Effect of Cooling Rate on Morphology of TiAl3 Particles in Al–4Ti Master Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Microstructural Analysis
3. Results and Discussion
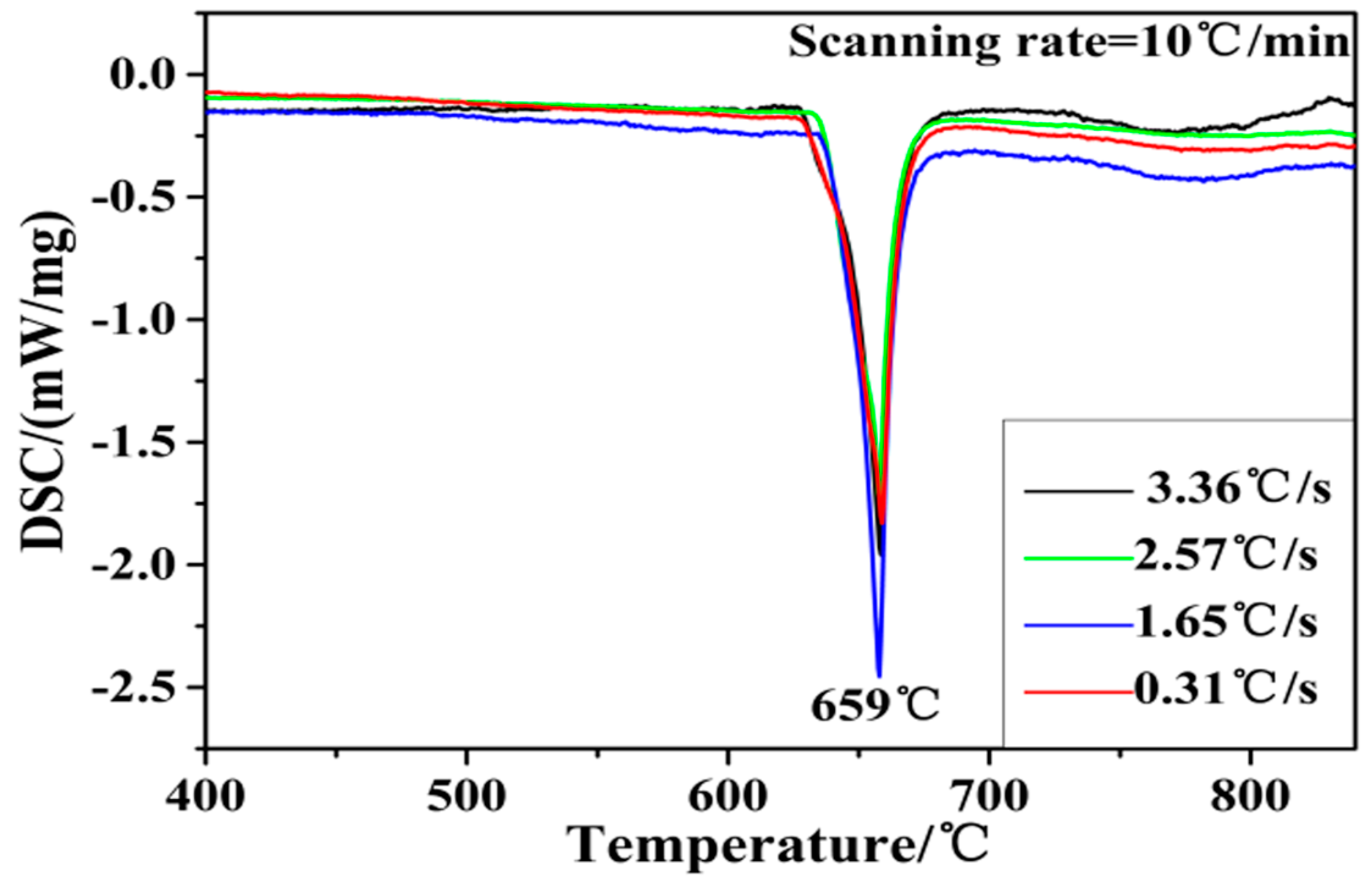
3.1. Microstructure of Al–4Ti Master Alloy
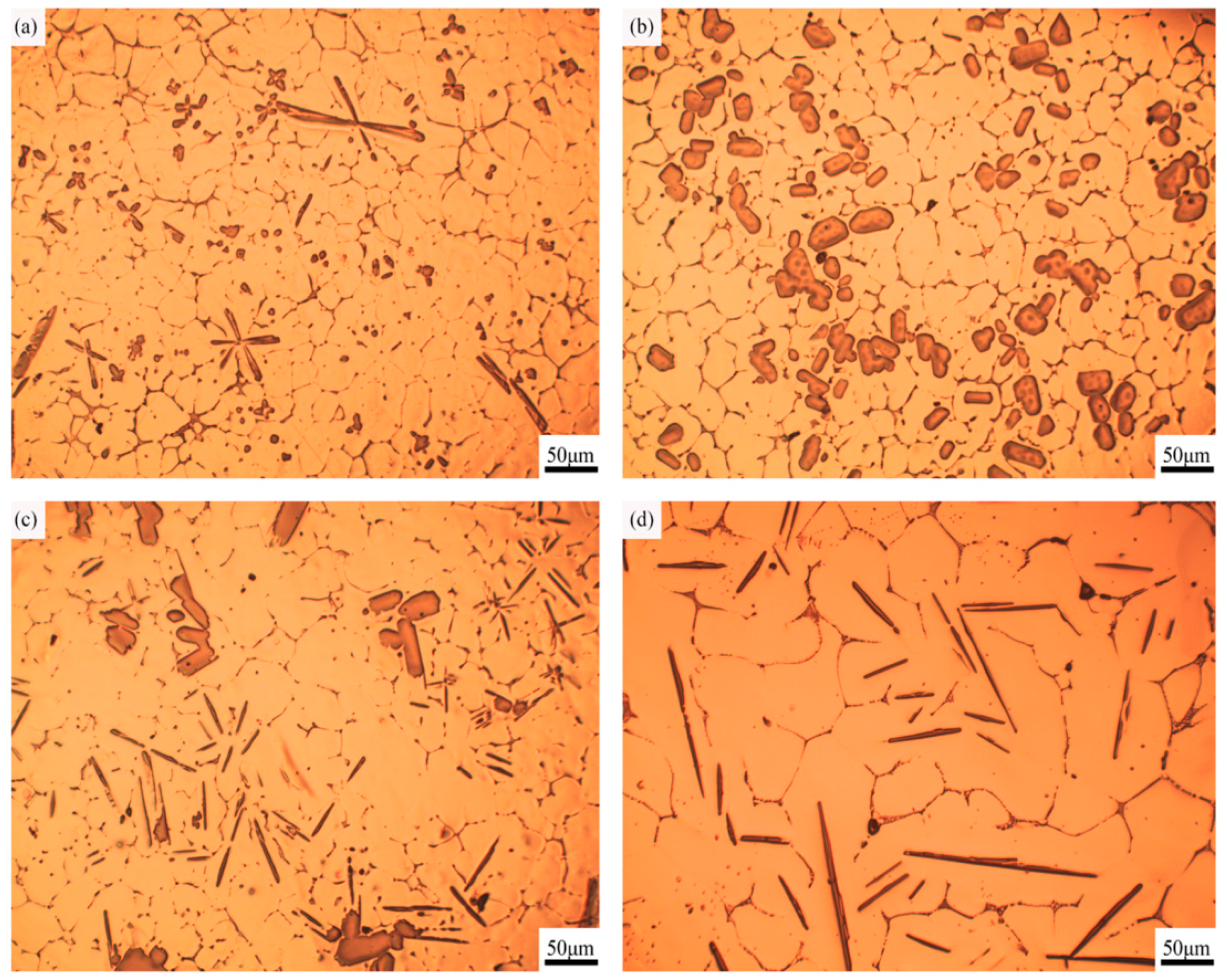
3.2. Morphology of TiAl3 Particles in Al-4Ti Master Alloy
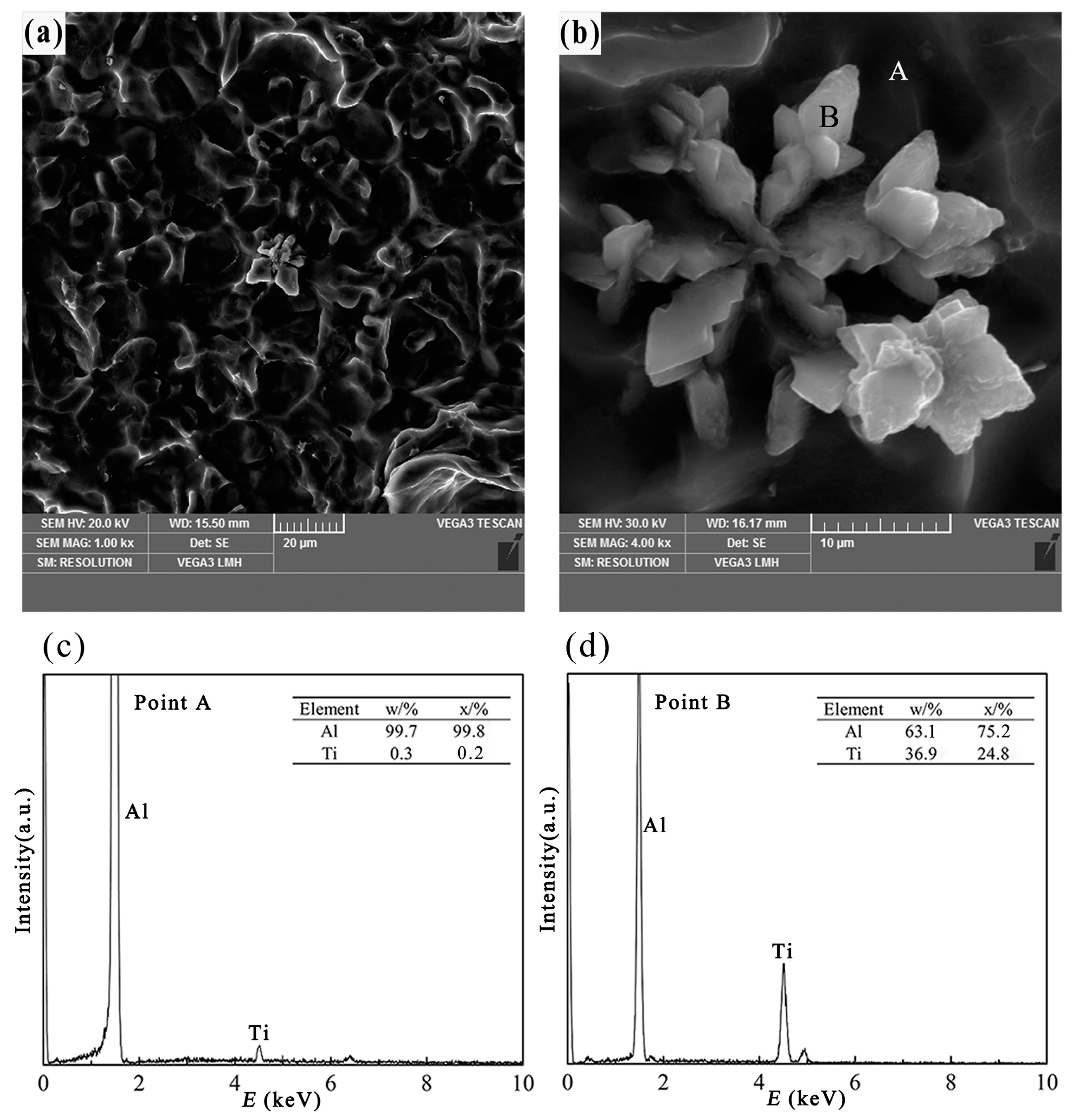
3.2.1. Petal-Like TiAl3 Particles
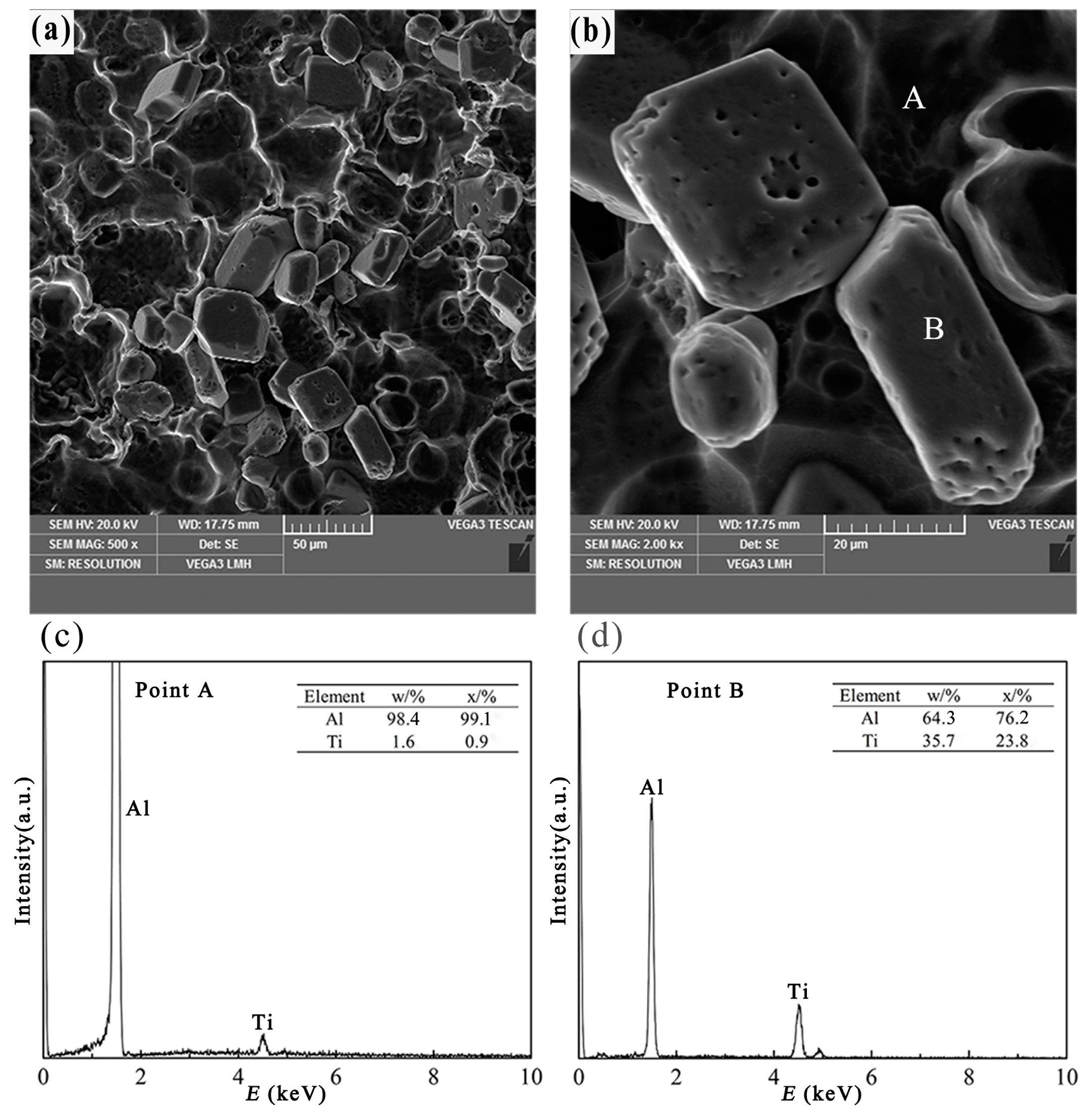
3.2.2. Blocky TiAl3 Particles
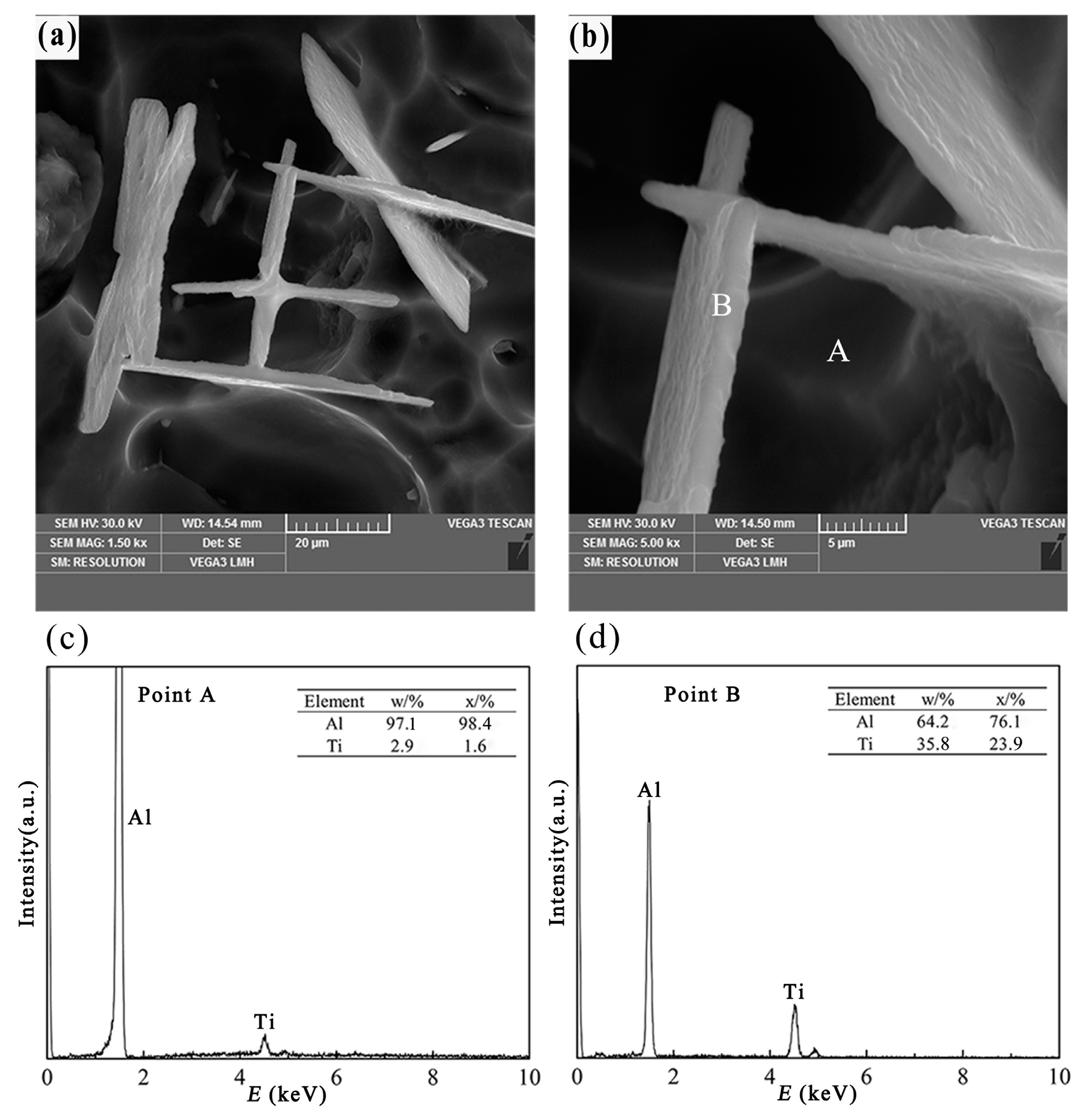
3.2.3. Flake-Like TiAl3 Particles
4. Conclusions
- Different morphologies of TiAl3 particles in Al–4Ti master alloy could be acquired by adopting different cooling rates. The petal-like, blocky, and flake-like TiAl3 particles were respectively acquired at the cooling rates of 3.36 K/s, 2.57 K/s, and 0.31 K/s.
- With the decrease of cooling rate, the morphology of TiAl3 particles in the prepared master alloy changed from petal-like to blocky and, finally, to flake-like. It was also found that the petal-like TiAl3 particles could be formed by thick plates during the solidification.
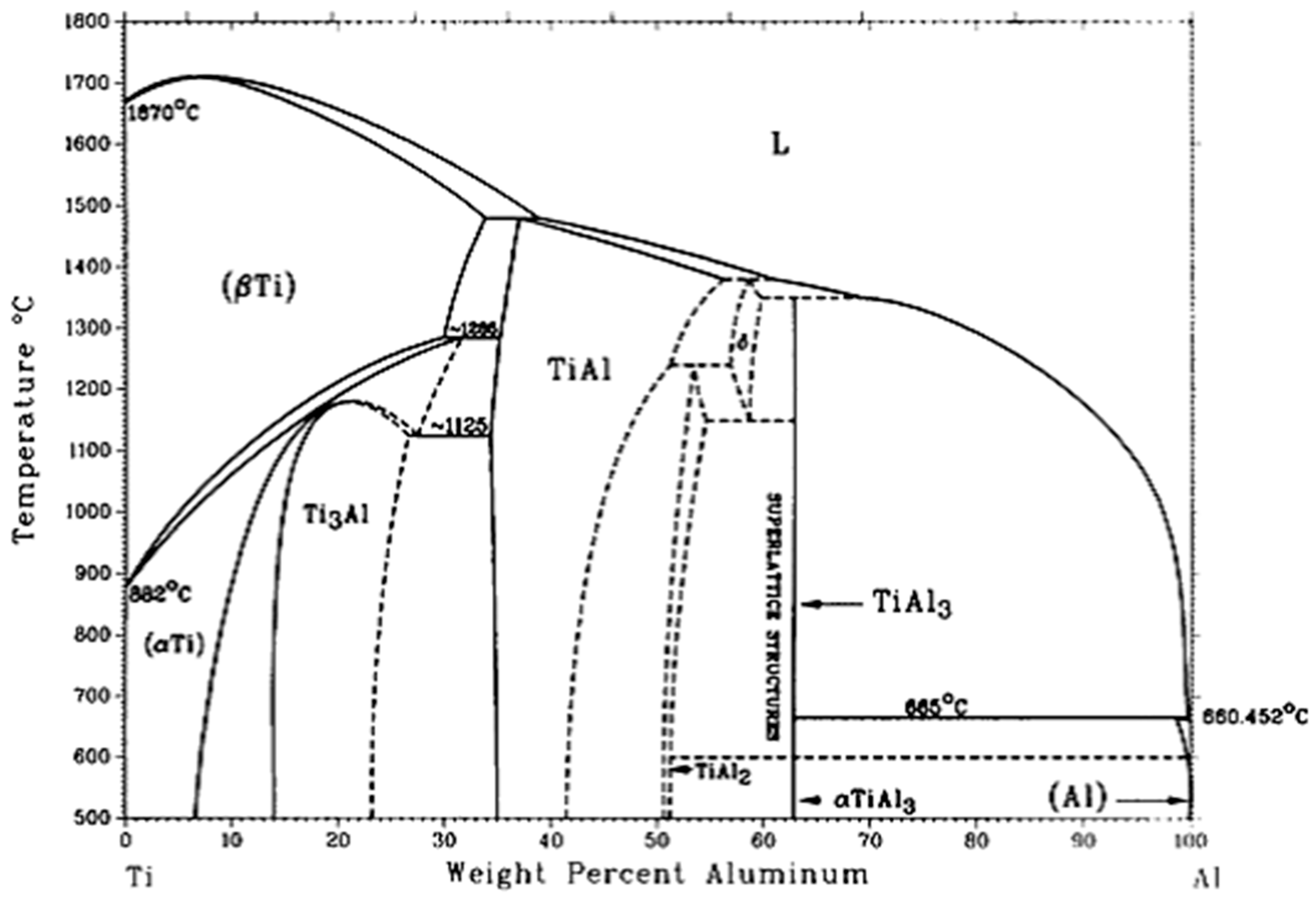
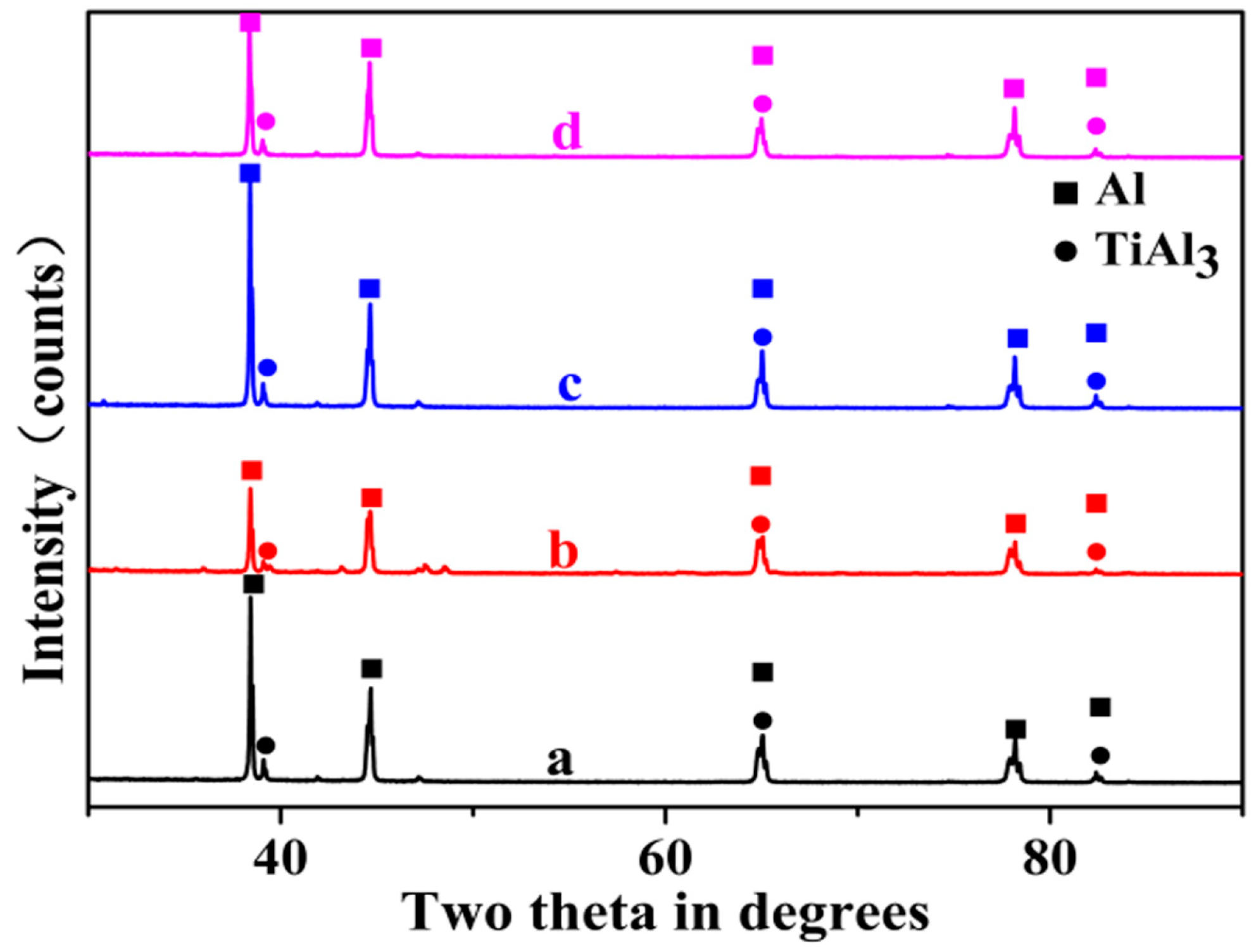
- The three main types of TiAl3 particles did not differ in composition. Moreover, the phase inversion temperature of Al–4Ti master alloy did not change with different particles morphologies.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hassanbeygi, V.; Shafyei, A. Investigation on microstructure and grain refining performance of a new type of Al-3Ti-1C master alloy. Open J. Met. 2014, 4, 49–55. [Google Scholar] [CrossRef]
- Kashyap, K.T.; Chandrashekar, T. Effects and mechanisms of grain refinement in aluminium alloys. Bull. Mater. Sci. 2001, 24, 345–353. [Google Scholar] [CrossRef]
- Ghadimi, H.; Nedjhad, S.H.; Eghbali, B. Enhanced grain refinement of cast aluminum alloy by thermal and mechanical treatment of Al-5Ti-B master alloy. Trans. Nonferr. Met. Soc. China 2013, 23, 1563–1569. [Google Scholar] [CrossRef]
- Kori, S.A.; Murty, B.S.; Chakraborty, M. Development of an efficient grain refiner for Al-7Si alloy and its modification with strontium. Mater. Sci. Eng. A 2000, 280, 58–61. [Google Scholar] [CrossRef]
- Krajewski, W.K.; Greer, A.L. EBSD study of ZnAl25 alloy inoculated with ZnTi4 master alloy. Mater. Sci. Forum 2006, 508, 281–286. [Google Scholar] [CrossRef]
- Krajewski, W.K. Determination of Al site preference in L12TiZn3—Base trialuminides. Mater. Sci. Forum 2006, 508, 615–619. [Google Scholar] [CrossRef]
- Krajewski, W.K.; Buraś, J.; Krajewski, P.K.; Greer, A.L.; Faerber, K.; Schumacher, P. New developments of Al-Zn cast alloys. Mater. Today Proc. 2015, 2, 4978–4983. [Google Scholar] [CrossRef]
- Auradi, V.; Kori, S.A. Effect of processing temperature on the microstructure of Al-7Ti master alloy and on refinement of a-Al dendrites in Al-7Si alloys. Adv. Mater. Lett. 2015, 6, 252–259. [Google Scholar] [CrossRef]
- Birol, Y. AlB3 master alloy to grain refine AlSi10Mg and AlSi12Cu aluminium foundry alloys. J. Alloys Compd. 2012, 513, 150–153. [Google Scholar] [CrossRef]
- Zhao, J.; He, J.; Tang, Q.; Wang, T.; Chen, J. Grain refinement efficiency in commercial-purity aluminum influenced by the addition of Al-4Ti master alloys with varying TiAl3 particles. Materials 2016, 9, 869. [Google Scholar] [CrossRef]
- Lee, M.S.; Terry, B.S. Effects of processing parameters on aluminide morphology in aluminium grain refining master alloys. Mater. Sci. Technol. 1991, 7, 608–612. [Google Scholar] [CrossRef]
- Li, P.; Kandalova, E.G.; Nikitin, V.I. Grain refining performance of Al-Ti master alloys with different microstructures. Mater. Lett. 2005, 59, 723–727. [Google Scholar] [CrossRef]
- Birol, Y. Effect of the salt addition practice on the grain refining efficiency of Al-Ti-B master alloys. J. Alloys Compd. 2006, 420, 207–212. [Google Scholar] [CrossRef]
- Birol, Y. The effect of holding conditions in the conventional halide salt process on the performance of Al-Ti-B grain refiner alloys. J. Alloys Compd. 2007, 427, 142–147. [Google Scholar] [CrossRef]
- Zhao, Z.; Guan, R.; Guan, X.; Zhang, J.; Sun, X.; Liu, H. Effects of electromagnetic stirring, shearing, and extrusion on TiB2 and TiAl3 particles in Al-5Ti-1B(wt. %) alloy. Mater. Manuf. Process. 2015, 30, 1223–1228. [Google Scholar] [CrossRef]
- Wang, X.; Jha, A.; Brydson, R. In situ fabrication of Al3Ti particle reinforced aluminium alloy metal-matrix composites. Mater. Sci. Eng. A 2004, 364, 339–345. [Google Scholar] [CrossRef]
- Alloy Phase Diagrams of ASM Handbook, 8th ed.; ASM International: Materials Park, OH, USA, 1992.
- Arnberg, L.; Bäckerud, L.; Klang, H. Intermetallic particles in Al-Ti-B-type master alloys for grain refinement of aluminium. Met. Technol. 1982, 9, 7–13. [Google Scholar] [CrossRef]
- Schumacher, P.; Greer, A.L.; Worth, J.; Evans, P.V.; Kearns, M.A.; Fisher, P.; Green, A.H. New studies of nucleation mechanisms in aluminium alloys: Implications for grain refinement practice. Mater. Sci. Technol. 1998, 14, 394–404. [Google Scholar] [CrossRef]
- Li, P.T.; Ma, X.G.; Li, Y.G.; Nie, J.F.; Liu, X.F. Effects of trace C addition on the microstructure and refining efficiency of Al-Ti-B master alloy. J. Alloys Compd. 2010, 503, 286–290. [Google Scholar] [CrossRef]
- Wang, T.; Gao, T.; Nie, J.; Li, P.; Liu, X. Influence of carbon source on the microstructure of Al-Ti-C master alloy and its grain refining efficiency. Mater. Charact. 2013, 83, 13–20. [Google Scholar] [CrossRef]
- Ding, W.W.; Zhang, X.Y.; Zhao, W.J.; Xia, T.D.; Zhang, H.X. Microstructure evolution and grain refining performance of new green kind of Al-5Ti-C master alloy. Int. J. Cast Met. Res. 2014, 28, 89–96. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, T.; Chen, J.; Fu, L.; He, J. Effect of Cooling Rate on Morphology of TiAl3 Particles in Al–4Ti Master Alloy. Materials 2017, 10, 238. https://doi.org/10.3390/ma10030238
Zhao J, Wang T, Chen J, Fu L, He J. Effect of Cooling Rate on Morphology of TiAl3 Particles in Al–4Ti Master Alloy. Materials. 2017; 10(3):238. https://doi.org/10.3390/ma10030238
Chicago/Turabian StyleZhao, Jianhua, Tao Wang, Jing Chen, Lu Fu, and Jiansheng He. 2017. "Effect of Cooling Rate on Morphology of TiAl3 Particles in Al–4Ti Master Alloy" Materials 10, no. 3: 238. https://doi.org/10.3390/ma10030238




