Antimicrobial Bamboo Materials Functionalized with ZnO and Graphene Oxide Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
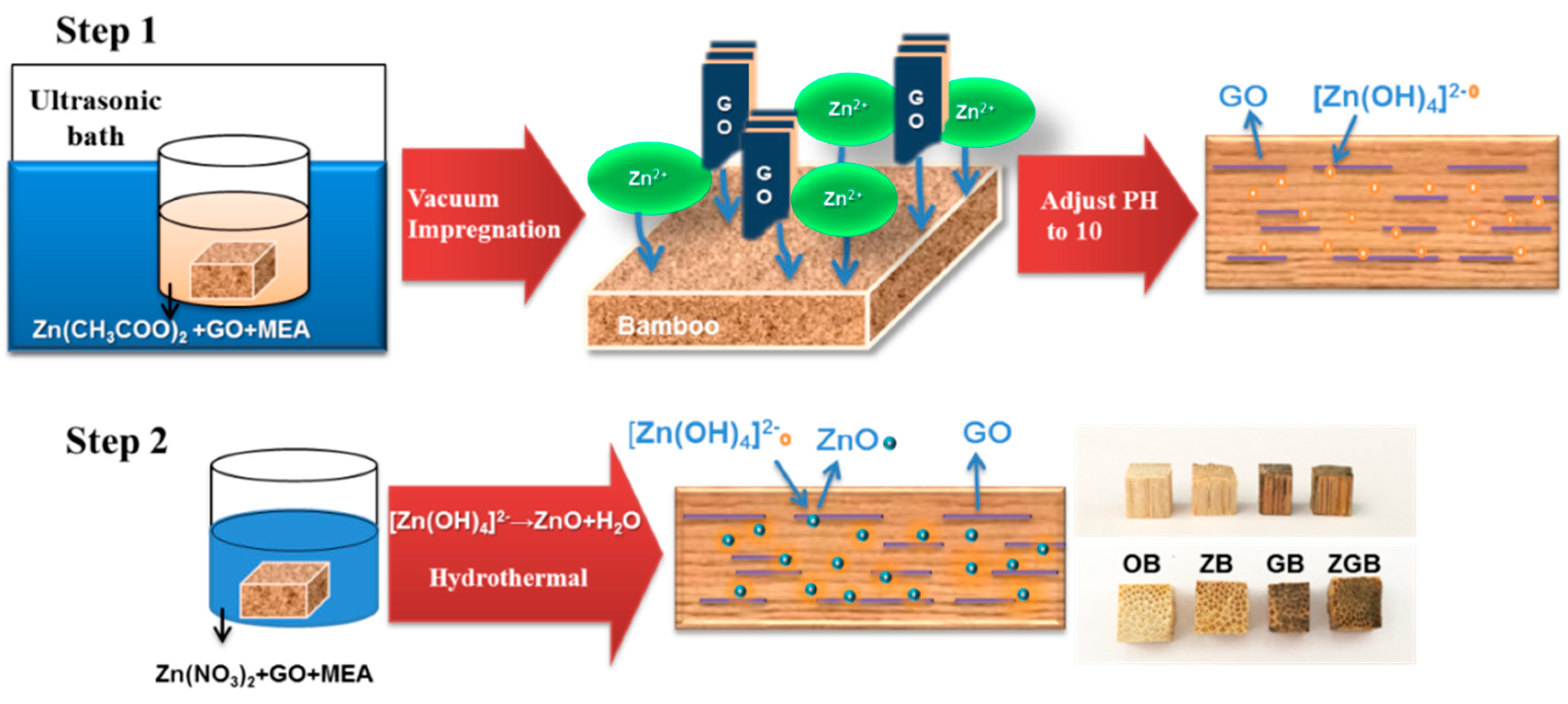
2.1. Preparation of Bamboo Composites
2.2. Characterization
2.3. Antibacterial Test
3. Results and Discussion
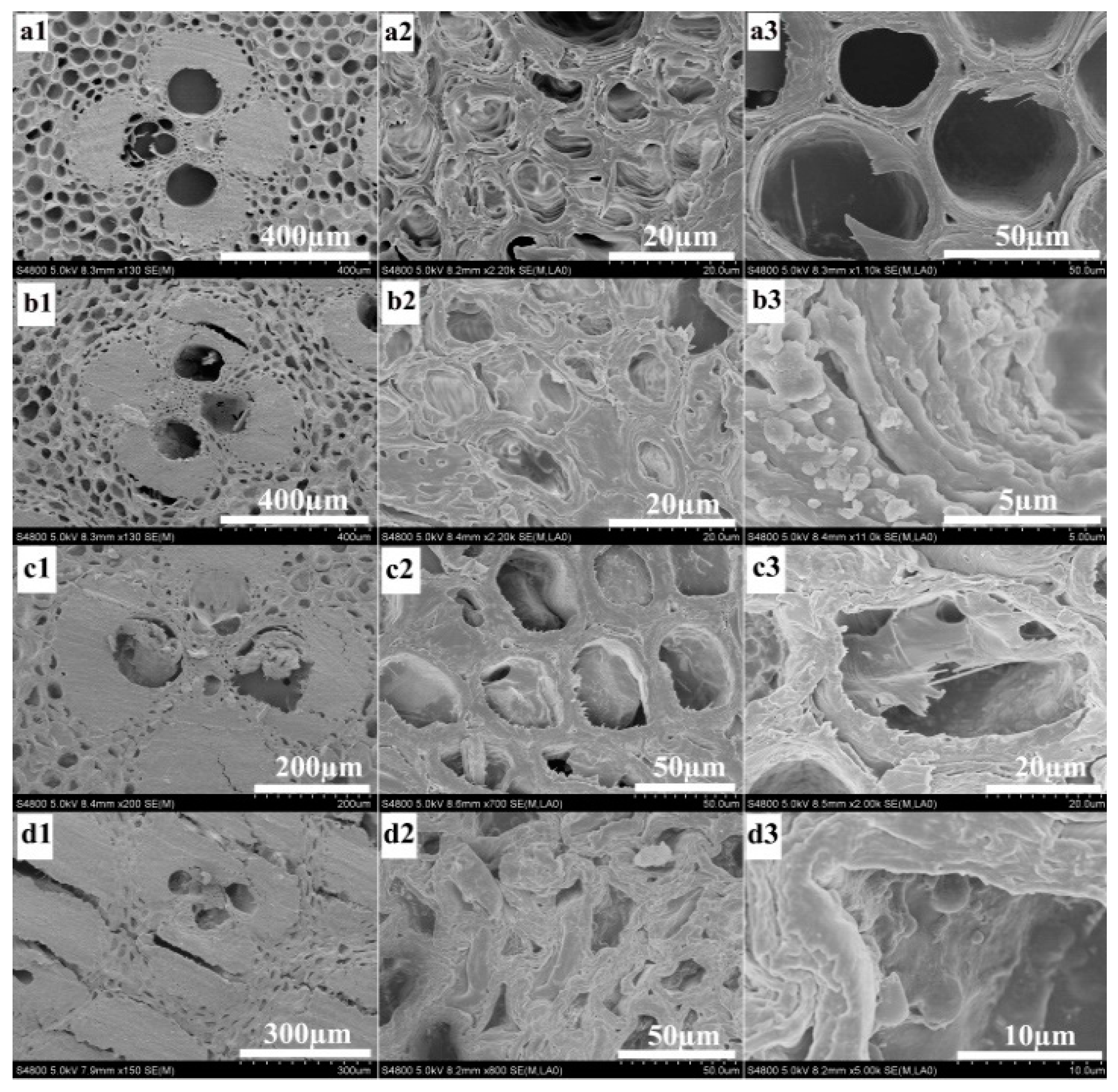
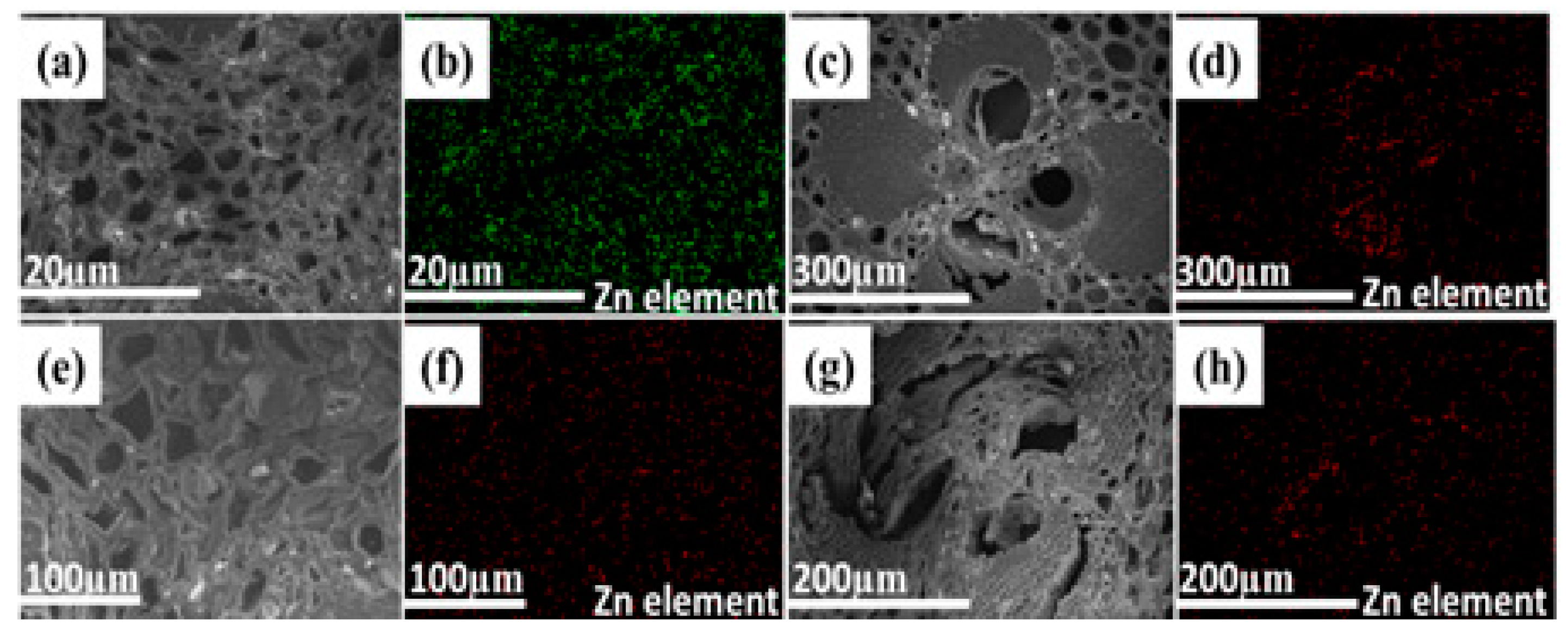
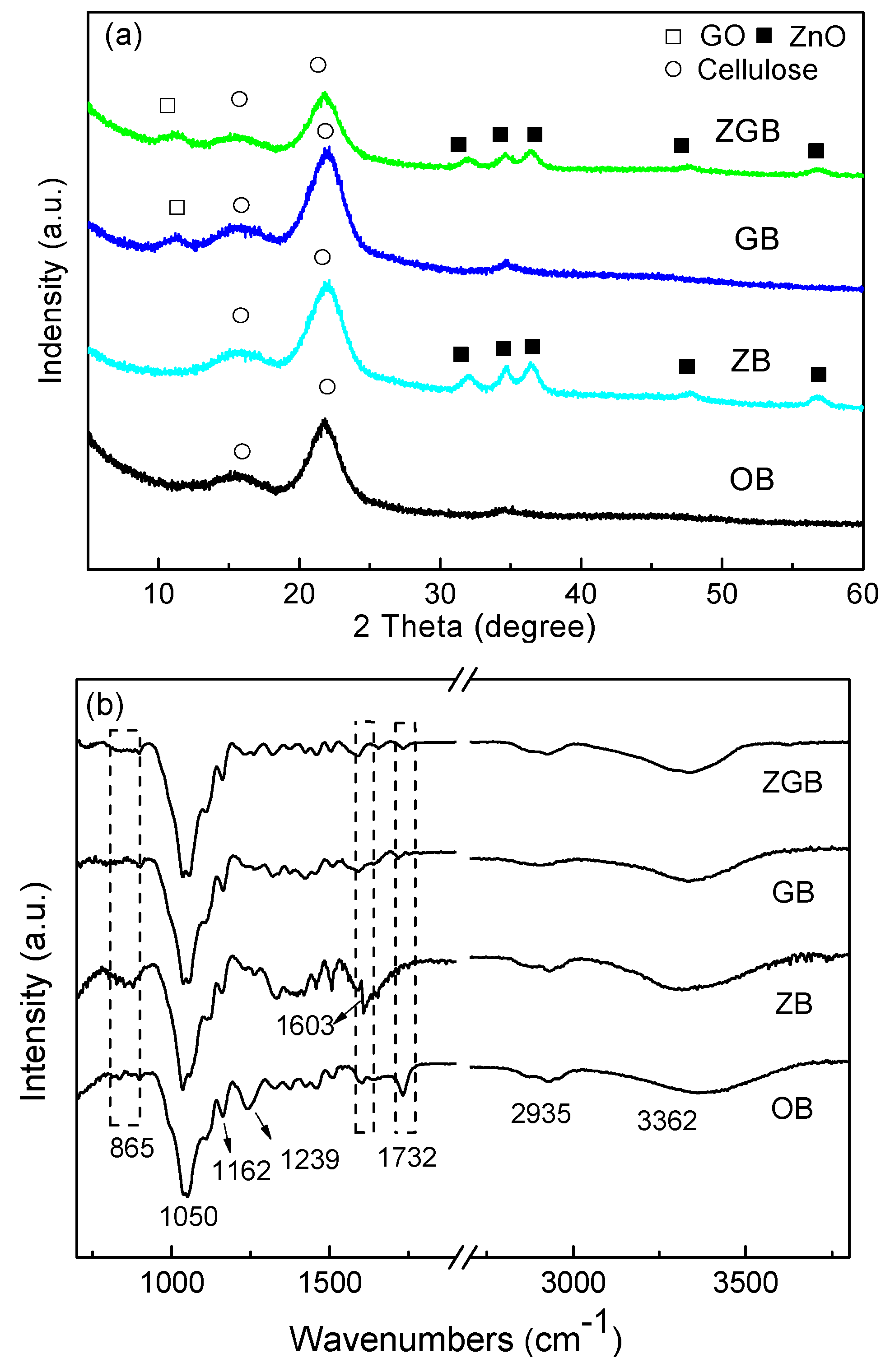
3.1. Synthesis and Characterization of Bamboo Composites
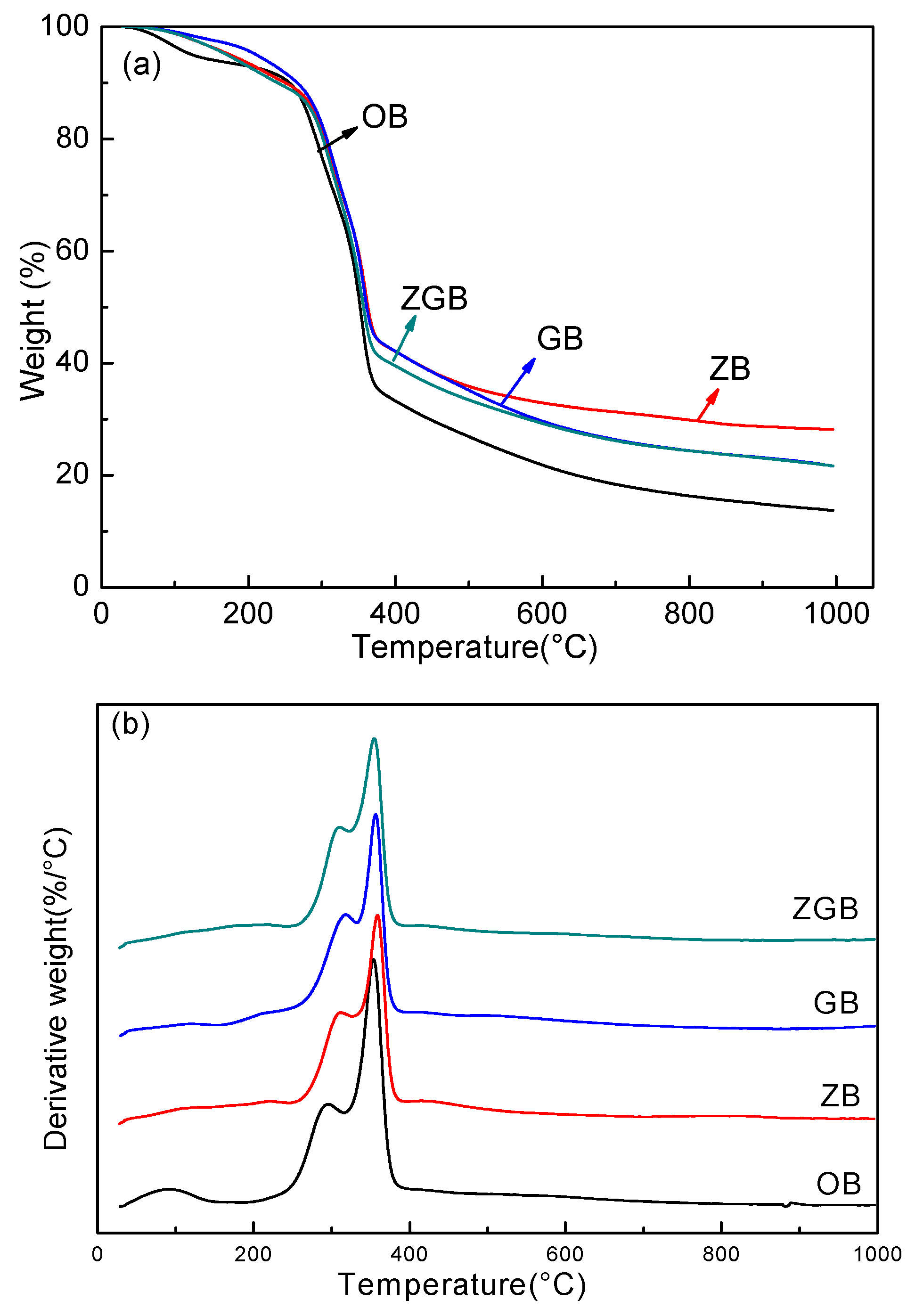
3.2. Thermal Stability
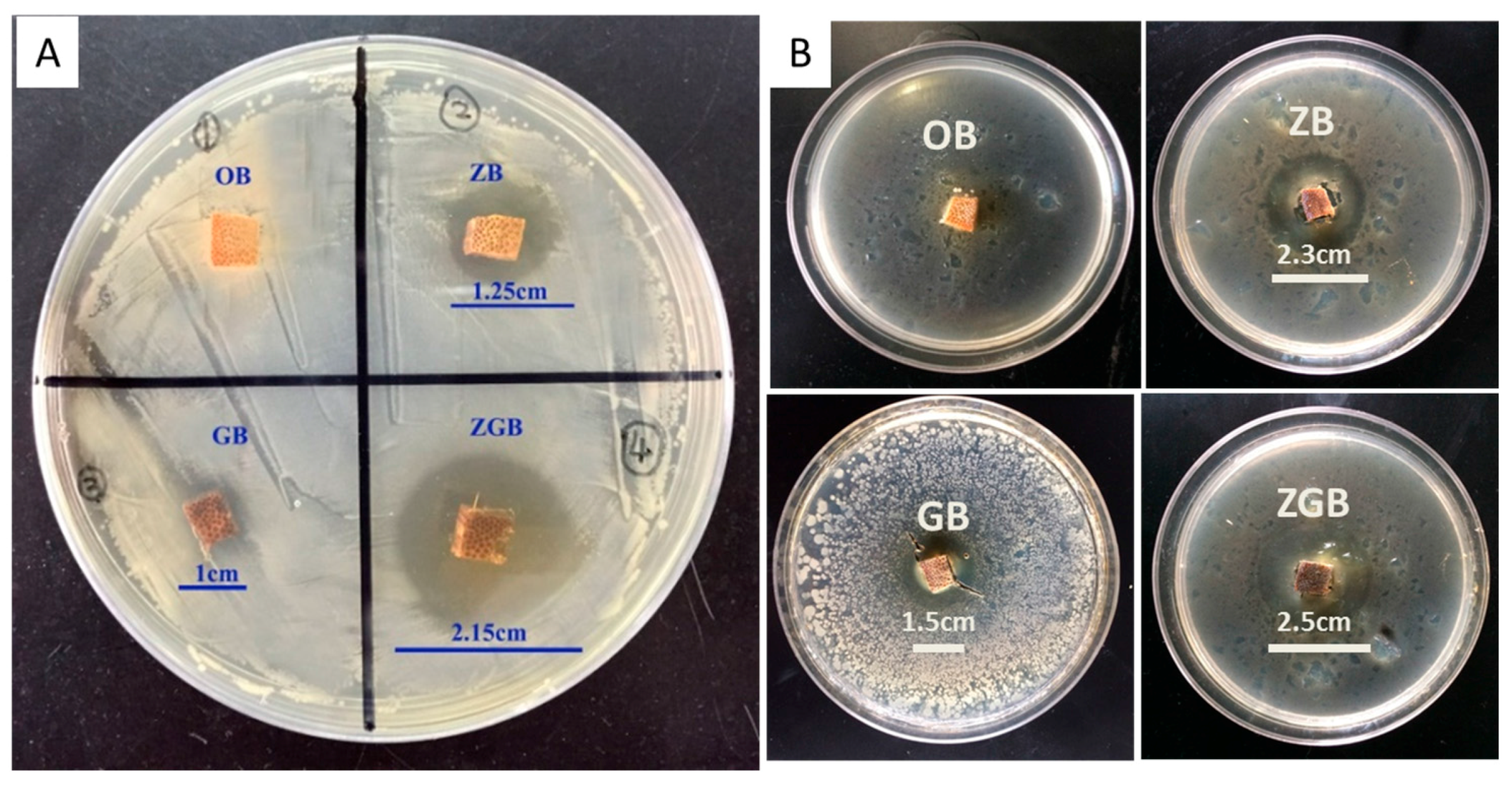
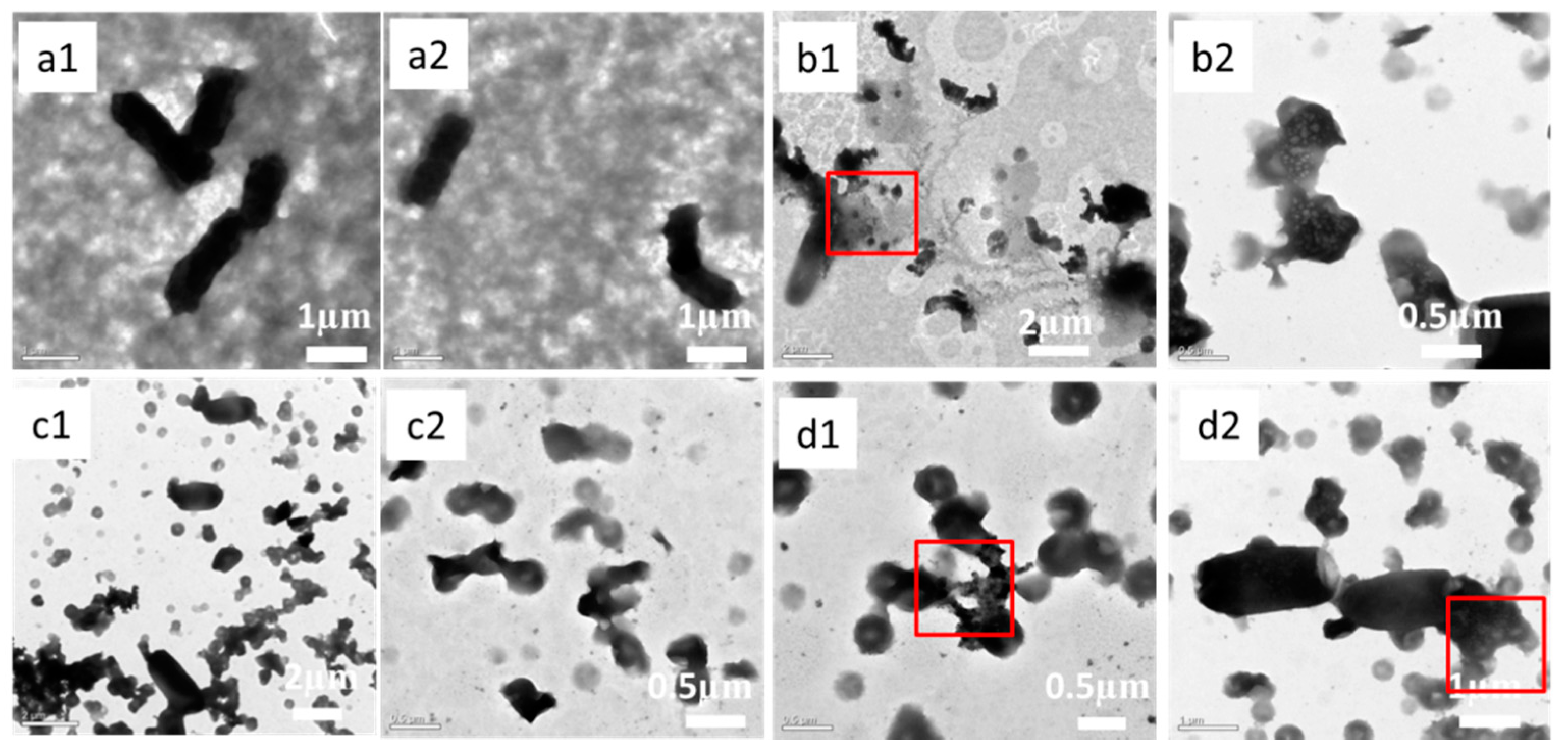
3.3. Antibacterial Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Z.J.; Jiang, Z.H.; Fei, B.H.; Cai, Z.Y.; Liu, X.E.; Yu, Y. Bamboo pellets: A potential and commercial pellets in China. Sci. Silvae Sin. 2012, 48, 140–144. [Google Scholar]
- Feng, X.; Feng, L.; Jin, M.; Zhai, J.; Jiang, L.; Zhu, D. Reversible super-hydrophobicity to super-hydrophilicity transition of aligned ZnO nanorod films. J. Am. Chem. Soc. 2004, 126, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Wang, X.C.; Fu, X.Z. Hierarchical macro/mesoporous TiO2/SiO2 and TiO2/ZrO2 nanocomposites for environmental photocatalysis. Energy Environ. Sci. 2009, 2, 872–877. [Google Scholar] [CrossRef]
- Li, J.P.; Sun, Q.F.; Yao, Q.F.; Wang, J.; Han, S.J.; Jin, C. Reversibly light-switchable wettability between super hydrophobicity and superhydrophilicity of hybrid ZnO/bamboo surfaces via alternation of UV irradiation and dark storage. Prog. Org. Coat. 2015, 87, 155–160. [Google Scholar] [CrossRef]
- Tam, K.H.; Djurišić, A.B.; Chan, C.M.N.; Xi, Y.Y.; Tse, C.W.; Leung, Y.H.; Chan, W.K.; Leung, F.C.C.; Au, D.W.T. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 2008, 516, 6167–6174. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Zhang, L.L.; Wen, D.S.; Ding, Y.L. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.; Brabec, C. Design rules for donors in bulk-heterojunction solar cells-towards 10% energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Wei, X.Q.; Zhang, Z.; Yu, Y.X.; Man, B.Y. Comparative study on structural and optical properties of ZnO thin films prepared by PLD using ZnO powder target and ceramic target. Opt. Laser Technol. 2009, 41, 530–534. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kimc, N.J.; Loss, R., Jr.; Surampalli, R.Y.; Hu, Z.Q. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Umer, R.; Samad, Y.A.; Zheng, L.X.; Liao, K. The effect of the ultrasonication pre-treatment of graphene oxide (GO) on the mechanical properties of GO/polyvinyl alcohol composites. Carbon 2013, 55, 321–327. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Novoselov, K.S. Graphene: New bridge between condensed matter physics and quantum electrodynamics. Solid State Commun. 2007, 143, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer grapheme. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, H.; Liu, X.F.; Wang, S.X.; Zhang, R. Anti-bacterial performances and biocompatibility of bacterial cellulose/graphene oxide composites. RSC Adv. 2015, 5, 4795–4803. [Google Scholar] [CrossRef]
- Gkikas, M.; Theodosopoulos, G.V.; Das, B.P.; Tsianou, M.; Iatrou, H.; Sakellariou, G. Gold-decorated graphene nanosheets composed of a biocompatible non-charged water-soluble polypeptide. Eur. Polym. J. 2014, 60, 106–113. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Murugan, R.; Palanisamy, S.; Prabhu, N.M.; Mahalingam, T.; Hayakaw, Y.; Ravi, G. Role of nickel doping on structural, optical, magnetic properties and antibacterial activity of ZnO nanoparticles. Mater. Res. Bull. 2016, 76, 48–61. [Google Scholar] [CrossRef]
- Raula, M.; Rashid, M.H.; Paira, T.K.; Dinda, E.; Mandal, T.K. Ascorbate-assisted growth of hierarchical ZnO nanostructures: Sphere, Spindle, and flower and their catalytic properties. Langmuir 2010, 26, 8769–8782. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, S.K.; Zhang, L.M.; You, T.T.; Xu, F. Adsorption of heavy metals by graphene oxide/cellulose hydrogel prepared from NaOH/urea aqueous solution. Materials 2016, 9, 582. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, S.; Wang, H.; Wang, B.; Jiang, J. Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr. Polym. 2008, 74, 659–665. [Google Scholar] [CrossRef]
- Yang, S.; Chen, F.; Shen, Q.; Lavernia, E.J.; Zhang, L.M. Microstructure and electrical properties of AZO/graphene nanosheets fabricated by spark plasma sintering. Materials 2016, 9, 638. [Google Scholar] [CrossRef]
- Fu, L.; Lai, G.S.; Zhang, H.L.; Yu, A.M. One-pot synthesis of multipod ZnO-carbon nanotube-reduced graphene oxide composites with high performance in photocatalysis. J. Nanosci. Nanotechnol. 2015, 15, 4325–4331. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.H.; Li, F.; Pham, T.S.H.; Yu, A.M.; Han, F.G.; Chen, L. Preparation of ZnO flower/reduced graphene oxide composite with enhanced photocatalytic performance under sunlight. Ceram. Int. 2015, 41, 4007–4013. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Structural and physico-mechanical characterization of bio-cellulose produced by a cell-free system. Carbohydr. Polym. 2016, 136, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, X.; Shen, Y.; Yoshino, K.; Feng, W. A mechanically strong, flexible and conductive film based on bacterial cellulose/graphene nanocomposite. Carbohydr. Polym. 2012, 87, 644–649. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Wang, X. Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2010, 2, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Wang, C.Y.; Liu, C.Y.; Zhang, M.; Ma, H.; Li, J. Fabrication of superhydrophobic spherical-like α-FeOOH films on the wood surface by a hydrothermal method. Coll. Surf. A 2012, 403, 29–34. [Google Scholar] [CrossRef]
- Satheesh, K.; Jayavel, R. Synthesis and electrochemical properties of reduced graphene oxide via chemical reduction using thiourea as a reducing agent. Mater. Lett. 2013, 113, 5–8. [Google Scholar] [CrossRef]
- Yazhini, K.B.; Prabu, H.G. Study on flame-retardant and UV-protection properties of cotton fabric functionalized with ppy–ZnO–CNT nanocomposite. RSC Adv. 2015, 5, 49062–49069. [Google Scholar] [CrossRef]
- Hu, W.B.; Peng, C.; Luo, W.J.; Lv, M.; Li, X.M.; Li, D.; Huang, Q.; Fan, C.H. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Sun, D.P.; Li, J.; Yang, X.J.; Yu, J.W.; Hao, Q.L.; Liu, W.M.; Liu, J.G.; Zou, Z.G.; Gu, J. In situ deposition of platinum nanoparticles on bacterial cellulose membranes and evaluation of PEM fuel cell performance. Electrochim. Acta 2009, 54, 6300–6305. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, X.; Li, L.C.; Huang, L.L.; Zhang, W.; Ye, J.D.; Hong, J.G. Preparation of nano-ZnO/regenerated cellulose composite particles via co-gelation and low-temperature hydrothermal synthesis. Mater. Lett. 2016, 175, 122–125. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Microbiol, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.-F.; Fievet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Achaby, M.E.; Essamlali, Y.; Miri, N.E.; Snik, A.; Abdelouahdi, K.; Fihri, A.; Zahouily, M.; Solhy, A. Graphene oxide reinforced chitosan/ polyvinylpyrrolidone polymer bio-nanocomposites. J. Appl. Polym. Sci. 2014, 131, 41042–41053. [Google Scholar] [CrossRef]
- Chen, J.N.; Peng, H.; Wang, X.P.; Shao, F.; Yuan, Z.D.; Han, H.Y. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Cao, A.N.; Jiang, Y.; Zhang, X.; Liu, J.H.; Liu, Y.F.; Wang, H.F. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, B.; Chen, X.; Mi, B.; Wei, P.; Fei, B.; Mu, X. Antimicrobial Bamboo Materials Functionalized with ZnO and Graphene Oxide Nanocomposites. Materials 2017, 10, 239. https://doi.org/10.3390/ma10030239
Zhang J, Zhang B, Chen X, Mi B, Wei P, Fei B, Mu X. Antimicrobial Bamboo Materials Functionalized with ZnO and Graphene Oxide Nanocomposites. Materials. 2017; 10(3):239. https://doi.org/10.3390/ma10030239
Chicago/Turabian StyleZhang, Junyi, Bo Zhang, Xiufang Chen, Bingbing Mi, Penglian Wei, Benhua Fei, and Xindong Mu. 2017. "Antimicrobial Bamboo Materials Functionalized with ZnO and Graphene Oxide Nanocomposites" Materials 10, no. 3: 239. https://doi.org/10.3390/ma10030239