Electrodeposited Organic Layers Formed from Aryl Diazonium Salts for Inhibition of Copper Corrosion
Abstract
:1. Introduction
2. Results
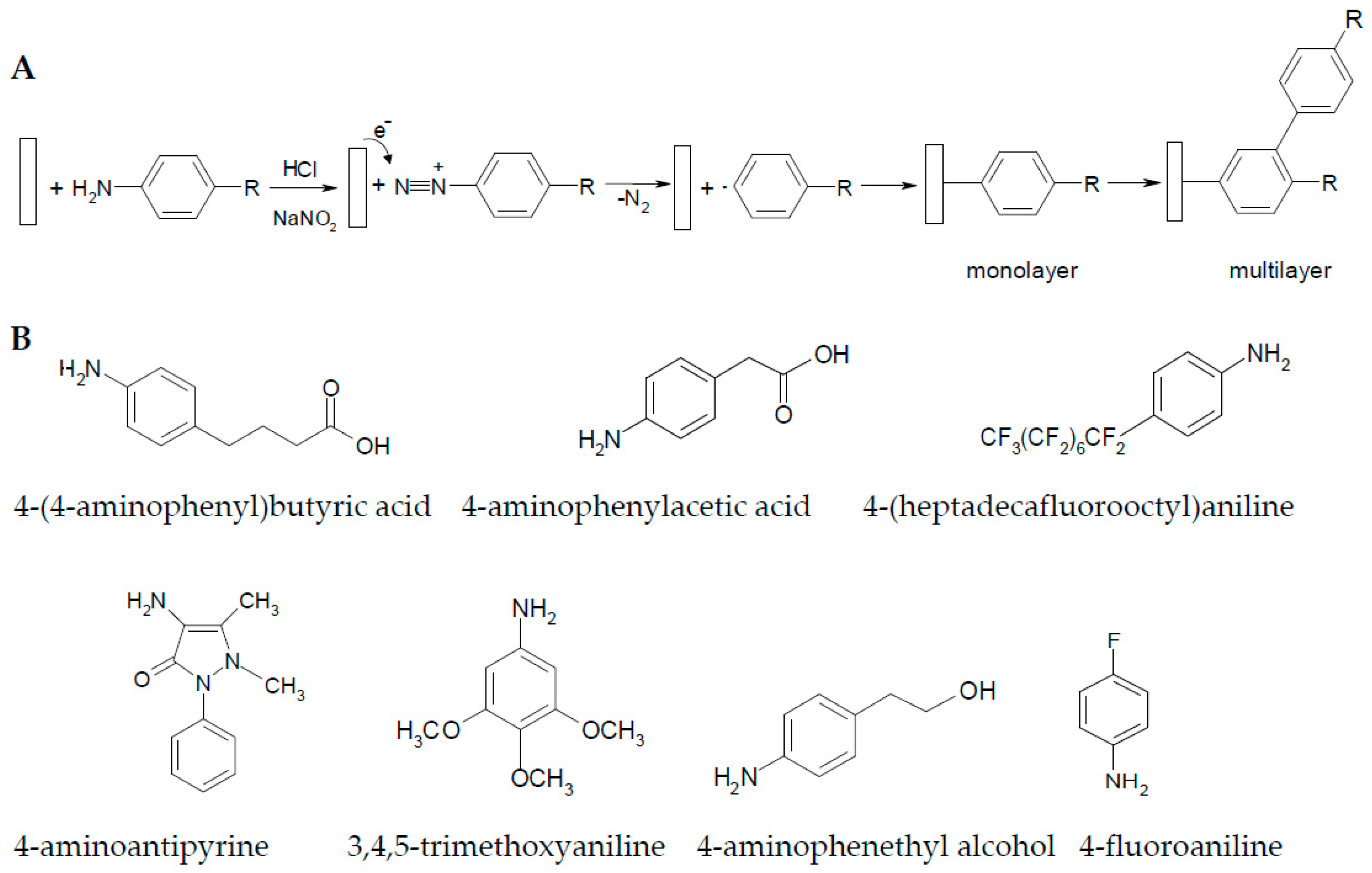
2.1. Influence of the Electrodeposition Process on the Copper Surface
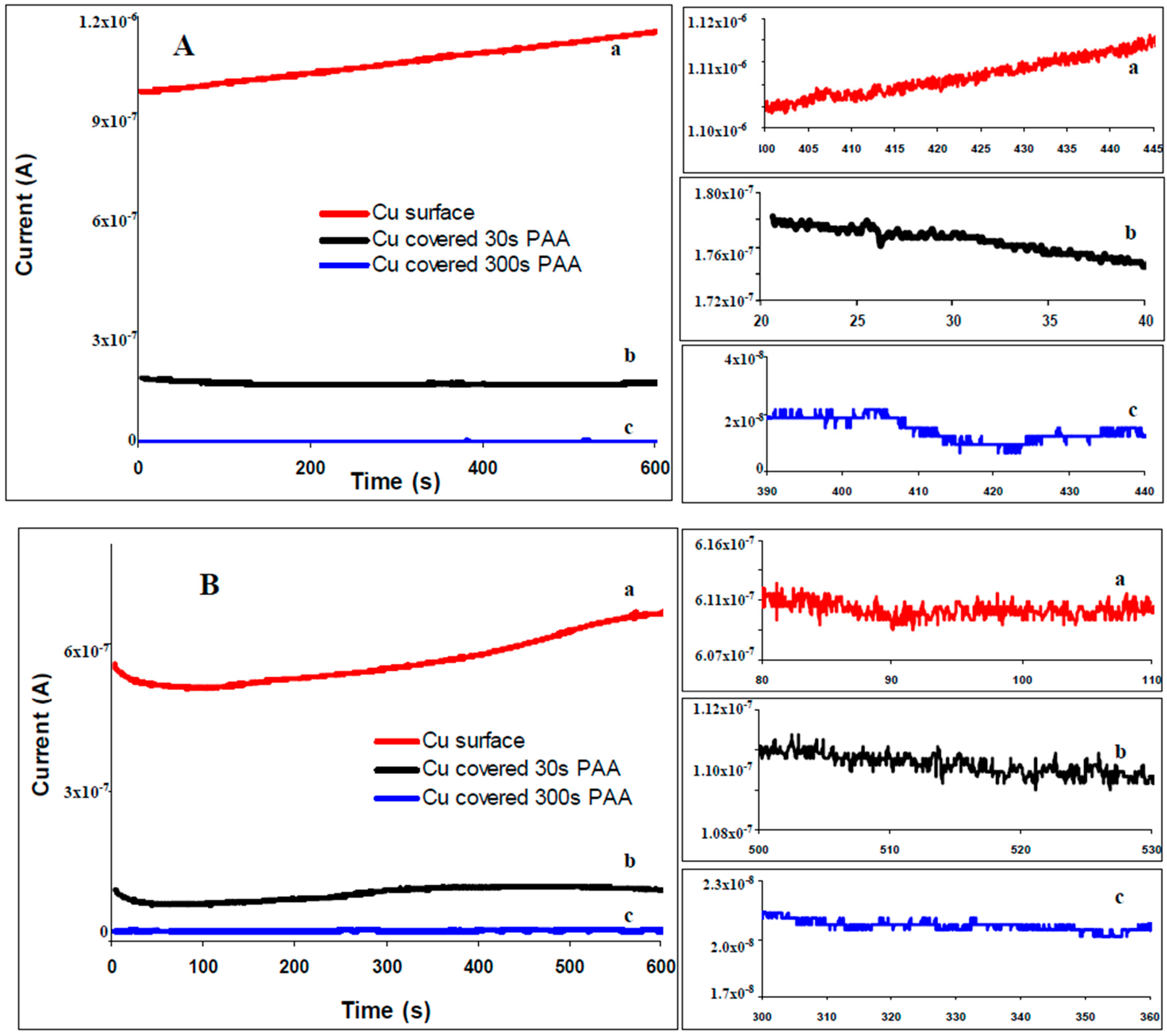
2.2. Electrogravimetric Study of the Layer Formation Process
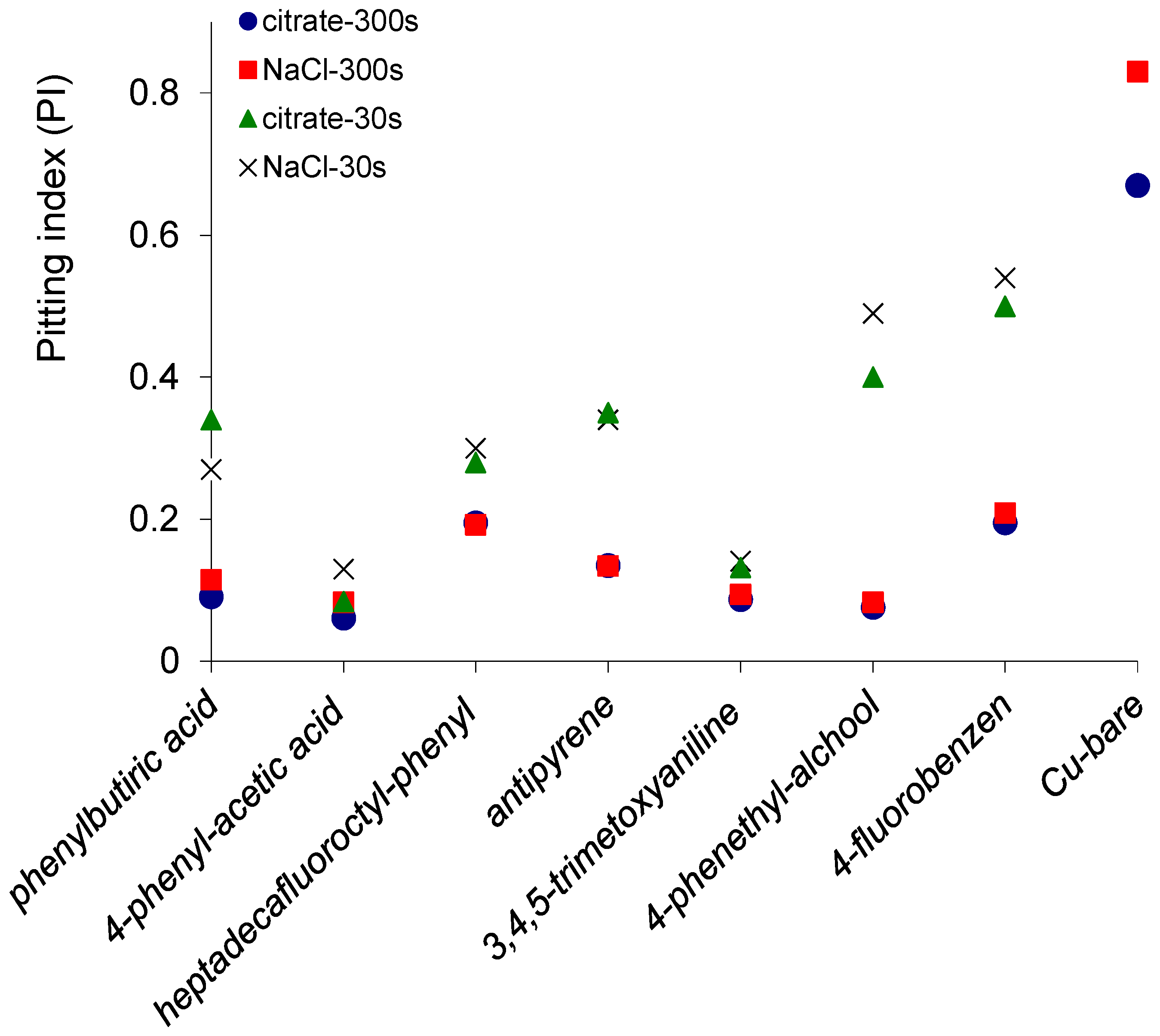
2.3. Electrochemical noise (ECN)
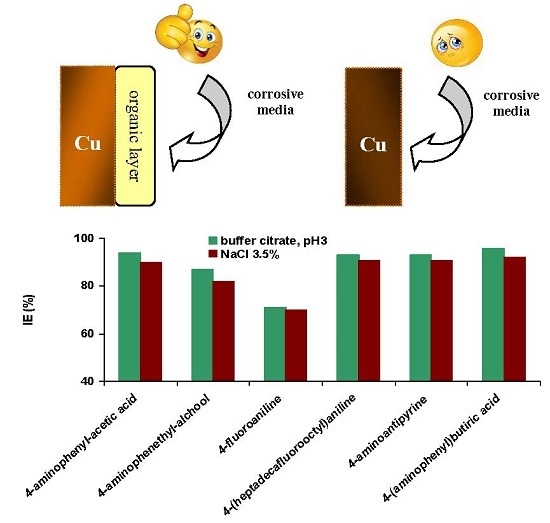
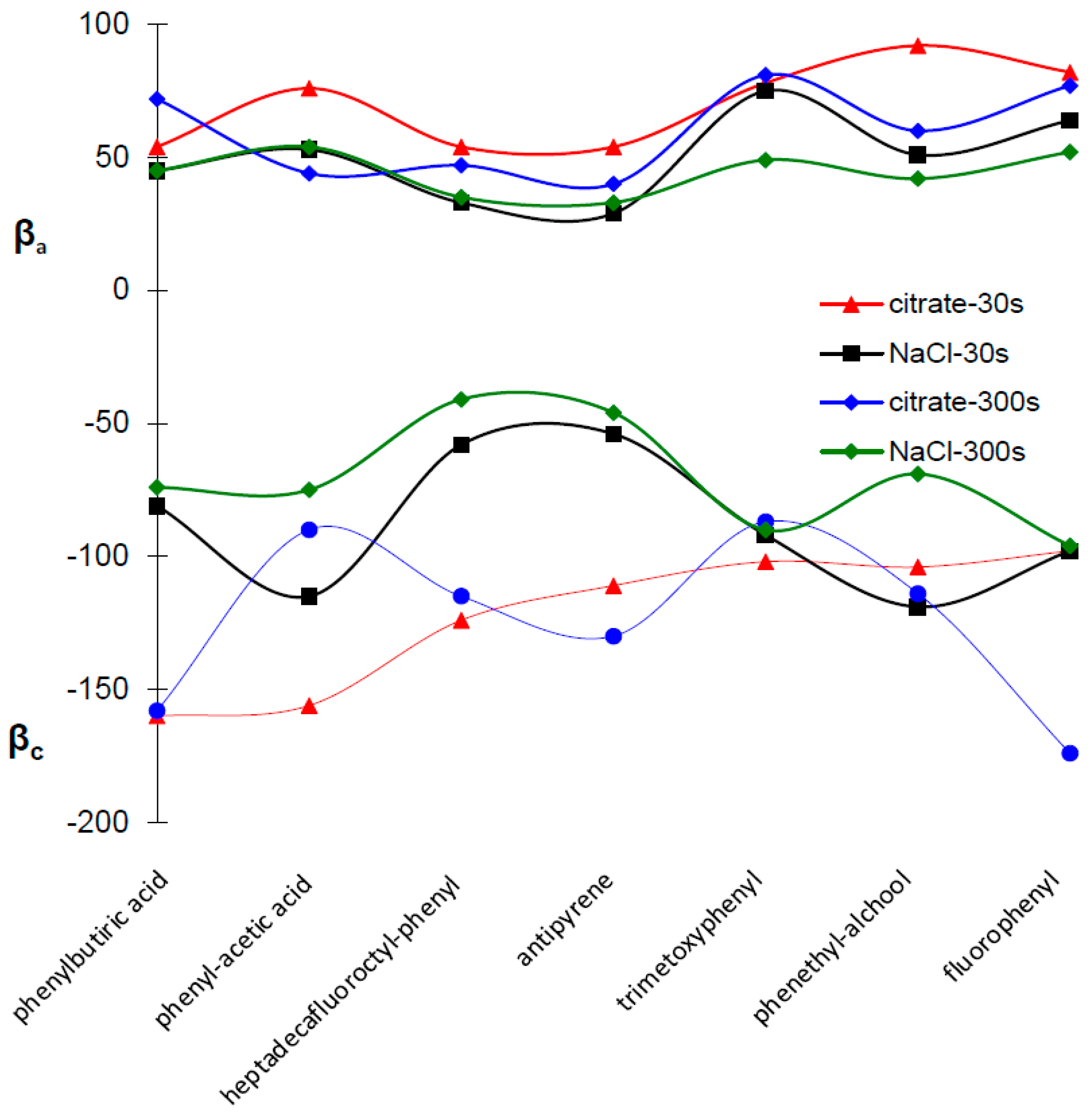
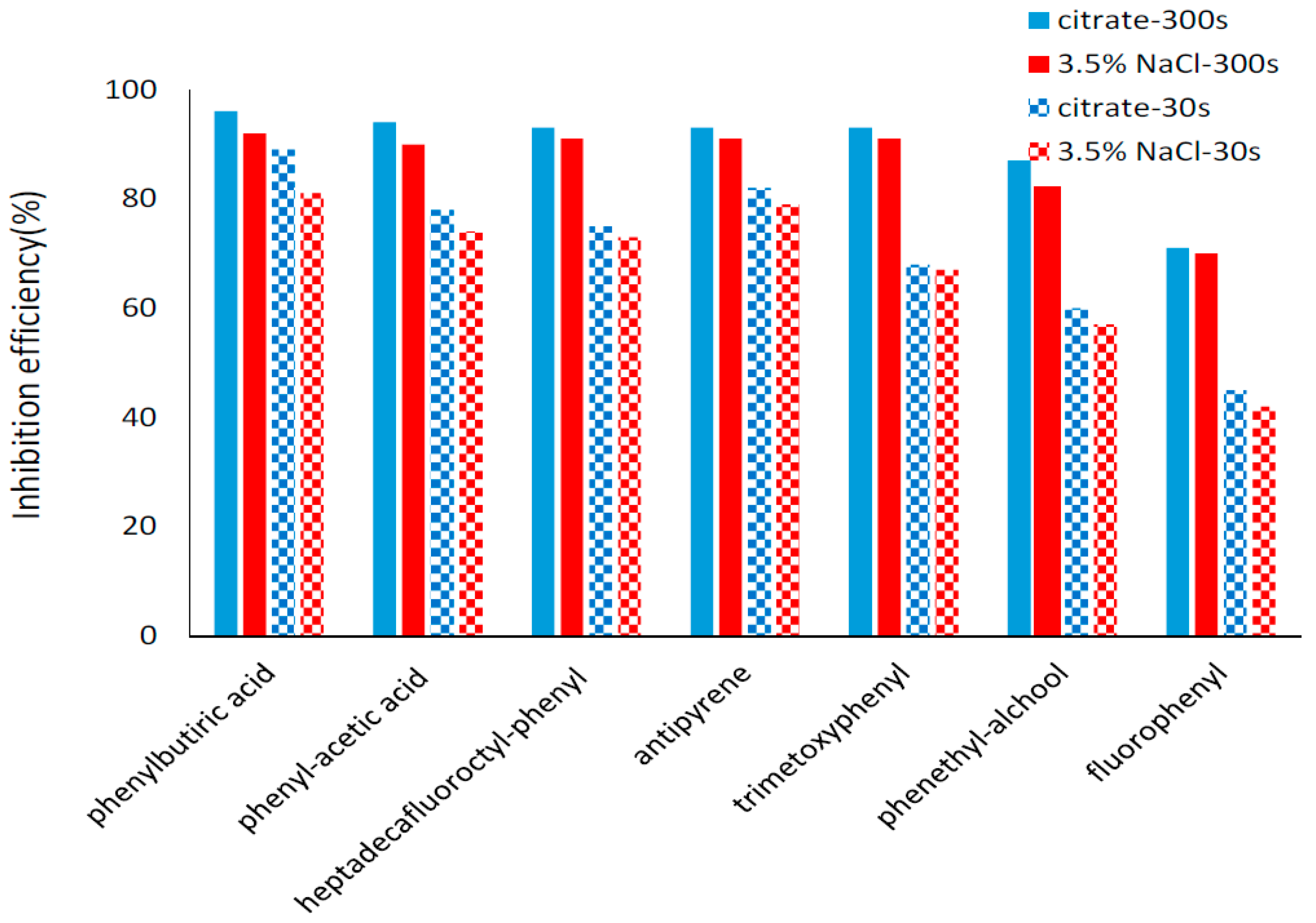
2.4. Potentiodynamic Polarization Measurements
3. Materials and Methods
3.1. Experimental Materials
3.2. Apparatus
3.3. Copper Electrodeposition on Gold SPE
3.4. Electrodeposition of Aryl Diazonium Salts on Copper Surfaces
3.5. Electrodeposition of Aryl Diazonium Salts on Copper Surfaces
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors–Principles, mechanisms and applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech Open Publisher: Rijeka, Croatia, 2014; pp. 366–379. [Google Scholar]
- Martinez Palou, R.; Olivares-Xomelt, O.; Likhanova, N.V. Environmentally friendly corrosion inhibitors. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: Rijeka, Croatia, 2014; pp. 431–465. [Google Scholar]
- Igual Muñoz, A.; García Antón, J.; Guiñón, J.L.; Pérez Herranz, V. Comparison of inorganic inhibitors of copper, nickel and copper–nickels in aqueous lithium bromide solution. Electrochim. Acta 2004, 50, 957–966. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Frignani, A.; Trabanelli, G.; Monticelli, C. Octadecyl-trimethoxy-silane film formed on copper in different conditions. Mater. Chem. Phys. 2007, 103, 340–344. [Google Scholar] [CrossRef]
- El-Haddad, M.N. Chitosan as a green inhibitor for copper corrosion in acidic medium. Int. J. Biol. Macromol. 2013, 55, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Romeiro, A.; Gouveia-Caridade, C.; Brett, C.M.A. Polyphenazine films as inhibitors of copper corrosion. J. Electroanal. Chem. 2013, 688, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–2. [Google Scholar]
- Kovačević, N.; Kokalj, A. The relation between adsorption bonding and corrosion inhibition of azole molecules on copper. Corros. Sci. 2013, 73, 7–17. [Google Scholar] [CrossRef]
- Tan, Y.S.; Srinivasan, M.P.; Pehkonen, S.O.; Chooi, S.Y.M. Effects of ring substituents on the protective properties of self-assembled benzenethiols on copper. Corros. Sci. 2006, 48, 840–862. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.M. Effects of 2-amino-5-ethylthio-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim. Acta 2006, 51, 4656–4662. [Google Scholar] [CrossRef]
- Ghelichkhaha, Z.; Sharifi-Aslb, S.; Farhadia, K.; Banisaieda, S.; Ahmadic, S.; Macdonald, D.D. L-cysteine/polydopamine nanoparticle-coatings for copper corrosion protection. Corros. Sci 2015, 91, 129–139. [Google Scholar] [CrossRef]
- Hurley, B.L.; McCreery, R.L. Covalent bonding of organic molecules to Cu and Al alloy 2024 t3 surfaces via diazonium ion reduction. J. Electrochem. Soc. 2004, 151, B252–B259. [Google Scholar] [CrossRef]
- Salas, B.V.; Stoytcheva, M.; Wiener, M.S.; Beltran, M.C.; Zlatev, R. Corrosion in the food industry and its control. In Food Industrial Processes—Methods and Equipment; Valdez, B., Ed.; InTech: Rijeka, Croatia, 2012; pp. 363–378. [Google Scholar]
- Zarras, P.; Buhrmaster, D.; Webber, C.; Anderson, N.; Stenger-Smith, J.D.; Goodman, P.A. Poly(2,5-bis(N-Methyl-N-Hexylamino)Phenylene Vinylene) (BAM-PPV) as pretreatment coating for aerospace applications: Laboratory and field studies. Materials 2014, 7, 8088–8104. [Google Scholar] [CrossRef]
- Chira, A.; Bucur, B.; Radulescu, M.C.; Galaon, T.; Radu, G.L. Study of electrochemically modified electrode with synthesized n-benzyl-4,4′-bipyridine with anti-fouling properties for oxygen and hydrogen peroxide detection. Int. J. Electrochem. Sci. 2014, 9, 4493–4511. [Google Scholar]
- Chira, A.; Bucur, B.; Bucur, M.P.; Radu, G.L. Electrode-modified with nanoparticles composed of 4,4′-bipyridine-silver coordination polymer for sensitive determination of Hg(II), Cu(II) and Pb(II). New J. Chem. 2014, 38, 5641–5646. [Google Scholar] [CrossRef]
- Pinson, J.; Podvorica, F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem. Soc. Rev. 2005, 34, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, S.; Kösemen, A.; Sen, Z.; Kılınç, N.; Harbeck, M. Poly(3-methylthiophene) thin films deposited electrochemically on QCMs for the sensing of volatile organic compounds. Sensors 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar] [PubMed]
- Zhang, X.; Rösicke, F.; Syritski, V.; Sun, G.; Reut, J.; Hinrichs, K.; Janietz, S.; Rappich, J. Influence of the para-substitutent of benzene diazonium salts and the solvent on the film growth during electrochemical reduction. Z. Phys. Chem. 2014, 228, 557–573. [Google Scholar] [CrossRef]
- Sherifa, E.M.; Park, S.M. Effects of 1,5-naphthalenediol on aluminum corrosion as a corrosion inhibitor in 0.50 M NaCl. J. Electrochem. Soc. 2005, 152, B205–B211. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.T.; Parka, S.M. Effects of o-vanillin as a brightener on zinc electrodeposition at iron electrodes. J. Electrochem. Soc. 2004, 151, C850–C854. [Google Scholar] [CrossRef]
- Finsgar, M.; Milosev, I.; Pihlar, B. Inhibition of copper corrosion studied by electrochemical and EQCN techniques. Acta Chim. Slov. 2007, 54, 591–597. [Google Scholar]
- Holcomb, G.R.; Covino, B.S.; Eden, D. Department of Energy Albany Research Center. 2001. Available online: http://www.osti.gov/scitech/servlets/purl/899598 (accessed on 16 January 2017). [Google Scholar]
- Jez, M.; Mitoraj, M.; Godlewska, E.; Jakubowska, M.; Bas, B. Evaluation of corrosion behaviour of selected metallic samples by electrochemical noise measurements. J. Solid State Electr. 2014, 18, 1635–1646. [Google Scholar] [CrossRef]
- Menchaca, C.; Castañeda, I.; Soto-Quintero, A.; Guardián, R.; Cruz, R.; García-Sánchez, M.A.; Uruchurtu, J. Characterization of a “Smart” hybrid varnish electrospun nylon benzotriazole copper corrosion protection coating. Int. J. Corros. 2012, 2012, 925958. [Google Scholar] [CrossRef]
- Kelly, R.; Inman, M.; Hudson, L. Analysis of electrochemical noise for type 410 stainless steel in chloride solutions. In Electrochemical Noise Measurement for Corrosion Applications; Kearns, J., Scully, J., Roberge, P., Reichert, D., Dawson, J., Eds.; ASTM International: West Conshohocken, PA, USA, 1996; pp. 101–113. [Google Scholar]
- Nagiub, A.M. Comparative electrochemical noise study of the corrosion of different alloys exposed to chloride media. Engineering 2014, 6, 1007–1016. [Google Scholar] [CrossRef]
- Klapper, H.S.; Goellner, J.; Burkert, A.; Heyn, A. Environmental factors affecting pitting corrosion of type 304 stainless steel investigated by electrochemical noise measurements under potentiostatic control. Corros. Sci. 2013, 75, 239–247. [Google Scholar] [CrossRef]
- Reid, S.A.; Eden, D.A. Assessment of Corrosion. A Method and Apparatus for Using EN to Asses Corrosion, Preferably with Skewness and Kurtosis Analysis Using Neutral Nets. U.S. Patent 6264824 B1, 2001. [Google Scholar]
- Chauhan, L.R.; Gunasekaran, G. Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros. Sci. 2007, 49, 1143–1161. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Wang, F. An investigation of benzimidazole derivative as corrosion inhibitor for mild steel in different concentration HCl solutions. Corros. Sci. 2011, 53, 113–121. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Xu, S.; Li, W. Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J. Colloid Interface Sci. 2016, 472, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, O.; Danaee, I.; Rashed, G.R.; RashvandAvei, M.H.; Maddahy, M.H. The inhibition effect of synthesized 4-hydroxybenzaldehyde-1,3propandiamine on the corrosion of mild steel in 1 M HCl. J. Mater. Eng. Perform. 2013, 22, 1054–1063. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gao, L.X.; Cai, Q.R.; Lee, K.Y. Inhibition of copper corrosion by modifying cysteine selfassembled film with alkylamine/alkylacid compounds. Mater. Corros. 2010, 61, 16–21. [Google Scholar] [CrossRef]
- Wang, C.T.; Chen, S.H.; Ma, H.Y.; Qi, C.S. Protection of copper corrosion by carbazole and N-vinylcarbazole self-assembled films in NaCl solution. J. Appl. Electrochem. 2003, 33, 179–186. [Google Scholar] [CrossRef]
- Singh, M.M.; Rastogi, R.B.; Upadhyay, B.N.; Yadav, M. Effect of substituents on corrosion inhibition efficiency of arylisothiocyanates and their condensation products with thiosemicarbazide for corrosion of copper in aqueous chloride solution. Indian J. Chem. Technol. 2003, 10, 414–419. [Google Scholar]
- Zhao, M.; Li, H.; Wen, L.; Yu, Z.; Zhang, S.; Han, Z. Synthesis and characterization of fluorine-containing polyurethane–acrylate core–shell emulsion. J. Appl. Polym. Sci. 2016, 133, 43357. [Google Scholar] [CrossRef]
- Rocklein, M.N.; George, S.M. Temperature-induced apparent mass changes observed during quartz crystal microbalance measurements of atomic layer deposition. Anal. Chem. 2003, 75, 4975–4982. [Google Scholar] [CrossRef]


| Organic Compound | Δm/cm2 (ng/cm2) | |
|---|---|---|
| Electrodeposition Time 30 (s) | Electrodeposition Time 300 (s) | |
| 4-aminophenylacetic acid | 39 | 388 |
| 4-aminophenethyl alcohol | 29 | 274 |
| 4-fluoroaniline | 26 | 266 |
| 4-(heptadecafluorooctyl)aniline | 36 | 377 |
| 4-aminoantipyrine | 40 | 416 |
| 4-phenylbutyric acid | 34 | 442 |
| 3,4,5-trimethoxyaniline | 38 | 435 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chira, A.; Bucur, B.; Radu, G.-L. Electrodeposited Organic Layers Formed from Aryl Diazonium Salts for Inhibition of Copper Corrosion. Materials 2017, 10, 235. https://doi.org/10.3390/ma10030235
Chira A, Bucur B, Radu G-L. Electrodeposited Organic Layers Formed from Aryl Diazonium Salts for Inhibition of Copper Corrosion. Materials. 2017; 10(3):235. https://doi.org/10.3390/ma10030235
Chicago/Turabian StyleChira, Ana, Bogdan Bucur, and Gabriel-Lucian Radu. 2017. "Electrodeposited Organic Layers Formed from Aryl Diazonium Salts for Inhibition of Copper Corrosion" Materials 10, no. 3: 235. https://doi.org/10.3390/ma10030235