.0 | 16.7 |
| (2) | TiC | 29.3 | 4.93 | −23.4 | 9.0 | 18.8 |
| (3) | Ti5Si3 | 18.1 | 4.32 | −15.6 | 3.4 | 7.1 |
| (4) | Ti3SiC2 | 52.1 | 4.53 | −2.0 | 1.1 | 2.3 |
| (5) | Si(g) | 0 | 2.34 | −100 | 2.9 | 6.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, X.; Jiang, Y. Pore Formation Process of Porous Ti3SiC2 Fabricated by Reactive Sintering. Materials 2017, 10, 163. https://doi.org/10.3390/ma10020163
Zhang H, Liu X, Jiang Y. Pore Formation Process of Porous Ti3SiC2 Fabricated by Reactive Sintering. Materials. 2017; 10(2):163. https://doi.org/10.3390/ma10020163
Chicago/Turabian StyleZhang, Huibin, Xinli Liu, and Yao Jiang. 2017. "Pore Formation Process of Porous Ti3SiC2 Fabricated by Reactive Sintering" Materials 10, no. 2: 163. https://doi.org/10.3390/ma10020163





