Large-Scale Surfactant-Free Synthesis of p-Type SnTe Nanoparticles for Thermoelectric Applications
Abstract
:1. Introduction
2. Results
2.1. Materials Synthesis
2.2. Materials Characterisation
2.3. Materials Performance Evaluation
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baxter, J.; Bian, Z.; Chen, G.; Danielson, D.; Dresselhaus, M.S.; Fedorov, A.G.; Fisher, T.S.; Jones, C.W.; Maginn, E.; Kortshagen, U.; et al. Nanoscale design to enable the revolution in renewable energy. Energy Environ. Sci. 2009, 2, 559–588. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.P.; Gogna, P. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Lan, Y.; Minnich, A.J.; Chen, G.; Ren, Z. Enhancement of thermoelectric figure-of-merit by a bulk nanostructuring approach. Adv. Funct. Mater. 2010, 20, 357–376. [Google Scholar] [CrossRef]
- Han, G.; Chen, Z.-G.; Drennan, J.; Zou, J. Indium selenides: Structural characteristics, synthesis and their thermoelectric performances. Small 2014, 10, 2747–2765. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.J.; Zhao, L.D.; Kanatzidis, M.G. Rationally designing high-performance bulk thermoelectric materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef] [PubMed]
- Tritt, T.M.; Subramanian, M.A. Thermoelectric materials, phenomena, and applications: A bird’s eye view. MRS Bull. 2006, 31, 188–194. [Google Scholar] [CrossRef]
- Zhao, L.D.; Lo, S.H.; Zhang, Y.S.; Sun, H.; Tan, G.J.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 2015, 351, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Lu, X.; Zhan, H.; Hui, S.; Tang, X.; Wang, G.; Dai, J.; Uher, C.; Wang, G.; Zhou, X. Broad temperature plateau for high ZTs in heavily doped p-type SnSe single crystals. Energy Environ. Sci. 2016, 9, 454–460. [Google Scholar] [CrossRef]
- Han, M.K.; Androulakis, J.; Kim, S.J.; Kanatzidis, M.G. Lead-free thermoelectrics: High figure of merit in p-type AgSnmSbTem+2. Adv. Energy Mater. 2012, 2, 157–161. [Google Scholar] [CrossRef]
- Chen, Y.; Nielsen, M.D.; Gao, Y.B.; Zhu, T.J.; Zhao, X.B.; Heremans, J.P. SnTe-AgSbTe2 thermoelectric alloys. Adv. Energy Mater. 2012, 2, 58–62. [Google Scholar] [CrossRef]
- Brebrick, R.F.; Strauss, A.J. Anomalous thermoelectric power as evidence for two-valence bands in SnTe. Phys. Rev. 1963, 131, 104–110. [Google Scholar] [CrossRef]
- Rogers, L.M. Valence band structure of SnTe. J. Phys. D Appl. Phys. 1968, 1, 845–852. [Google Scholar] [CrossRef]
- Santhanam, S.; Chaudhuri, A.K. Transport-properties of SnTe interpreted by means of a 2 valence band model. Mater. Res. Bull. 1981, 16, 911–917. [Google Scholar] [CrossRef]
- Brebrick, R.F. Deviations from stoichiometry and electrical properties in SnTe. J. Phys. Chem. Solids 1963, 24, 27–36. [Google Scholar] [CrossRef]
- Kafalas, J.A.; Brebrick, R.F.; Strauss, A.J. Evidence that SnTe is semiconductor. Appl. Phys. Lett. 1964, 4, 93–94. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, B.L.; Lan, Y.C.; Lukas, K.; Liu, W.S.; Esfarjani, K.; Opeil, C.; Broido, D.; Chen, G.; Ren, Z.F. High thermoelectric performance by resonant dopant indium in nanostructured SnTe. Proc. Natl. Acad. Sci. USA 2013, 110, 13261–13266. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.J.; Zhao, L.D.; Shi, F.Y.; Doak, J.W.; Lo, S.H.; Sun, H.; Wolverton, C.; Dravid, V.P.; Uher, C.; Kanatzidis, M.G. High thermoelectric performance of p-type SnTe via a synergistic band engineering and nanostructuring approach. J. Am. Chem. Soc. 2014, 136, 7006–7017. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Shenoy, U.S.; Anand, S.; Waghmare, U.V.; Biswas, K. Mg alloying in SnTe facilitates valence band convergence and optimizes thermoelectric properties. Chem. Mater. 2015, 27, 581–587. [Google Scholar] [CrossRef]
- Tan, G.J.; Shi, F.Y.; Doak, J.W.; Sun, H.; Zhao, L.D.; Wang, P.L.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Extraordinary role of Hg in enhancing the thermoelectric performance of p-type SnTe. Energy Environ. Sci. 2015, 8, 267–277. [Google Scholar] [CrossRef]
- Wu, H.J.; Chang, C.; Feng, D.; Xiao, Y.; Zhang, X.; Pei, Y.L.; Zheng, L.; Wu, D.; Gong, S.K.; Chen, Y.; et al. Synergistically optimized electrical and thermal transport properties of SnTe via alloying high-solubility MnTe. Energy Environ. Sci. 2015, 8, 3298–3312. [Google Scholar] [CrossRef]
- Banik, A.; Shenoy, U.S.; Saha, S.; Waghmare, U.V.; Biswas, K. High power factor and enhanced thermoelectric performance of SnTe-AgInTe2: Synergistic effect of resonance level and valence band convergence. J. Am. Chem. Soc. 2016, 138, 13068–13075. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Biswas, K. Lead-free thermoelectrics: Promising thermoelectric performance in p-type SnTe1-xSex system. J. Mater. Chem. A 2014, 2, 9620–9625. [Google Scholar] [CrossRef]
- Tan, G.J.; Shi, F.Y.; Sun, H.; Zhao, L.D.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. SnTe-AgBiTe2 as an efficient thermoelectric material with low thermal conductivity. J. Mater. Chem. A 2014, 2, 20849–20854. [Google Scholar] [CrossRef]
- Tan, G.J.; Shi, F.Y.; Hao, S.Q.; Chi, H.; Zhao, L.D.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Codoping in SnTe: Enhancement of thermoelectric performance through synergy of resonance levels and band convergence. J. Am. Chem. Soc. 2015, 137, 5100–5112. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Su, X.L.; Tan, X.M.; Zheng, G.; She, X.Y.; Yan, Y.G.; Tang, X.F.; Uher, C. Ultra-fast non-equilibrium synthesis and phase segregation in InxSn1-xTe thermoelectrics by SHS-PAS processing. J. Mater. Chem. C 2015, 3, 8550–8558. [Google Scholar] [CrossRef]
- Banik, A.; Vishal, B.; Perumal, S.; Datta, R.; Biswas, K. The origin of low thermal conductivity in Sn1-xSbxTe: Phonon scattering via layered intergrowth nanostructures. Energy Environ. Sci. 2016, 9, 2011–2019. [Google Scholar] [CrossRef]
- Zhao, L.D.; Zhang, X.; Wu, H.J.; Tan, G.J.; Pei, Y.L.; Xiao, Y.; Chang, C.; Wu, D.; Chi, H.; Zheng, L.; et al. Enhanced thermoelectric properties in the counter-doped SnTe system with strained endotaxial SrTe. J. Am. Chem. Soc. 2016, 138, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Wang, J.L.; Cheng, Z.X.; Sun, Q.; Li, Z.; Dou, S.X. Lead-free SnTe-based thermoelectrics: Enhancement of thermoelectric performance by doping with Gd/Ag. J. Mater. Chem. A 2016, 4, 7936–7942. [Google Scholar] [CrossRef]
- Pei, Y.Z.; Zheng, L.L.; Li, W.; Lin, S.Q.; Chen, Z.W.; Wang, Y.Y.; Xu, X.F.; Yu, H.L.; Chen, Y.; Ge, B.H. Interstitial point defect scattering contributing to high thermoelectric performance in SnTe. Adv. Electron. Mater. 2016, 2, 1600019. [Google Scholar] [CrossRef]
- Zhou, M.; Gibbs, Z.M.; Wang, H.; Han, Y.M.; Xin, C.N.; Li, L.F.; Snyder, G.J. Optimization of thermoelectric efficiency in SnTe: The case for the light band. Phys. Chem. Chem. Phys. 2014, 16, 20741–20748. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tan, X.J.; Xu, J.T.; Liu, G.Q.; Shao, H.Z.; Fu, Y.J.; Wang, X.; Liu, Z.; Xu, J.Q.; Jiang, H.C.; et al. Valence band engineering and thermoelectric performance optimization in SnTe by Mn-alloying via a zone-melting method. J. Mater. Chem. A 2015, 3, 19974–19979. [Google Scholar] [CrossRef]
- Mehta, R.J.; Zhang, Y.L.; Karthik, C.; Singh, B.; Siegel, R.W.; Borca-Tasciuc, T.; Ramanath, G. A new class of doped nanobulk high-figure-of-merit thermoelectrics by scalable bottom-up assembly. Nature Mater. 2012, 11, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fang, H.; Yang, H.; Jauregui, L.A.; Chen, Y.P.; Wu, Y. Design principle of telluride-based nanowire heterostructures for potential thermoelectric applications. Nano Lett. 2012, 12, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kirk, B.; Jauregui, L.A.; Yang, H.; Xu, X.; Chen, Y.P.; Wu, Y. Rational synthesis of ultrathin n-type Bi2Te3 nanowires with enhanced thermoelectric properties. Nano Lett. 2011, 12, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Chen, Z.-G.; Yang, L.; Hong, M.; Drennan, J.; Zou, J. Rational design of Bi2Te3 polycrystalline whiskers for thermoelectric applications. ACS Appl. Mater. Interfaces 2015, 7, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shao, S.; Li, N.; McCall, K.; Wang, J.; Zhang, S.X. Single crystalline nanostructures of topological crystalline insulator SnTe with distinct facets and morphologies. Nano Lett. 2013, 13, 5443–5448. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jung, Y.; Disa, A.S.; Walker, F.J.; Ahn, C.H.; Cha, J.J. Synthesis of SnTe nanoplates with {100} and {111} surfaces. Nano Lett. 2014, 14, 4183–4188. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Wang, Q.S.; Wang, Z.M.; Zhan, X.Y.; Xu, K.; Wang, F.M.; Mirza, M.; He, J. Weak antilocalization effect of topological crystalline Insulator Pb1-xSnxTe nanowires with tunable composition and distinct {100} facets. Nano Lett. 2015, 15, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Cai, K.M.; Li, J.; Huang, Y.; Wang, Z.X.; Xu, K.; Wang, F.; Zhan, X.Y.; Wang, F.M.; Wang, K.Y.; et al. Rational design of ultralarge Pb1-xSnxTe nanoplates for exploring crystalline symmetry-protected topological transport. Adv. Mater. 2016, 28, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Korkosz, R.J.; Luo, Z.S.; Riba, P.; Cadavid, D.; Ortega, S.; Cabot, A.; Kanatzidis, M.G. Electron doping in bottom-up engineered thermoelectric nanomaterials through HCl-mediated ligand displacement. J. Am. Chem. Soc. 2015, 137, 4046–4049. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Popuri, S.R.; Greer, H.F.; Llin, L.F.; Bos, J.W.G.; Zhou, W.Z.; Paul, D.J.; Ménard, H.; Knox, A.R.; Montecucco, A.; Siviter, J.; Man, E.A.; Li, W.-G.; Paul, M.C.; Gao, M.; Sweet, T.; Freer, R.; Azough, F.; Baig, H.; Mallick, T.K.; Gregory, D.H. Chlorine-enabled electron doping in solution-synthesised SnSe thermoelectric nanomaterials. Adv. Energy Mater. 2017. (In press) [Google Scholar] [CrossRef]
- Cadavid, D.; Ibanez, M.; Shavel, A.; Dura, O.J.; Lopez de la Torre, M.A.; Cabot, A. Organic ligand displacement by metal salts to enhance nanoparticle functionality: Thermoelectric properties of Ag2Te. J. Mater. Chem. A 2013, 1, 4864–4870. [Google Scholar] [CrossRef]
- Han, G.; Popuri, S.R.; Greer, H.F.; Bos, J.W.G.; Zhou, W.Z.; Knox, A.R.; Montecucco, A.; Siviter, J.; Man, E.A.; Macauley, M.; et al. Facile surfactant-free synthesis of p-type SnSe nanoplates with exceptional thermoelectric power factors. Angew. Chem. Int. Ed. 2016, 55, 6433–6437. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Li, Z.; Lu, G.Q.; Dou, S.X. Robust scalable synthesis of surfactant-free thermoelectric metal chalcogenide nanostructures. Nano Energy 2015, 15, 193–204. [Google Scholar] [CrossRef]
- Zhang, W.X.; Cheng, Y.W.; Hu, J.Q.; Zhan, J.H.; Yu, W.C.; Yang, L.; Qian, Y.T. Low temperature synthesis of ultrafine SnTe powder in ethanol solvent. Chem. Lett. 2000, 29, 446–447. [Google Scholar] [CrossRef]
- An, C.H.; Tang, K.B.; Hai, B.; Shen, G.Z.; Wang, C.R.; Qian, Y.T. Solution-phase synthesis of monodispersed SnTe nanocrystallites at room temperature. Inorg. Chem. Commun. 2003, 6, 181–184. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Heiss, W.; Shevchenko, E.V.; Lee, J.S.; Schwinghammer, H.; Alivisatos, A.P.; Talapin, D.V. SnTe nanocrystals: A new example of narrow-gap semiconductor quantum dots. J. Am. Chem. Soc. 2007, 129, 11354–11355. [Google Scholar] [CrossRef]
- Ning, J.J.; Men, K.K.; Xiao, G.J.; Zou, B.; Wang, L.; Dai, Q.Q.; Liu, B.B.; Zou, G.T. Synthesis of narrow band gap SnTe nanocrystals: Nanoparticles and single crystal nanowires via oriented attachment. Cryst. Eng. Comm. 2010, 12, 4275–4279. [Google Scholar] [CrossRef]
- Xu, Y.; Al-Salim, N.; Hodgkiss, J.M.; Tilley, R.D. Solution synthesis and optical properties of SnTe nanocrystals. Cryst. Growth Des. 2011, 11, 2721–2723. [Google Scholar] [CrossRef]
- Li, Z.L.; Chen, Y.D.; Li, J.F.; Chen, H.; Wang, L.J.; Zheng, S.Q.; Lu, G.W. Systhesizing SnTe nanocrystals leading to thermoelectric performance enhancement via an ultra-fast microwave hydrothermal method. Nano Energy 2016, 28, 78–86. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, S.; Chen, H. Enhanced electronic transport properties of Se-doped SnTe1−xSex nanoparticles by microwave-assisted solvothermal method. J. Electron. Mater. 2016. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Bierly, J.N.; Muldawer, L.; Beckman, O. The continuous rhombohedral-gubic transformation in GeTe-SnTe alloys. Acta Metall. 1963, 11, 447–454. [Google Scholar] [CrossRef]
- Gamsjäger, H.; Gajda, T.; Sangster, J.; Saxena, S.; Voigt, W. Chemical thermodynamics of tin. In Nuclear Energy Agency Data Bank; Peronne, J., Ed.; Organisation for Economic Co-Operation and Development: Paris, France, 2012. [Google Scholar]
- Lin, S.Q.; Li, W.; Chen, Z.W.; Shen, J.W.; Ge, B.H.; Pei, Y.Z. Tellurium as a high-performance elemental thermoelectric. Nature Commun. 2016, 7, 10287. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.L.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- PDF-2 Release 2008. Joint Committee on Powder Diffraction Standards (JCPDS)-International Centre for Diffraction Data (ICDD). Avaliable online: http://www.icdd.com (accessed on 24 February 2017).
- Zhang, B. Lead Chalcogenide Nano-Composites: Synthesis, Thermal and Electrical Transport Properties; Clemson University: Clemson, SC, USA, 2008. [Google Scholar]

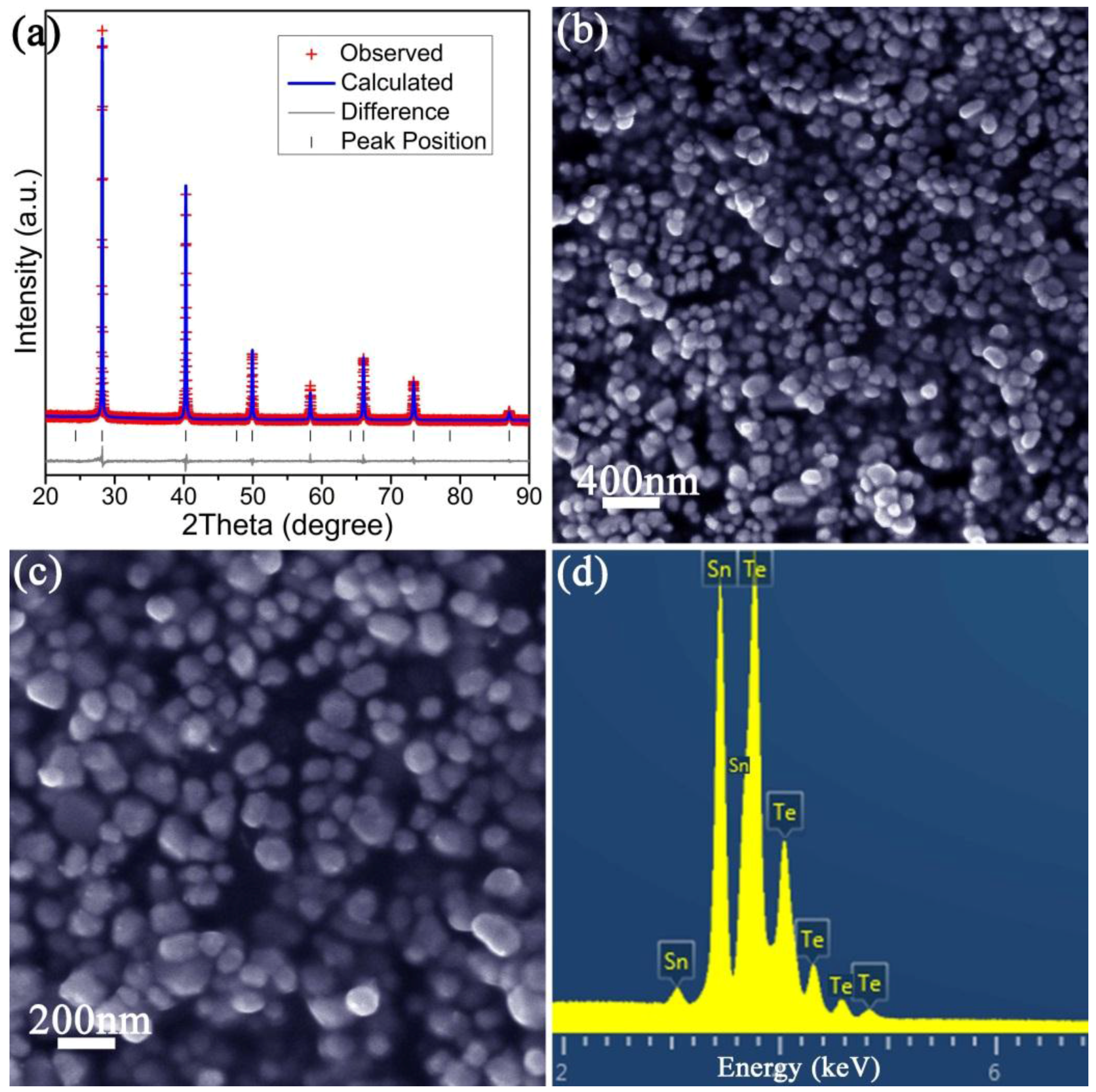
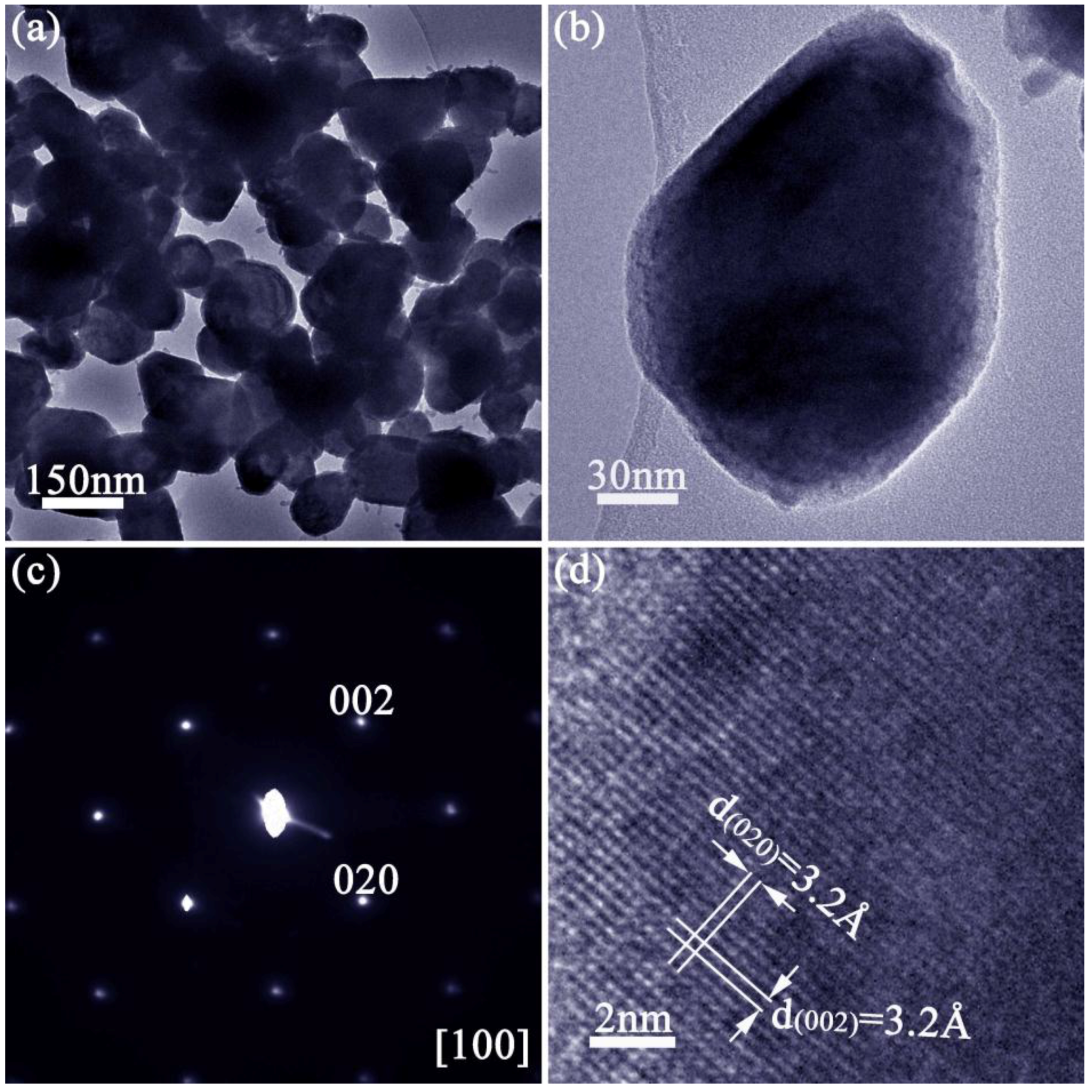
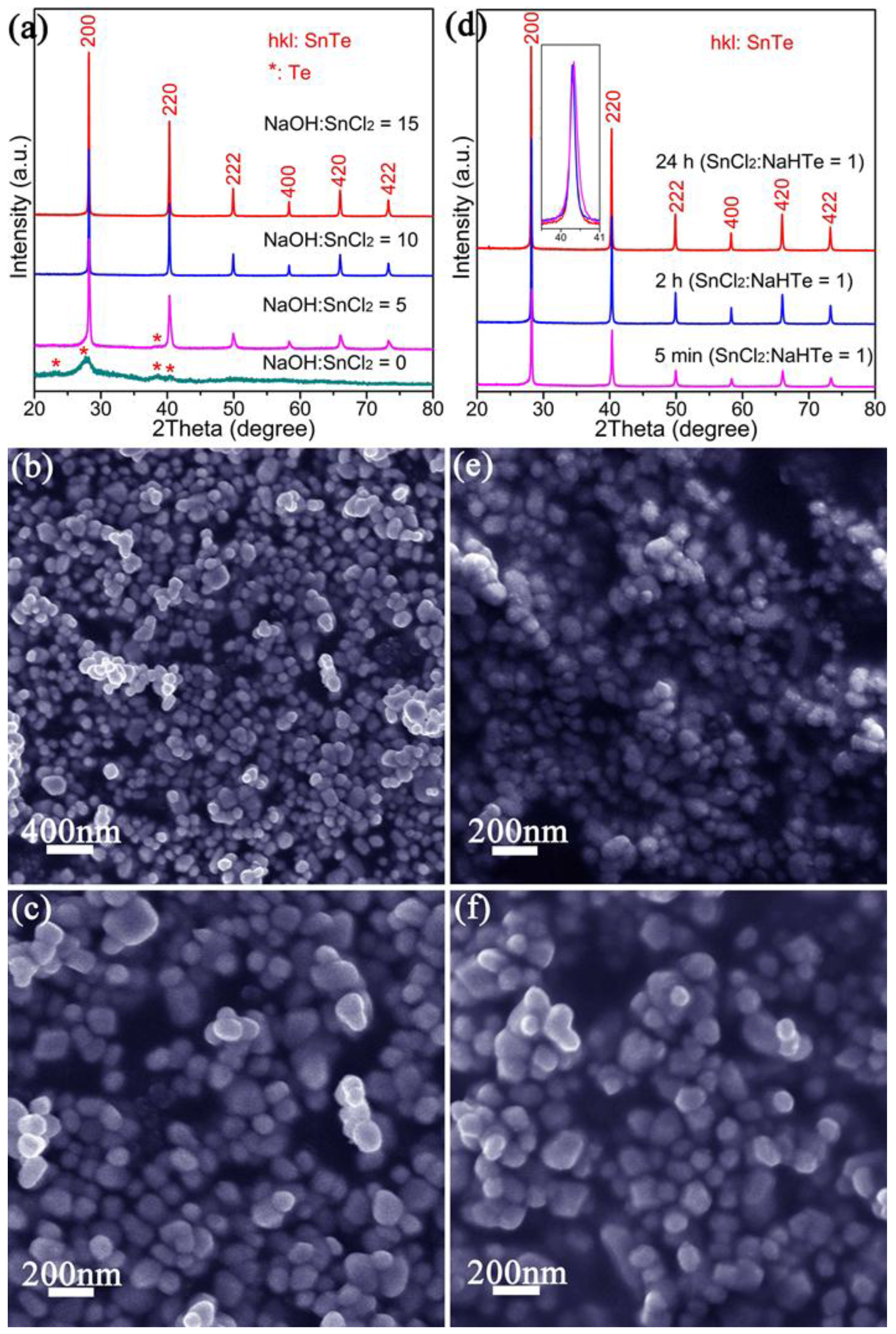
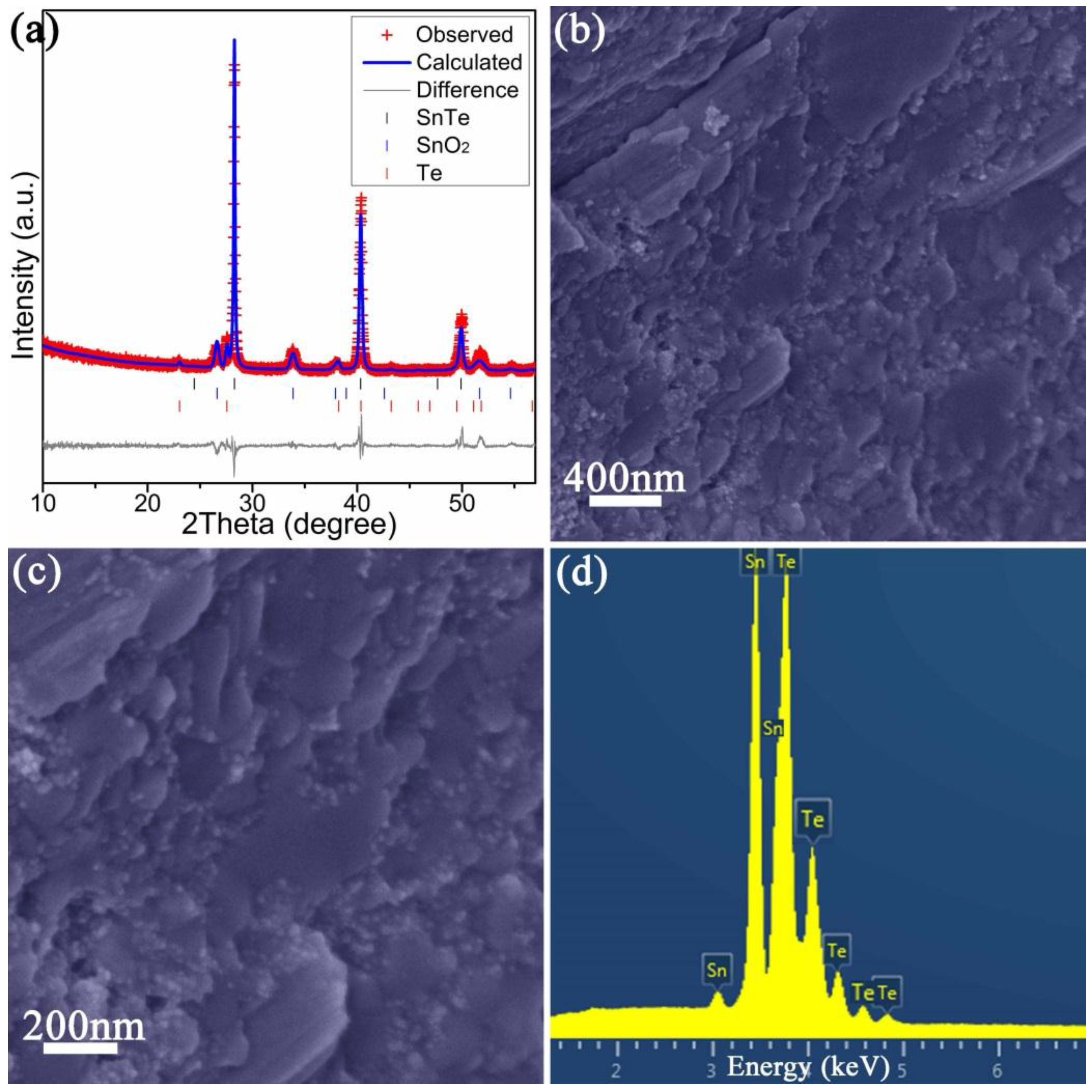
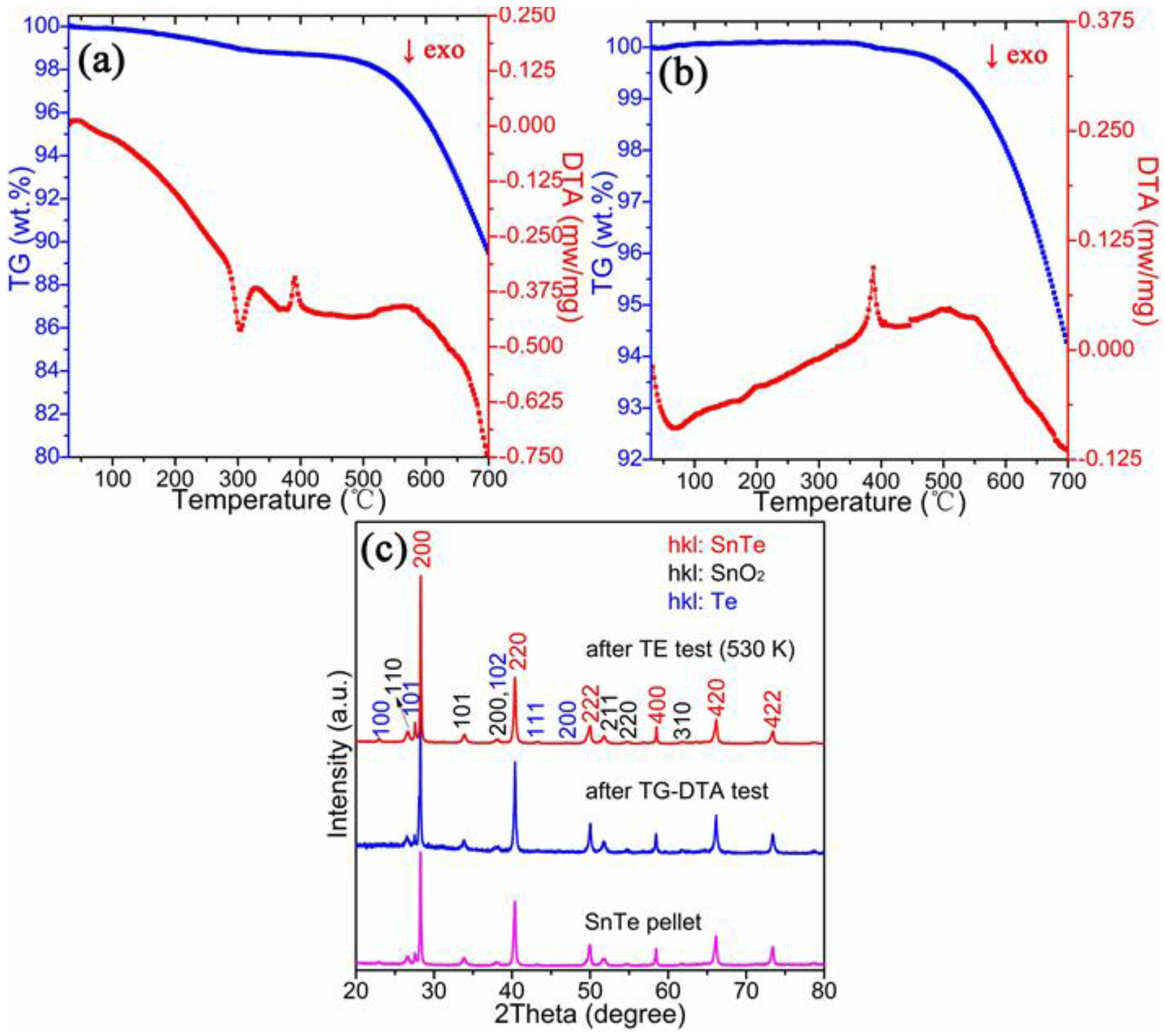
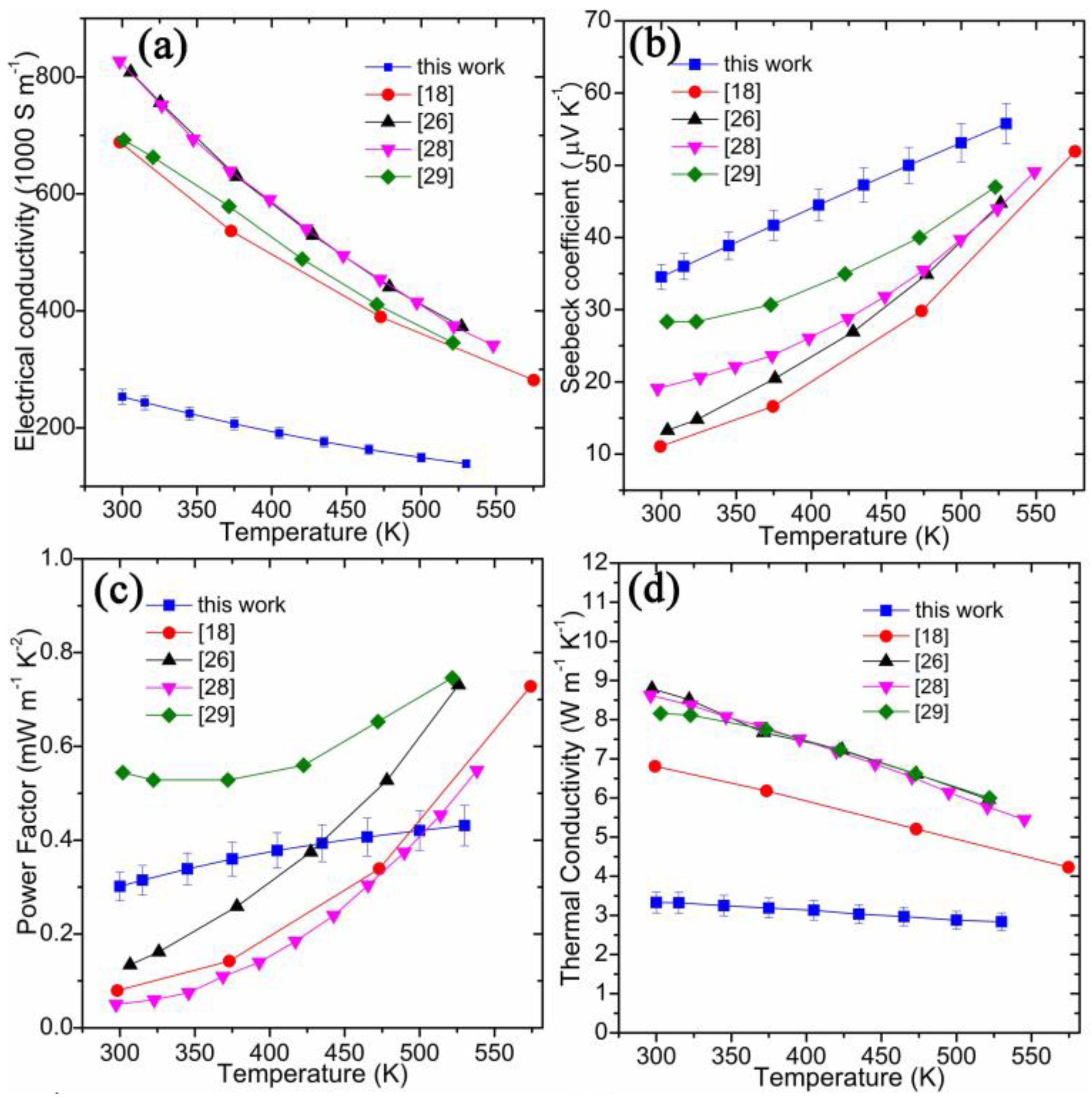
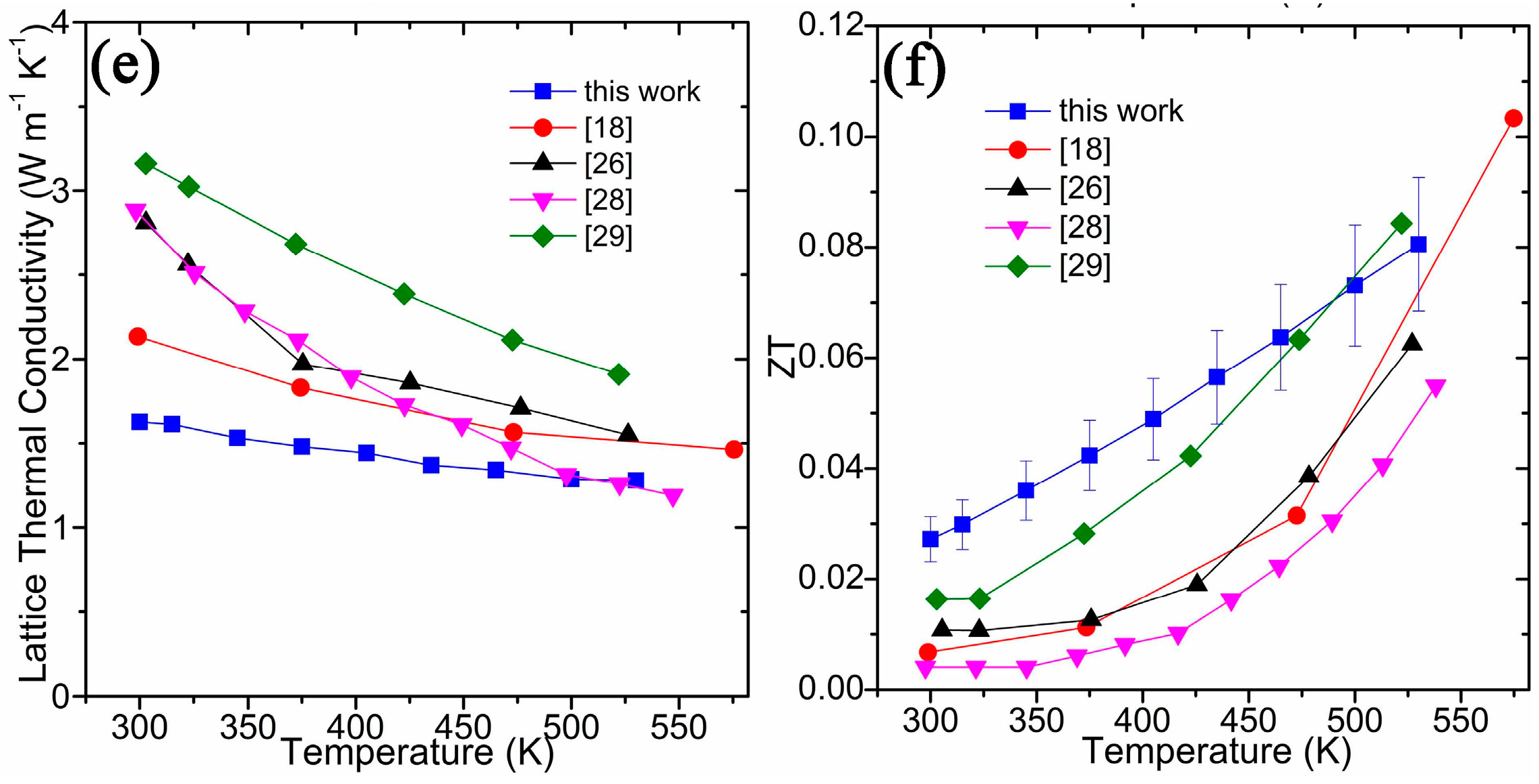
| Chemical Formula | SnTe |
|---|---|
| Crystal System | Cubic |
| Space Group | |
| a (Å) | 6.3234(1) |
| Volume (Å3) | 252.84(1) |
| Z | 4 |
| Formula Weight (g·mol−1) | 246.29 |
| Calculated density (g·cm−3) | 6.470 |
| Rwp | 0.1181 |
| Rp | 0.0832 |
| χ2 | 1.449 |
| Atom | Wyckoff Symbol | x | y | z | 100*Uiso/Å2 | Occupancy |
|---|---|---|---|---|---|---|
| Sn | 4a | 0 | 0 | 0 | 3.87(8) | 1 |
| Te | 4b | 0.5 | 0.5 | 0.5 | 2.49(7) | 1 |
| Chemical Formula | SnTe | SnO2 | Te |
|---|---|---|---|
| Crystal System | Cubic | Tetragonal | Trigonal |
| Space Group | Fmm (225) | P42/mnm (136) | P3121 (152) |
| a (Å) | 6.3441(7) | 4.7610(10) | 4.4836(16) |
| b (Å) | |||
| c (Å) | 3.2008(10) | 5.9551(34) | |
| Volume (Å3) | 255.33(8) | 72.55(3) | 103.67(5) |
| Z | 4 | 2 | 3 |
| Formula Weight (g·mol−1) | 246.29 | 150.69 | 127.6 |
| Calculated density (g·cm−3) | 6.407 | 6.898 | 6.131 |
| Phase fraction (wt %) | 72.3(1) | 20.4(3) | 7.3(2) |
| Rwp | 0.1497 | ||
| Rp | 0.1120 | ||
| χ2 | 2.359 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Zhang, R.; Popuri, S.R.; Greer, H.F.; Reece, M.J.; Bos, J.-W.G.; Zhou, W.; Knox, A.R.; Gregory, D.H. Large-Scale Surfactant-Free Synthesis of p-Type SnTe Nanoparticles for Thermoelectric Applications. Materials 2017, 10, 233. https://doi.org/10.3390/ma10030233
Han G, Zhang R, Popuri SR, Greer HF, Reece MJ, Bos J-WG, Zhou W, Knox AR, Gregory DH. Large-Scale Surfactant-Free Synthesis of p-Type SnTe Nanoparticles for Thermoelectric Applications. Materials. 2017; 10(3):233. https://doi.org/10.3390/ma10030233
Chicago/Turabian StyleHan, Guang, Ruizhi Zhang, Srinivas R. Popuri, Heather F. Greer, Michael J. Reece, Jan-Willem G. Bos, Wuzong Zhou, Andrew R. Knox, and Duncan H. Gregory. 2017. "Large-Scale Surfactant-Free Synthesis of p-Type SnTe Nanoparticles for Thermoelectric Applications" Materials 10, no. 3: 233. https://doi.org/10.3390/ma10030233





