Adsorption Behavior of High Stable Zr-Based MOFs for the Removal of Acid Organic Dye from Water
Abstract
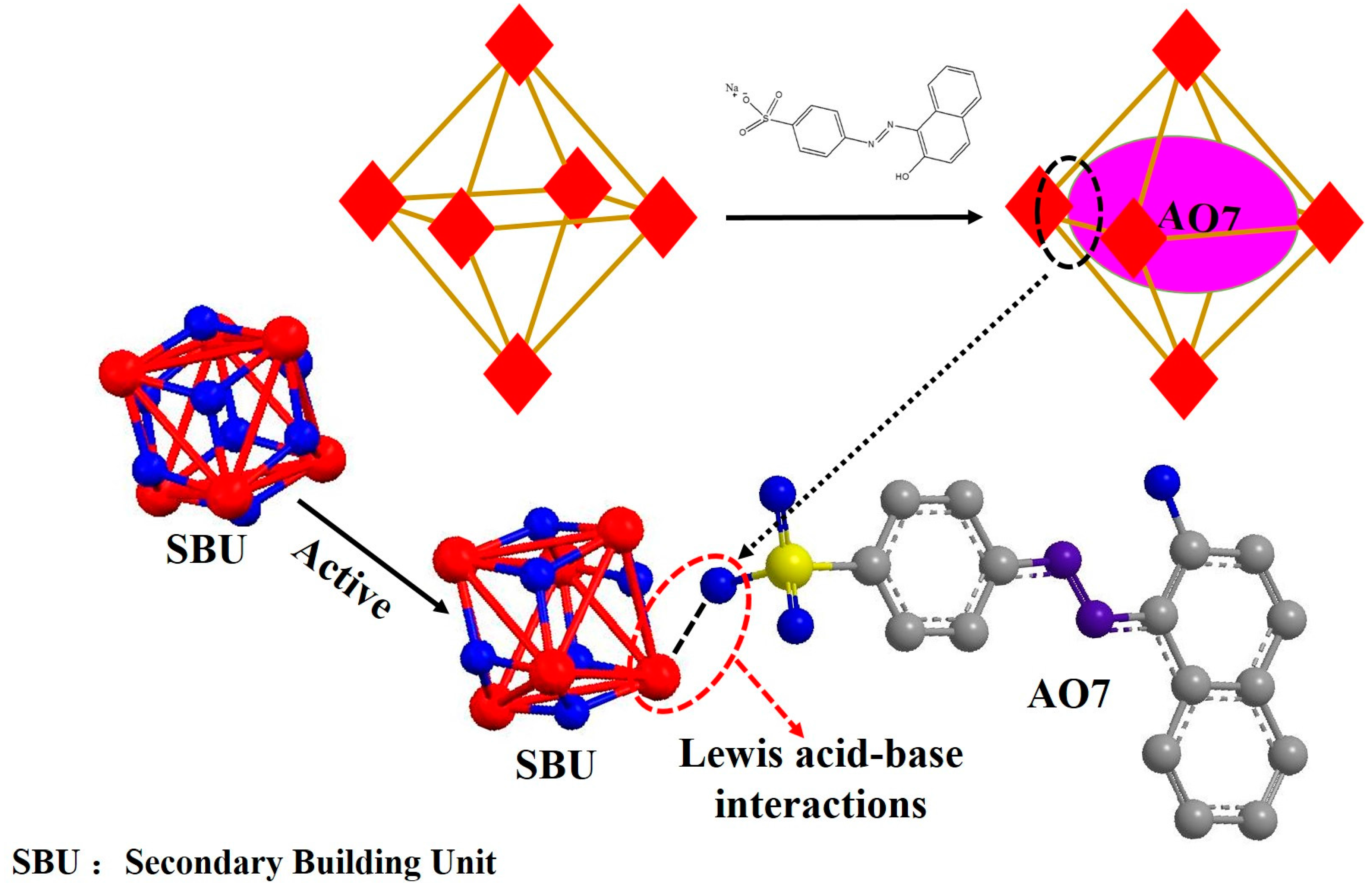
:1. Introduction
2. Results and Discussion
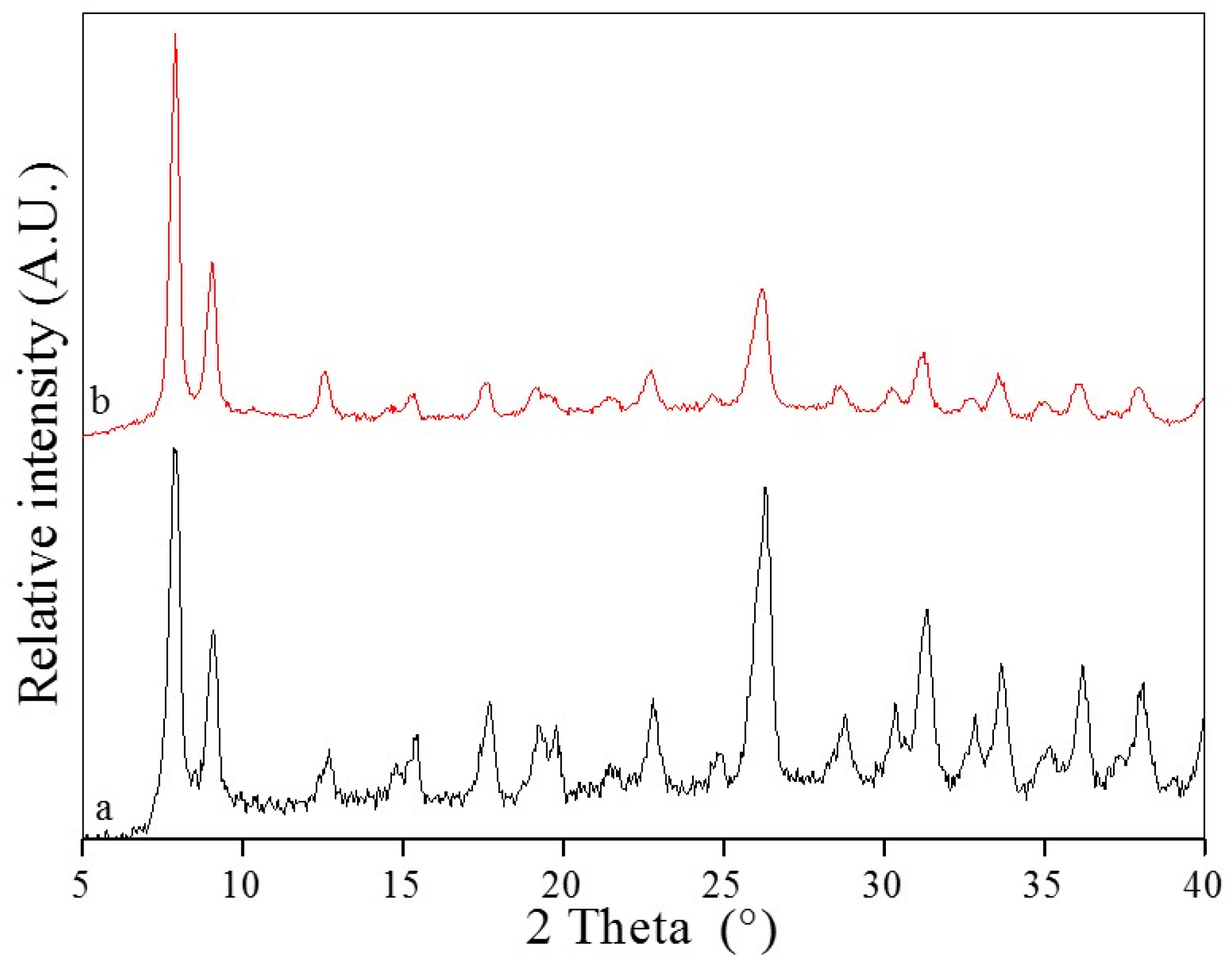
2.1. Powder X-ray Diffraction (PXRD)
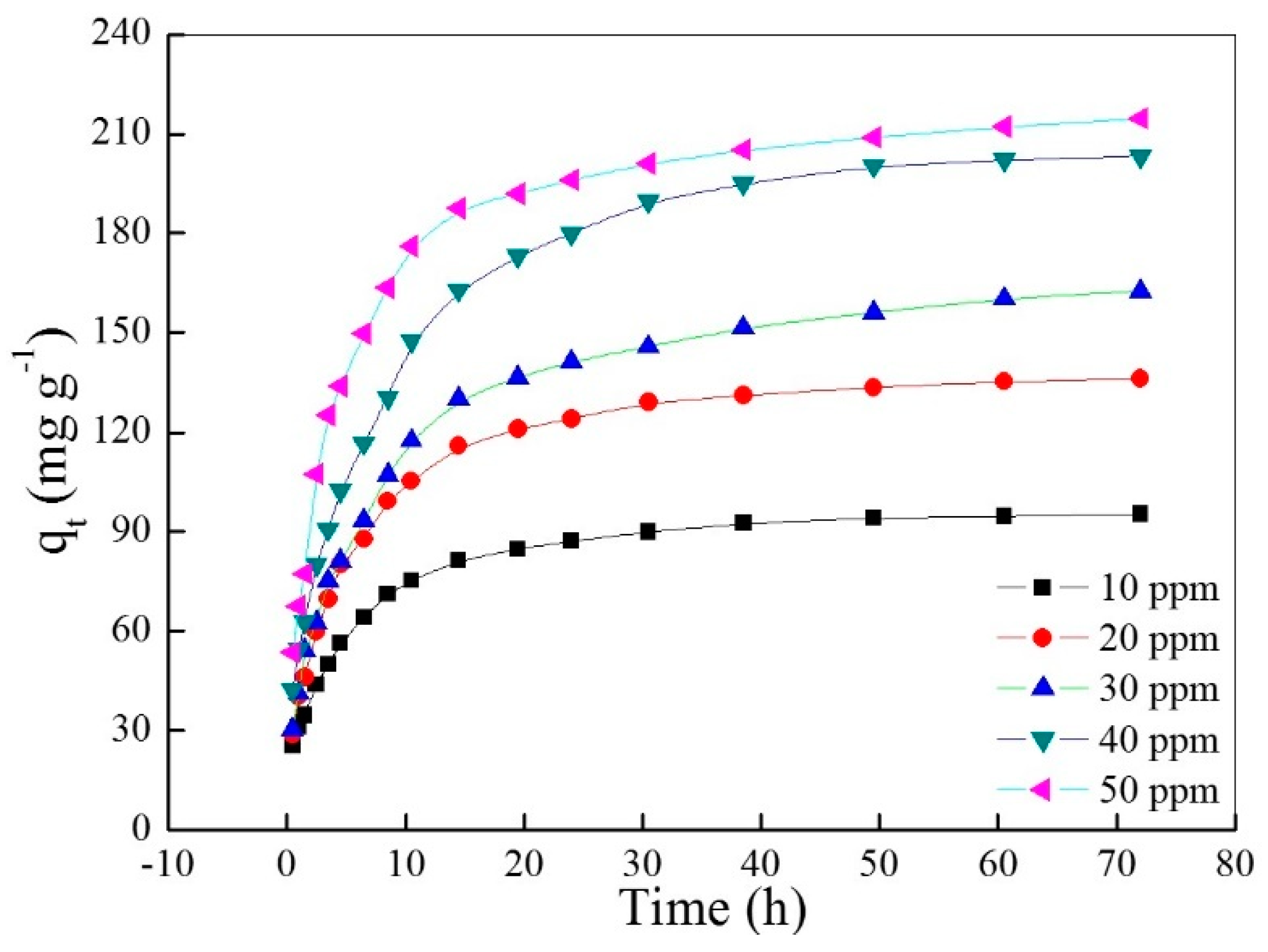
2.2. Adsorption Kinetic Studies
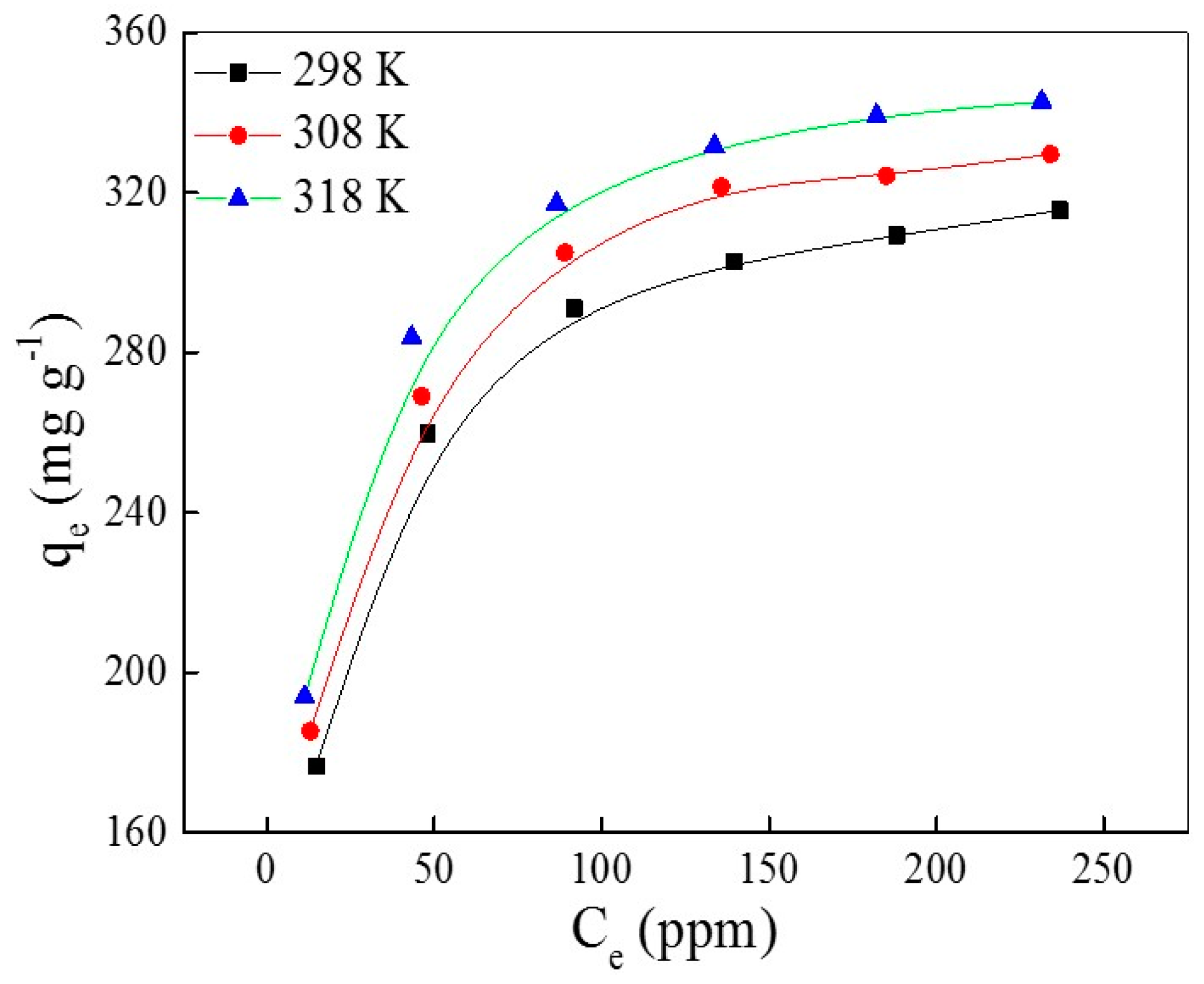
2.3. Adsorption Isotherm Studies
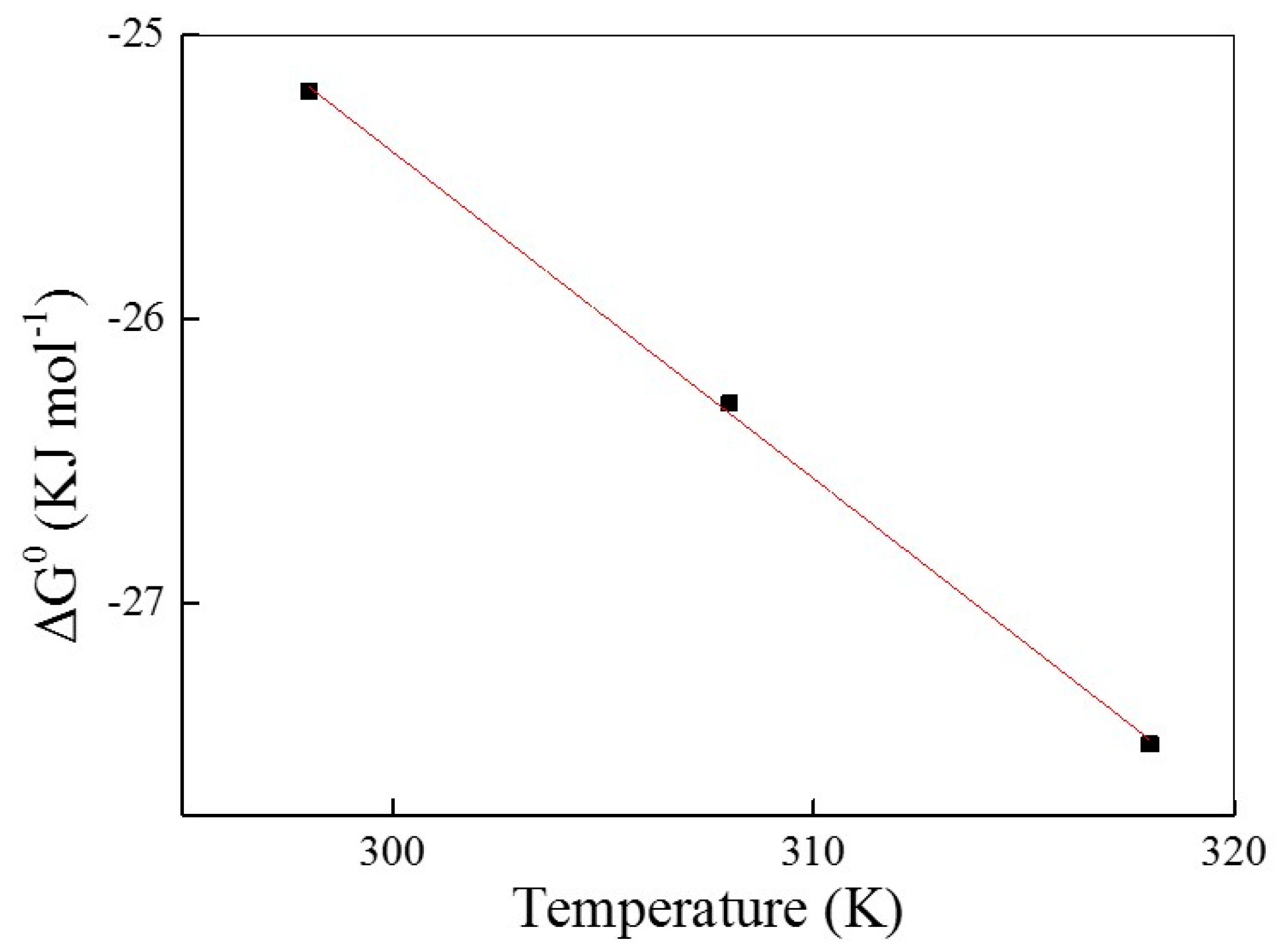
2.4. Adsorption Thermodynamics
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpaa, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Cambie, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noel, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpaa, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.X.; Su, Y.M.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling-an overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigments 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Tang, Y.L.; Rong, N.N.; Liu, F.L.; Chu, M.S.; Dong, H.M.; Zhang, Y.H.; Xiao, P. Enhancement of the photoelectrochemical performance of CuWO4 films for water splitting by hydrogen treatment. Appl. Surf. Sci. 2016, 361, 133–140. [Google Scholar] [CrossRef]
- Santiago, D.E.; Espino-Estevez, M.R.; Gonzalez, G.V.; Arana, J.; Gonzalez-Diaz, O.; Dona-Rodriguez, J.M. Photocatalytic treatment of water containing imazalil using an immobilized TiO2 photoreactor. Appl. Catal. Gen. 2015, 498, 1–9. [Google Scholar] [CrossRef]
- Gondal, M.A.; Chang, X.F.; Ali, M.A.; Yamani, Z.H.; Zhou, Q.; Ji, G.B. Adsorption and degradation performance of Rhodamine B over BiOBr under monochromatic 532 nm pulsed laser exposure. Appl. Catal. Gen. 2011, 397, 192–200. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Pei, X.; Zhang, S.; Feng, X.; Zhou, J.; Wang, B. Water Purification: Adsorption over Metal-Organic Frameworks. Chin. J. Chem. 2016, 34, 175–185. [Google Scholar] [CrossRef]
- Wang, S.B.; Peng, Y.L. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; Gomez-Serrano, V.; Alvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R. Advances in application of natural clay and its composites in removal of biological, organic, and inorganic contaminants from drinking water. Adv. Mater. Sci. Eng. 2011, 2011, 872531. [Google Scholar] [CrossRef]
- Namazi, H.; Heydari, A.; Pourfarzolla, A. Synthesis of glycoconjugated polymer based on polystyrene and nanoporous β-cyclodextrin to remove copper (II) from water pollution. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 1–6. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.M.; Zhao, X.D.; Xie, L.T.; Liu, D.H.; Yang, Q.Y.; Zhong, C.L. Treatment of waste water using metal-organic frameworks. Prog. Chem. 2012, 24, 1646–1655. [Google Scholar]
- Zheng, L.; Ding, A.Z.; Ding, W.C. Compare Study of a New Type Adsorbent and 2 Traditional Adsorbents on Their Efficiency for As (V) Adsorption. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010; pp. 1–4.
- Ongkudon, C.M.; Kansil, T.; Wong, C. Challenges and strategies in the preparation of large-volume polymer-based monolithic chromatography adsorbents. J. Sep. Sci. 2014, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Samadder, S.R. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Liu, J. Surface modification of activated carbon for enhanced adsorption of perfluoroalkyl acids from aqueous solutions. Chemosphere 2016, 144, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Vinitnantharat, S.; Kositchaiyong, S.; Chiarakorn, S. Removal of fluoride in aqueous solution by adsorption on acid activated water treatment sludge. Appl. Surf. Sci. 2010, 256, 5458–5462. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Du, Y.; Ma, J. Novel synthesis of carbon spheres supported nanoscale zero-valent iron for removal of metronidazole. Appl. Surf. Sci. 2016, 390, 50–59. [Google Scholar] [CrossRef]
- Han, T.T.; Li, C.F.; Guo, X.Y.; Huang, H.L.; Liu, D.H.; Zhong, C.L. In-situ synthesis of SiO2@MOF composites for high-efficiency removal of aniline from aqueous solution. Appl. Surf. Sci. 2016, 390, 506–512. [Google Scholar] [CrossRef]
- Ding, L.H.; Rahimi, P.; Hawkins, R.; Bhatt, S.; Shi, Y. Naphthenic acid removal from heavy oils on alkaline earth-metal oxides and ZnO catalysts. Appl. Catal. Gen. 2009, 371, 121–130. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2004, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Application of metal-organic frameworks. Polym. Int. 2017. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.J.; Allan, D.R.; Muszkiewicz, A.; Morrison, C.A.; Moggach, S.A. The effect of high pressure on MOF-5: Guest-induced modification of pore size and content at high pressure. Angew. Chem. 2011, 123, 11334–11337. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jobic, H.; Salles, F.; Kolokolov, D.; Guillerm, V.; Serre, C.; Maurin, G. Probing the Dynamics of CO2 and CH4 within the Porous Zirconium Terephthalate UiO-66(Zr): A synergic combination of neutron scattering measurements and molecular simulations. Chem. Eur. J. 2011, 17, 8882–8889. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.J.; Zhang, W.Q.; Zhang, J.S.; Luo, G.X.; Jia, Y.; Deng, M.L.; Ling, Y. Facile preparation of UiO-66 nanoparticles with tunable sizes in a continuous flow microreactor and its application in drug delivery. Microporous Mesoporous Mater. 2016, 220, 148–154. [Google Scholar] [CrossRef]
- Zhao, X.; Bu, X.; Wu, T.; Zheng, S.T.; Wang, L.; Feng, P. Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat. Commun. 2013, 4, 2344. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-C.; Xia, Y.; Ma, N.; Shi, J.-J.; Jiang, T.; Chiang, T.-C.; Zhang, Z.-C.; Tsen, W.-C. Adsorptive removal of acid orange 7 from aqueous solution with metal-organic framework material, iron (III) trimesate. Desalination Water Treat. 2016, 57, 3218–3226. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Absorption Gelöster Stoffe; PA Norstedt & Söner: Stockholm, Sweden, 1898; pp. 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part II.—Liquids. J. Am. Chem. Soc. 1915, 38, 102–105. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, e470. [Google Scholar]
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.J. Review—Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
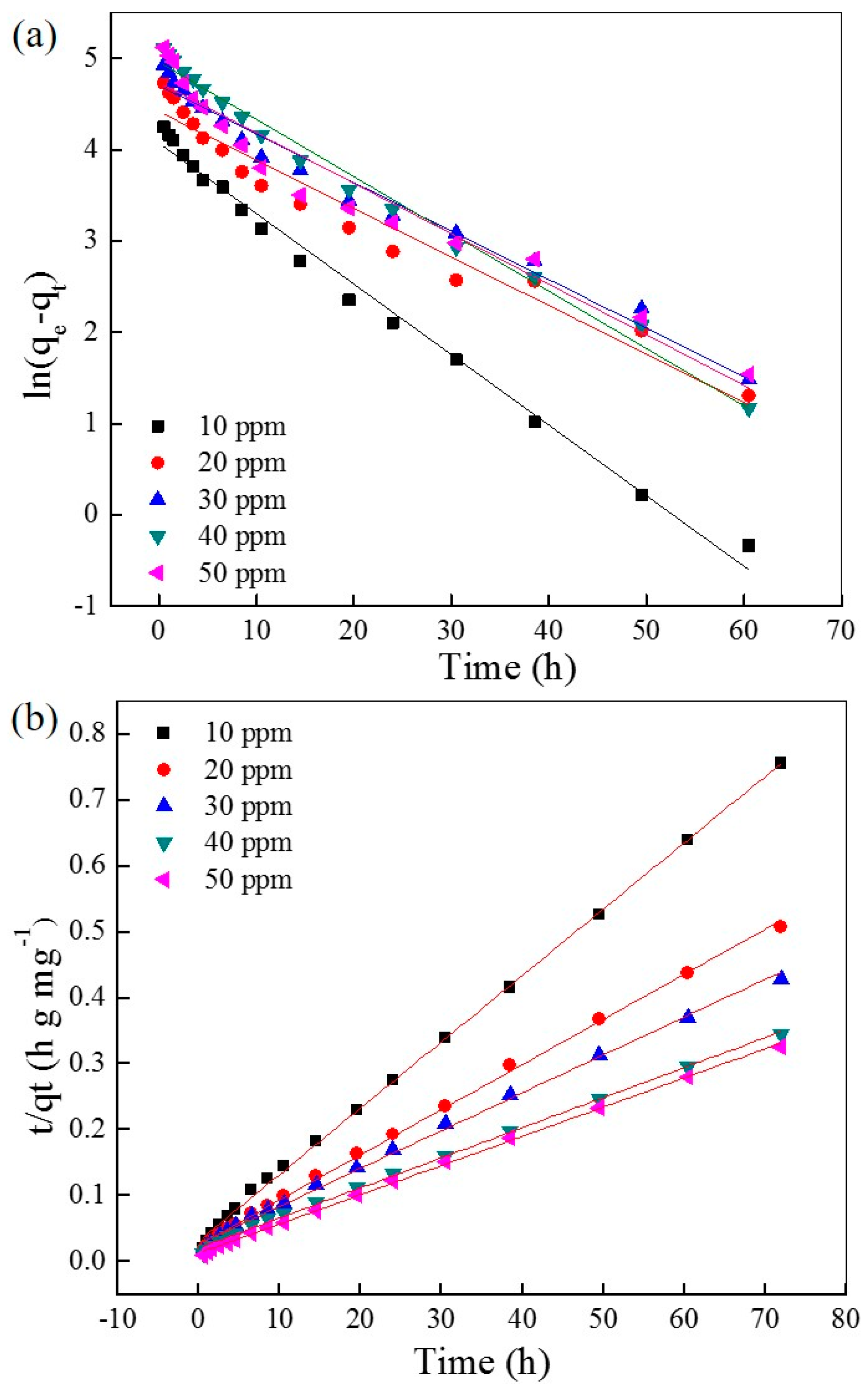

| C0 ppm | qe mg·g−1 | k1 10−2·h−1 | R2 |
|---|---|---|---|
| 10 | 58.6 | 7.72 | 0.9899 |
| 20 | 82.6 | 5.31 | 0.9510 |
| 30 | 110 | 5.31 | 0.9755 |
| 40 | 142 | 6.27 | 0.9878 |
| 50 | 114 | 5.53 | 0.9363 |
| C0 ppm | qe mg·g−1 | k2 10−3 g·mg−1·h−1 | R2 |
|---|---|---|---|
| 10 | 99.5 | 3.11 | 0.9985 |
| 20 | 146 | 1.82 | 0.9973 |
| 30 | 175 | 1.21 | 0.9979 |
| 40 | 219 | 0.987 | 0.9991 |
| 50 | 226 | 1.50 | 0.9990 |
| Temperature (K) | qs (mg·g−1) | KL (104 L·mol−1) | R2 | ΔG0 (kJ·mol−1) | ΔH0 (kJ·mol−1) | ΔS0 (J·mol−1·K−1) |
|---|---|---|---|---|---|---|
| 298 | 332 | 2.64 | 0.9999 | −25.2 | 9.09 | 115 |
| 308 | 346 | 2.93 | 0.9999 | −26.3 | ||
| 318 | 358 | 3.32 | 0.9999 | −27.5 |
| Temprature (K) | KF (mg·g−1) (L·mg−1)n | nF | R2 |
|---|---|---|---|
| 298 | 108 | 4.87 | 0.9092 |
| 308 | 118 | 5.01 | 0.9225 |
| 318 | 130 | 5.31 | 0.9229 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.-D.; Tsai, F.-C.; Ma, N.; Xia, Y.; Liu, H.-L.; Zhan, X.-Q.; Yu, X.-Y.; Zeng, X.-Z.; Jiang, T.; Shi, D.; et al. Adsorption Behavior of High Stable Zr-Based MOFs for the Removal of Acid Organic Dye from Water. Materials 2017, 10, 205. https://doi.org/10.3390/ma10020205
Zhang K-D, Tsai F-C, Ma N, Xia Y, Liu H-L, Zhan X-Q, Yu X-Y, Zeng X-Z, Jiang T, Shi D, et al. Adsorption Behavior of High Stable Zr-Based MOFs for the Removal of Acid Organic Dye from Water. Materials. 2017; 10(2):205. https://doi.org/10.3390/ma10020205
Chicago/Turabian StyleZhang, Ke-Deng, Fang-Chang Tsai, Ning Ma, Yue Xia, Huan-Li Liu, Xue-Qing Zhan, Xiao-Yan Yu, Xiang-Zhe Zeng, Tao Jiang, Dean Shi, and et al. 2017. "Adsorption Behavior of High Stable Zr-Based MOFs for the Removal of Acid Organic Dye from Water" Materials 10, no. 2: 205. https://doi.org/10.3390/ma10020205





