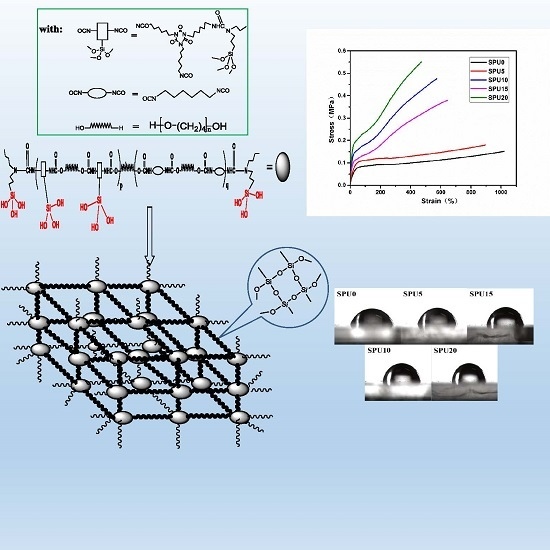
Preparation and Characterization of Polyurethanes with Cross-Linked Siloxane in the Side Chain by Sol-Gel Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
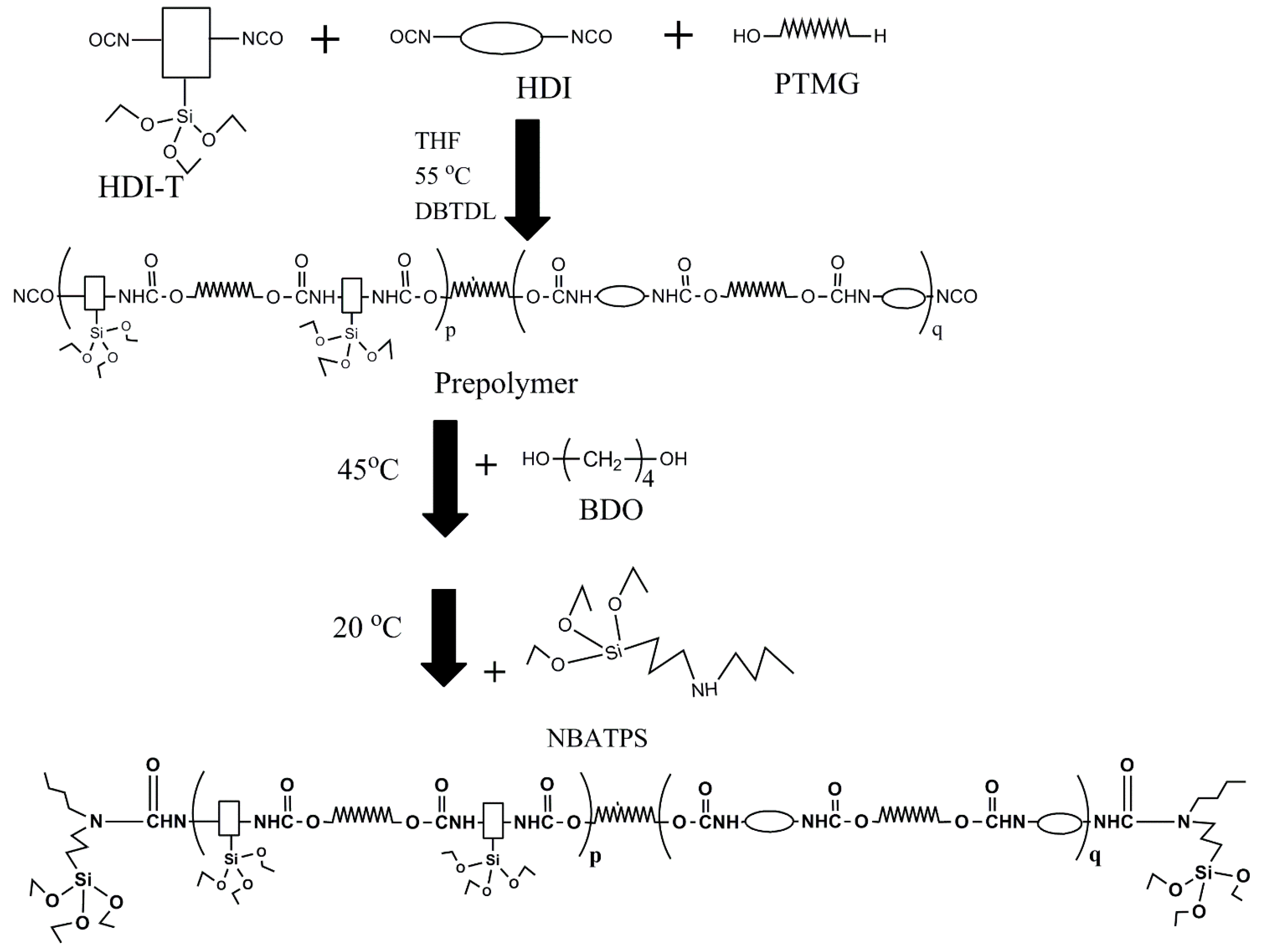
2.2.1. Preparation of HDI-3 Grafted NBATPS
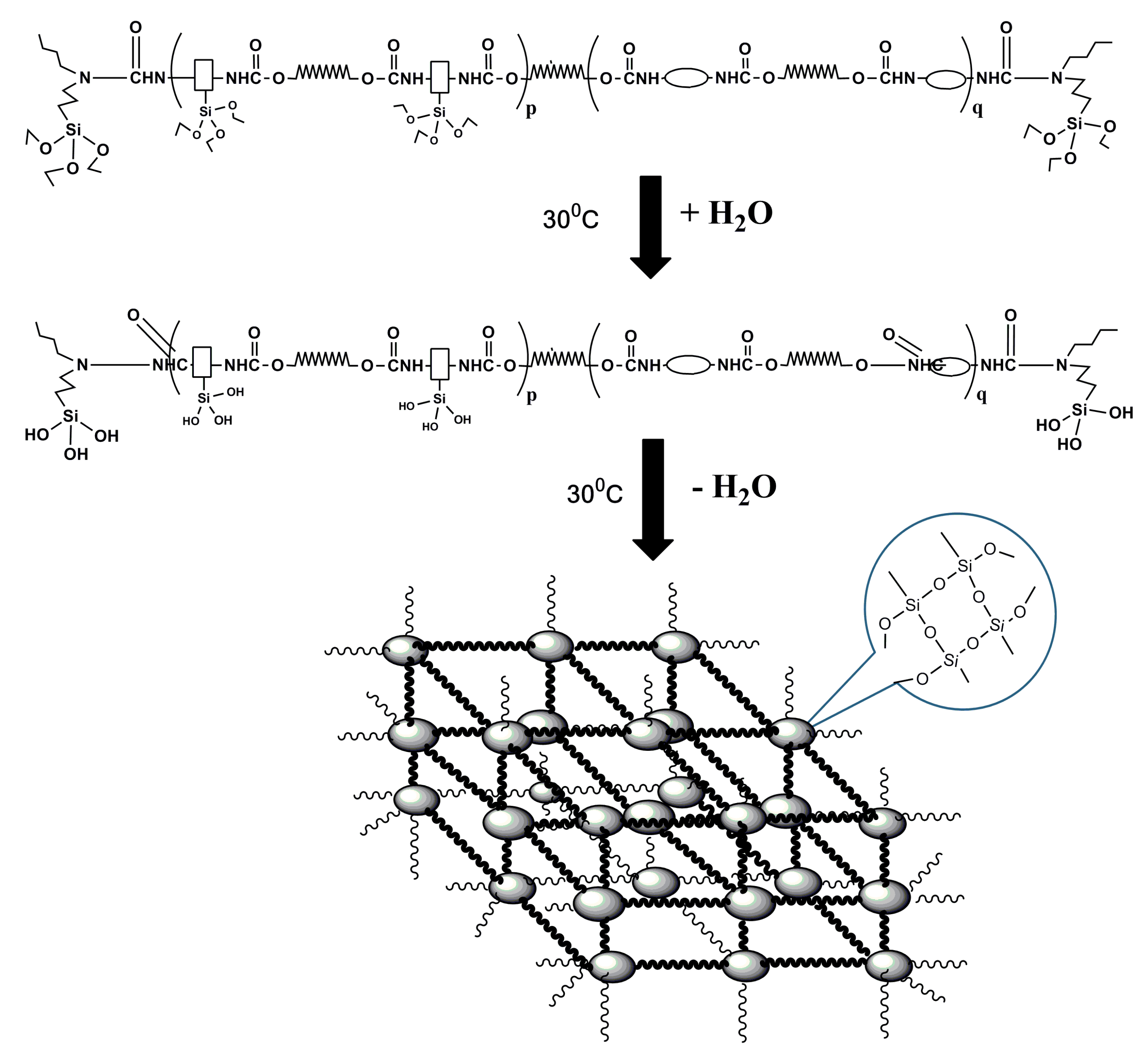
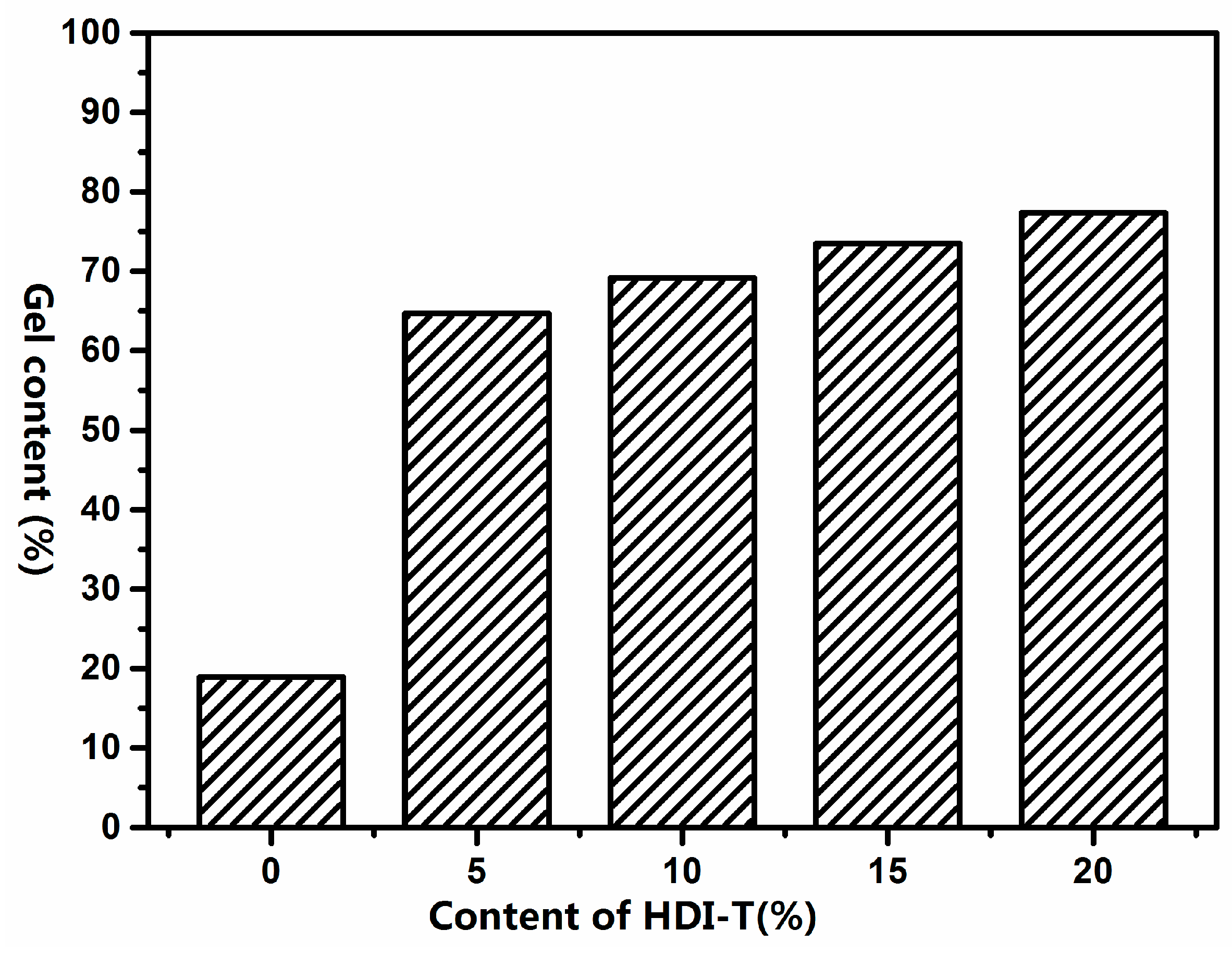
2.2.2. Synthesis of Silicone/Polyurethane (SPU) Hybrids
2.2.3. Preparation of Silicone/Polyurethane (SPU) Films
2.3. Characterization
3. Results and Discussion
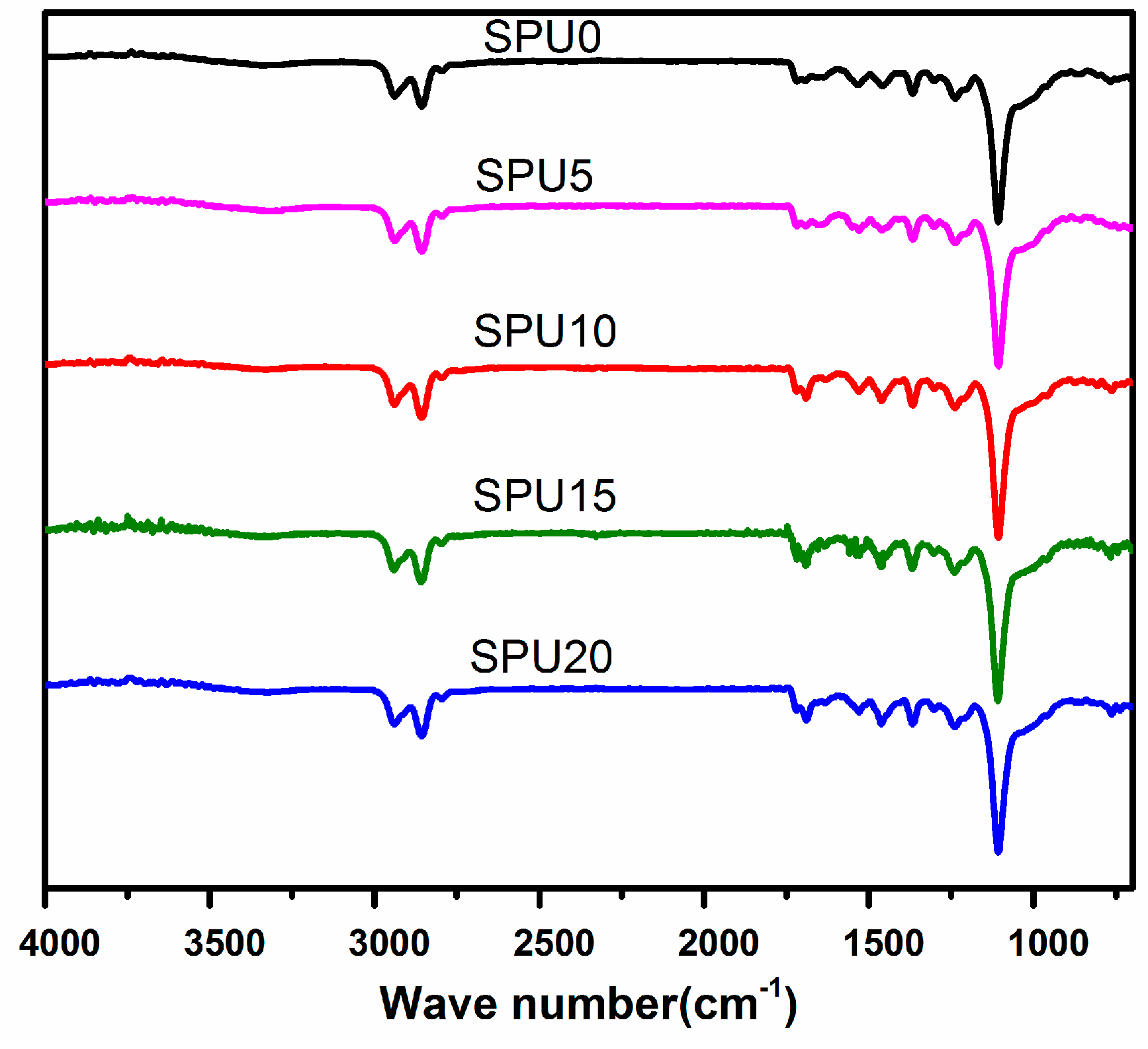
3.1. Infrared Spectroscopy
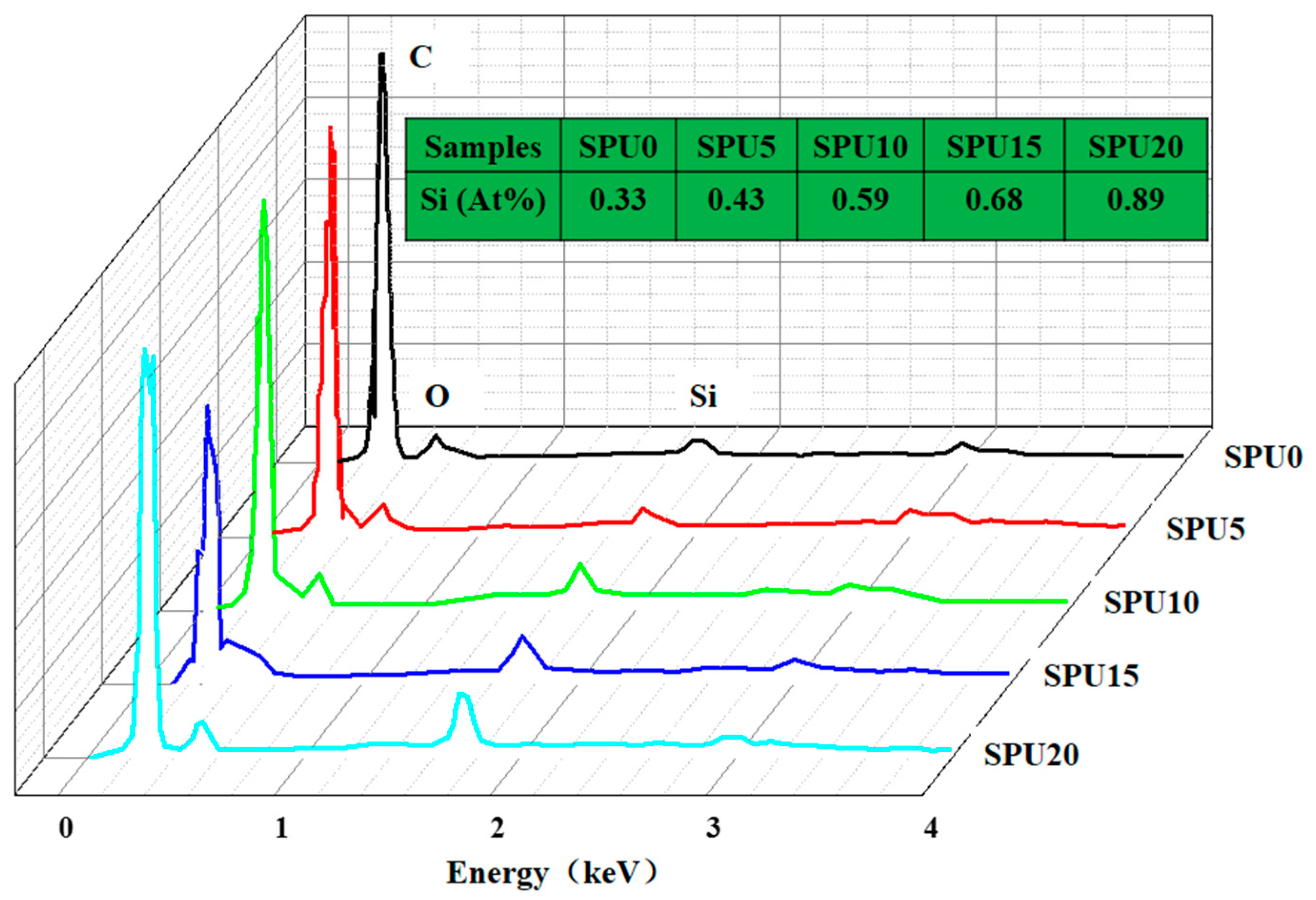
3.2. XPS Analysis of SPU Films
3.3. DSC Analysis of SPU Films
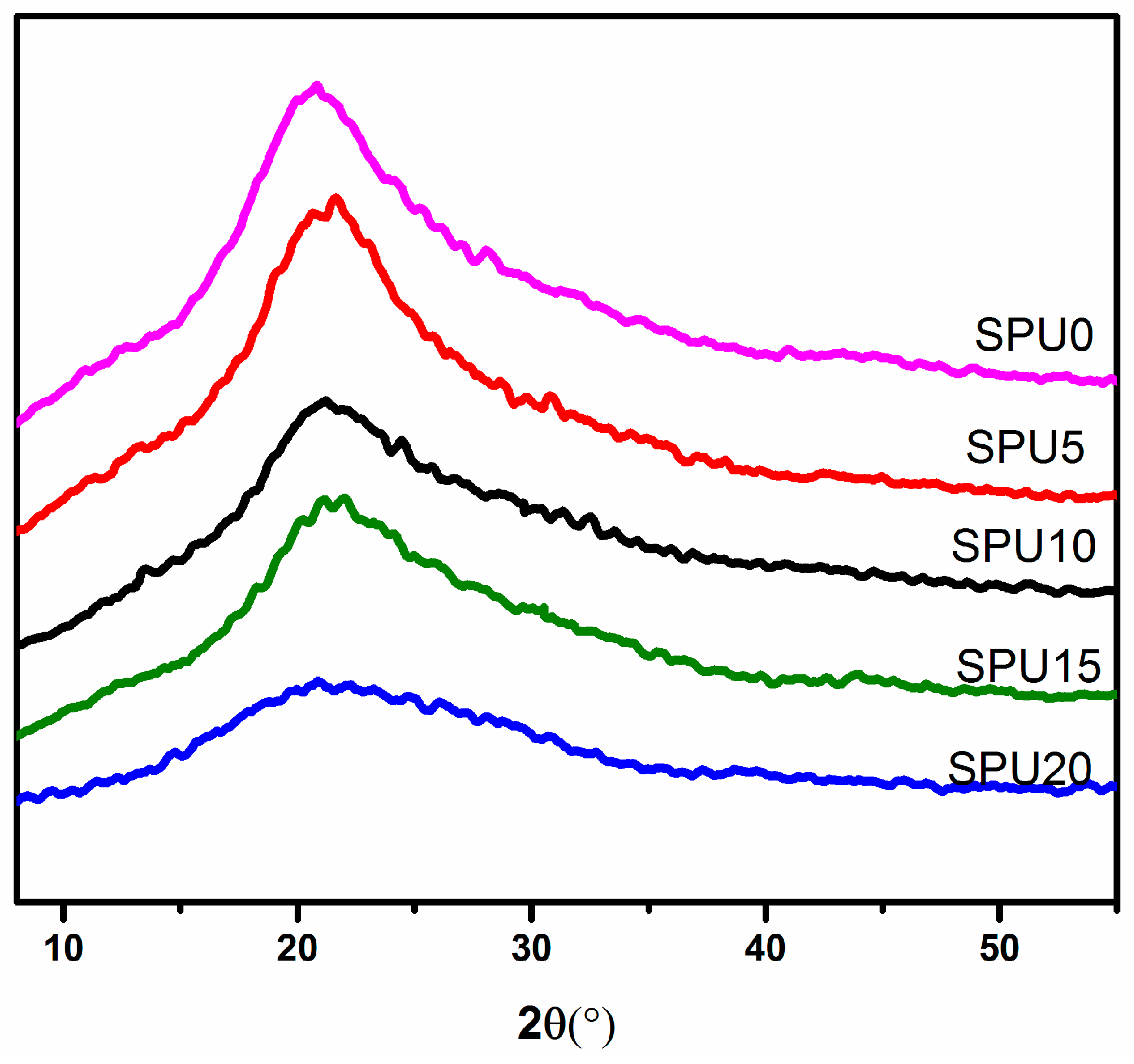
3.4. X-ray Diffraction Analysis
3.5. SEM Analysis of Surface Morphologies
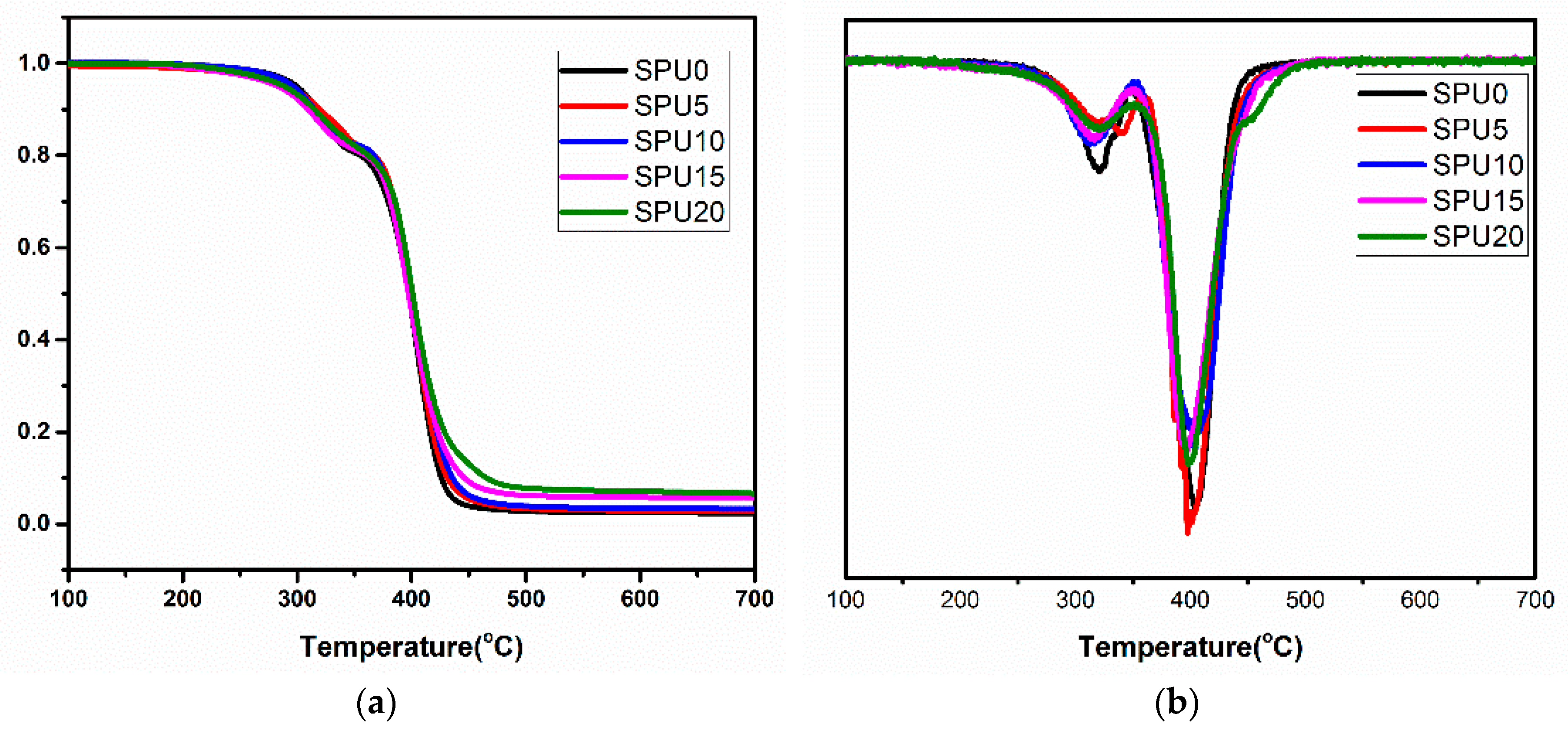
3.6. Thermal Properties of SPU Films
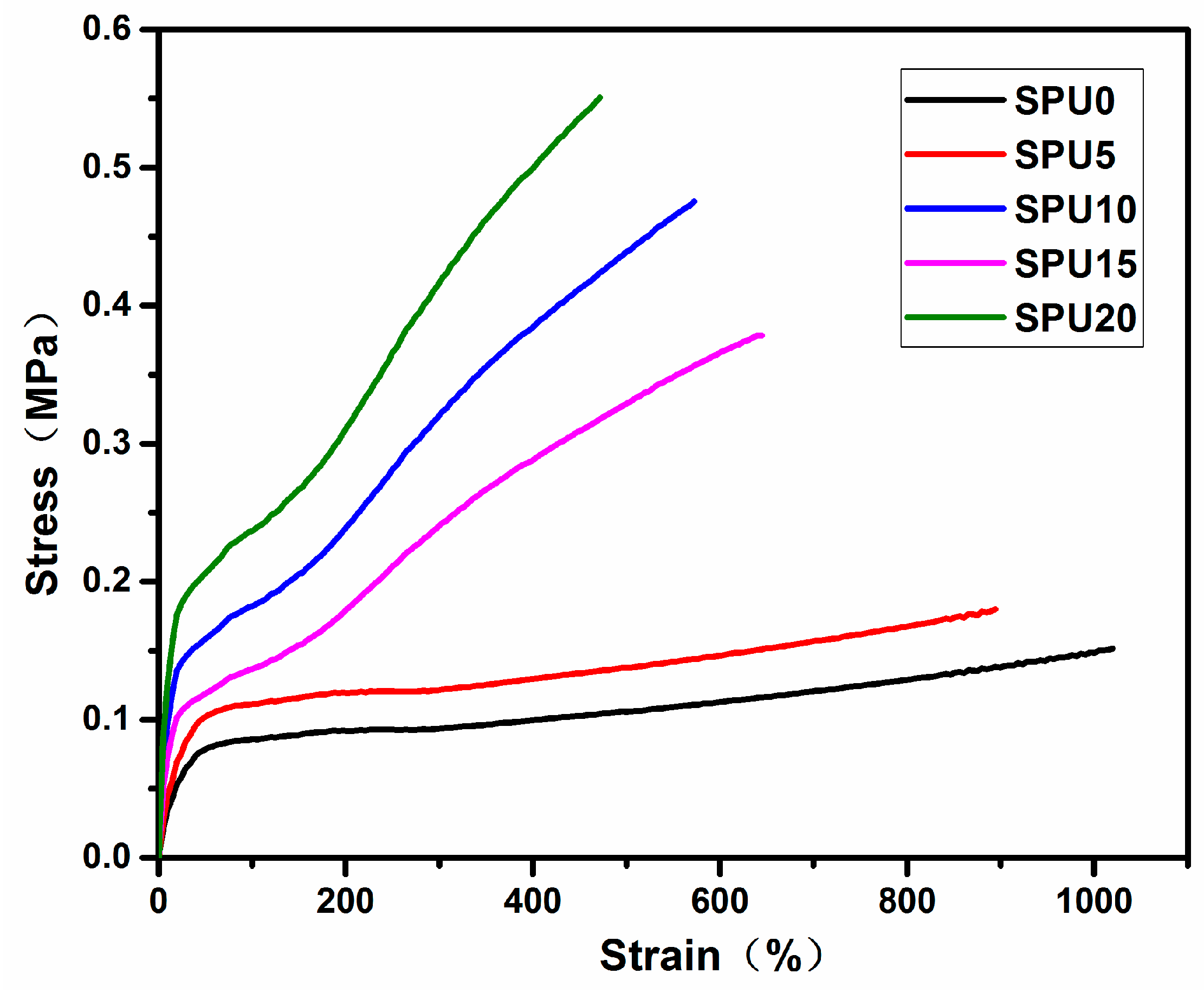
3.7. Mechanical Properties of SPU Films
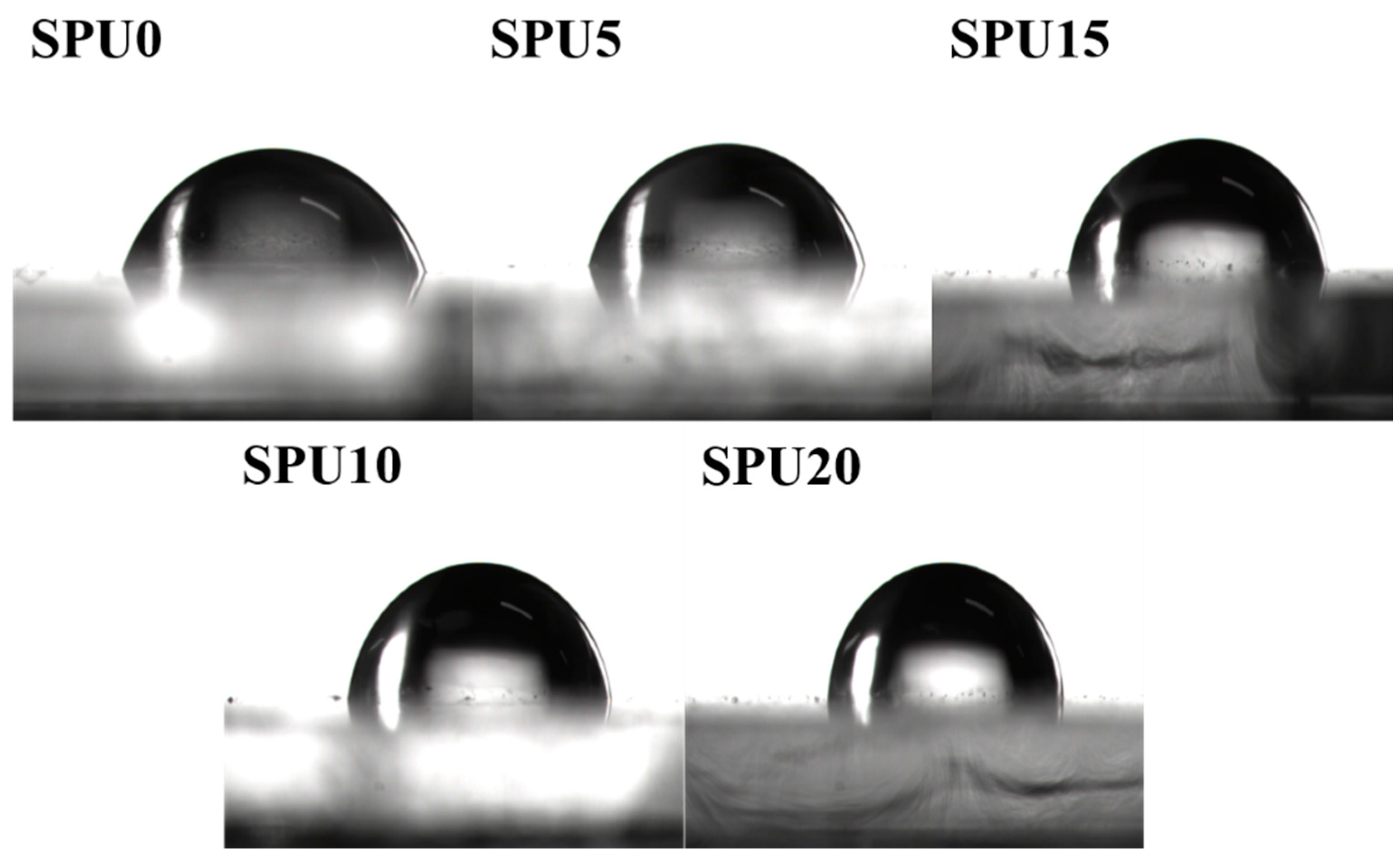
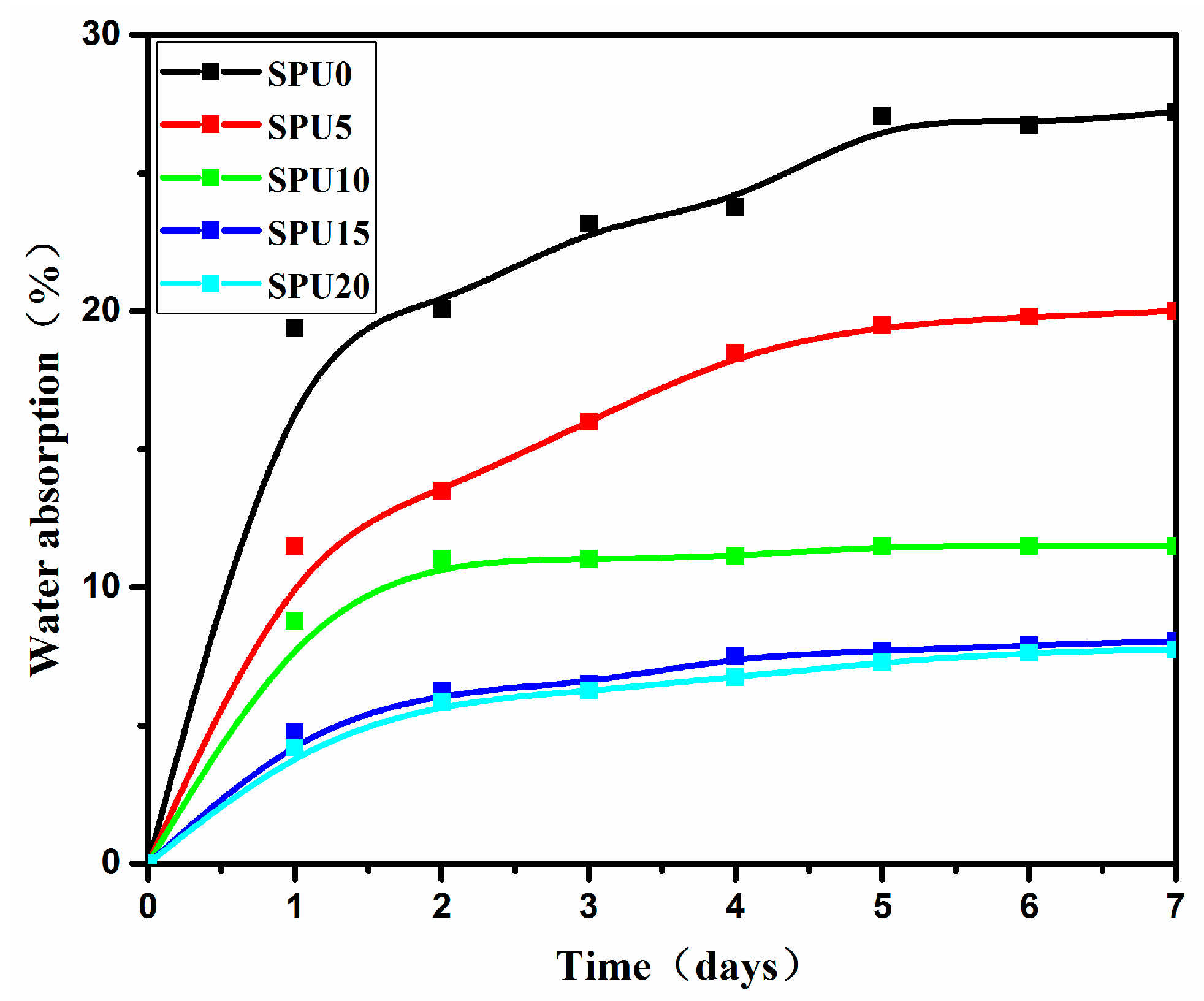
3.8. Surface Property and Water Absorption of SPU Films
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Zhang, Q.; Hu, J.; Gong, S. Preparation and characterization of aqueous polyurethane dispersions with well-defined soft segments. J. Appl. Polym. Sci. 2011, 122, 3064–3070. [Google Scholar] [CrossRef]
- Jóźwiak, A.B.; Kielty, C.M.; Black, R.A. Surface functionalization of polyurethane for the immobilization of bioactive moieties on tissue scaffolds. J. Mater. Chem. 2008, 18, 2240–2248. [Google Scholar] [CrossRef]
- Osman, M.A.; Mittal, V.; Morbidelli, M.; Suter, U.W. Polyurethane adhesive nanocomposites as gas permeation barrier. Macromolecules 2003, 36, 9851–9858. [Google Scholar] [CrossRef]
- Ihata, O.; Kayaki, Y.; Ikariya, T. Synthesis of Thermoresponsive Polyurethane from 2-Methylaziridine and Supercritical Carbon Dioxide. Angew. Chem. Int. Ed. 2004, 43, 717–719. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Zhang, X.X.; Wang, J.P.; Wang, X.C. Polyurethane foam containing microencapsulated phase-change materials with styrene–divinybenzene co-polymer shells. J. Mater. Sci. 2009, 44, 3141–3147. [Google Scholar] [CrossRef]
- Li, Y.-J.; Tomita, T.; Tanda, K.; Nakaya, T. Synthesis and hemocompatibility evaluation of novel segmented polyurethanes with phosphatidylcholine polar headgroups. Chem. Mater. 1998, 10, 1596–1603. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Ferguson, J. Polyurethane elastomers. Prog. Polym. Sci. 1991, 16, 695–836. [Google Scholar] [CrossRef]
- Vlad, S.; Spiridon, I.; Grigoras, C.V.; Drobota, M.; Nistor, A. Thermal, mechanical and wettability properties of some branched polyetherurethane elastomers. e-Polymers 2013, 9, 1618–7229. [Google Scholar] [CrossRef]
- Vlad, S.; Oprea, S. Effect of polyols on the physico-mechanical properties of some polyurethanes. J. Optoelectron. Adv. Mater. 2007, 9, 994–999. [Google Scholar]
- Buma, P.; Ramrattan, N.V.; van Tienen, T.G.; Veth, R.P. Tissue engineering of the meniscus. Biomaterials 2004, 25, 1523–1532. [Google Scholar] [CrossRef]
- Yeganeh, H.; Moeini, H.R. Novel Polyurethane Electrical Insulator Coatings Based on Amide-Ester-Ether Polyols Derived from Castor Oil and Re-cycled Poly(ethylene terphthalate). High Perform. Polym. 2007, 19, 113–126. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Dai, J.; Bai, C. Synthesis and properties of PDMS modified waterborne polyurethane–acrylic hybrid emulsion by solvent-free method. Prog. Organ. Coat. 2008, 63, 238–244. [Google Scholar] [CrossRef]
- Malay, O.; Oguz, O.; Kosak, C.; Yilgor, E.; Yilgor, I.; Menceloglu, Y.Z. Polyurethaneurea–silica nanocomposites: Preparation and investigation of the structure–property behavior. Polymer 2013, 54, 5310–5320. [Google Scholar] [CrossRef]
- Fan, Q.; Fang, J.; Chen, Q.; Yu, X. Synthesis and properties of polyurethane modified with aminoethylaminopropyl poly (dimethyl siloxane). J. Appl. Polym. Sci. 1999, 74, 2552–2558. [Google Scholar] [CrossRef]
- Okkema, A.; Fabrizius, D.; Grasel, T.; Cooper, S.; Zdrahala, R. Bulk, surface and blood-contacting properties of polyether polyurethanes modified with polydimetnylsiloxane macroglycols. Biomaterials 1989, 10, 23–32. [Google Scholar] [CrossRef]
- Jeon, H.T.; Jang, M.K.; Kim, B.K.; Kim, K.H. Synthesis and characterizations of waterborne polyurethane–silica hybrids using sol–gel process. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 559–567. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, X.; Dai, J.; Wang, J. Synthesis of UV crosslinkable waterborne siloxane–polyurethane dispersion PDMS-PEDA-PU and the properties of the films. J. Coat. Technol. Res. 2008, 5, 251–257. [Google Scholar] [CrossRef]
- Subramani, S.; Lee, J.Y.; Choi, S.W.; Kim, J.H. Waterborne trifunctionalsilane-terminated polyurethane nanocomposite with silane-modified clay. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2747–2761. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A. The influence of the ionic concentration, concentration of the polymer, degree of neutralization and chain extension on aqueous polyurethane dispersions prepared by the acetone process. Polymer 2006, 47, 1805–1811. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B.; Jeong, H. Aqueous dispersion of polyurethane ionomers from hexamethylene diisocyanate and trimellitic anhydride. Colloid Polym. Sci. 1991, 269, 895–900. [Google Scholar] [CrossRef]
- Xu, J.; Shi, W.; Pang, W. Synthesis and shape memory effects of Si–O–Si cross-linked hybrid polyurethanes. Polymer 2006, 47, 457–465. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Yao, C.-T.; Hsieh, C.-F.; Yang, H.-C.; Wu, C.-P. Preparation and properties of amino-terminated anionic waterborne-polyurethane–silica hybrid materials through a sol–gel process in the absence of an external catalyst. Eur. Polym. J. 2008, 44, 2777–2783. [Google Scholar] [CrossRef]
- Subramani, S.; Lee, J.; Cheong, I.; Kim, J. Synthesis and characterization of water-borne crosslinked silylated polyurethane dispersions. J. Appl. Polym. Sci. 2005, 98, 620–631. [Google Scholar] [CrossRef]
- Fei, G.; Shen, Y.; Wang, H.; Shen, Y. Effects of polydimethylsiloxane concentration on properties of polyurethane/polydimethylsiloxane hybrid dispersions. J. Appl. Polym. Sci. 2006, 102, 5538–5544. [Google Scholar] [CrossRef]
- Jaisankar, S.N.; Ramalingam, S.; Subramani, H.; Mohan, R.; Saravanan, P.; Samanta, D.; Mandal, A.B. Cloisite-g-Methacrylic acid copolymer nanocomposites by graft from method for leather processing. Indust. Eng. Chem. Res. 2013, 52, 1379–1387. [Google Scholar] [CrossRef]
- Hu, Y.; Samanta, D.; Parelkar, S.S.; Hong, S.W.; Wang, Q.; Russell, T.P.; Emrick, T. Ferritin–polymer conjugates: Grafting chemistry and integration into nanoscale assemblies. Adv. Funct. Mater. 2010, 20, 3603–3612. [Google Scholar] [CrossRef]
- Ghosh, S.; Maity, S.; Jana, T. Polybenzimidazole/silica nanocomposites: Organic-inorganic hybrid membranes for PEM fuel cell. J. Mater. Chem. 2011, 21, 14897–14906. [Google Scholar] [CrossRef]
- Meera, K.M.S.; Sankar, R.M.; Murali, A.; Jaisankar, S.N.; Mandal, A.B. Sol–gel network silica/modified montmorillonite clay hybrid nanocomposites for hydrophobic surface coatings. Colloids Surf. B Biointerfaces 2012, 90, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Dahmouche, K.; Santilli, C.V.; Pulcinelli, S.H.; Craievich, A.F. Small-angle X-ray scattering study of sol-gel-derived siloxane-PEG and siloxane-PPG hybrid materials. J. Phys. Chem. B 1999, 103, 4937–4942. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Lambert, J.B.; Gurusamy-Thangavelu, S.A.; Ma, K. The silicate-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 2010, 327, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yang, D.; Xiao, M.; Han, J.; Nie, J. Preparation of silica/polyurethane nanocomposites by UV-induced polymerization from surfaces of silica. J. Appl. Polym. Sci. 2009, 111, 1936–1941. [Google Scholar] [CrossRef]
- Sen, P.; Mukherjee, S.; Patra, A.; Bhattacharyya, K. Solvation dynamics of DCM in a DPPC vesicle entrapped in a sodium silicate derived sol-gel matrix. J. Phys. Chem. B 2005, 109, 3319–3323. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Wicks, D.A.; Madbouly, S.A.; Otaigbe, J.U. Nanostructured polyurethane/POSS hybrid aqueous dispersions prepared by homogeneous solution polymerization. Macromolecules 2006, 39, 7037–7043. [Google Scholar] [CrossRef]
- Hao, T.; Liu, X.; Hu, G.H.; Jiang, T.; Zhang, Q. Preparation and characterization of polyurethane/POSS hybrid aqueous dispersions from mono-amino substituted POSS. Polym. Bull. 2016, 1–13. [Google Scholar] [CrossRef]
- Meera, K.M.S.; Sankar, R.M.; Jaisankar, S.N.; Mandal, A.B. Physicochemical Studies on Polyurethane/Siloxane Cross-Linked Films for Hydrophobic Surfaces by the Sol–Gel Process. J. Phys. Chem. B 2013, 117, 2682–2694. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, Y.; Ou, C.; Xia, Z.; Zhong, L. Synthesis and characterization of waterborne polyurethanes with alkoxy silane groups in the side chains for potential application in waterborne ink. Prog. Organ. Coat. 2016, 92, 85–94. [Google Scholar] [CrossRef]
- Pergal, M.V.; Džunuzović, J.V.; Poręba, R.; Ostojić, S.; Radulović, A.; Špírková, M. Microstructure and properties of poly(urethane-siloxane)s based on hyperbranched polyester of the fourth pseudo generation. Prog. Organ. Coat. 2013, 76, 743–756. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Tian, X.; Zheng, K.; Cheng, Q. Effect of 3-Aminopropyltriethoxysilane on polycarbonate based waterborne polyurethane transparent coatings. Prog. Organ. Coat. 2014, 77, 1073–1078. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Li, X.; Chen, W. Synthesis of vegetable oil-based waterborne polyurethane/silver-halloysite antibacterial nanocomposites. Compos. Sci. Technol. 2016, 126, 86–93. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Yang, L.; Zlatanić, A.; Zhang, W.; Javni, I. Network structure and properties of polyurethanes from soybean oil. J. Appl. Polym. Sci. 2007, 105, 2717–2727. [Google Scholar] [CrossRef]
- Allauddin, S.; Narayan, R.; Raju, K.V.S.N. Synthesis and Properties of Alkoxysilane Castor Oil and Their Polyurethane/Urea–Silica Hybrid Coating Films. ACS Sustain. Chem. Eng. 2013, 1, 910–918. [Google Scholar] [CrossRef]
- Khan, F.; Kwek, D.; Kronfli, E.; Ahmad, S. Crosslinking of ethylene–propylene (–diene) terpolymer elastomer initiated by an excimer laser. Polym. Degrad. Stab. 2007, 92, 1640–1644. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Y.L.; Hu, C.P. Polyurethaneurea aqueous dispersions prepared with diethyltoluenediamine as chain extender. J. Coat. Technol. Res. 2007, 4, 59–66. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Pan, Y.-W.; Chuang, C.-N.; Guo, J.-J.; Chen, S.-H.; Wang, C.-K.; Hsieh, K.-H. Fabrication and characterization of waterborne polyurethane (WPU) with aluminum trihydroxide (ATH) and mica as flame retardants. J. Polym. Res. 2015, 22, 1–9. [Google Scholar] [CrossRef]
- Niu, Z.; Bian, F. Synthesis and characterization of multiple cross-linking UV-curable waterborne polyurethane dispersions. Iran. Polym. J. 2012, 21, 221–228. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Song, W.; Li, Z.; Wang, X. Alkoxysilane functionalized polyurethane/polysiloxane copolymers: Synthesis and the effect of end-capping agent. Polym. Bull. 2007, 59, 53–63. [Google Scholar] [CrossRef]




| Samples | HDI (mol) | HDI-T (mol) | PTMG (mol) | BDO (mol) | NBAPTS (mol) |
|---|---|---|---|---|---|
| SPU0 | 0.0480 | 0 | 0.02 | 0.02 | 0.016 |
| SPU5 | 0.0456 | 0.0024 | 0.02 | 0.02 | 0.016 |
| SPU10 | 0.0432 | 0.0048 | 0.02 | 0.02 | 0.016 |
| SPU15 | 0.0408 | 0.0072 | 0.02 | 0.02 | 0.016 |
| SPU20 | 0.0384 | 0.0096 | 0.02 | 0.02 | 0.016 |
| Sample | SPU0 | SPU5 | SPU10 | SPU15 | SPU20 |
|---|---|---|---|---|---|
| Crystallization temperature (°C) | −22.4 | −23.9 | −25.4 | −31.1 | −31.5 |
| Melting temperature (°C) | 29.1 | 26.3 | 25.7 | 23.8 | 19.8 |
| Sample | Si Concentration/% | |
|---|---|---|
| Theoretical | Experimental | |
| SPU0 | 0.40 | 0.33 |
| SPU5 | 0.45 | 0.43 |
| SPU10 | 0.50 | 0.59 |
| SPU15 | 0.55 | 0.68 |
| SPU20 | 0.60 | 0.89 |
| HDI-T Content (%) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Pencil Hardness |
|---|---|---|---|---|
| 0 | 0.41 | 0.15 | 1019.7 | HB |
| 5 | 0.53 | 0.18 | 894.6 | H |
| 10 | 1.02 | 0.38 | 644.5 | 2H |
| 15 | 1.36 | 0.48 | 572.3 | 2H |
| 20 | 1.77 | 0.55 | 471.4 | 2H |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Hao, T.-H.; Hu, G.-H.; Shi, D.; Huang, D.; Jiang, T.; Zhang, Q.-C. Preparation and Characterization of Polyurethanes with Cross-Linked Siloxane in the Side Chain by Sol-Gel Reactions. Materials 2017, 10, 247. https://doi.org/10.3390/ma10030247
Zhao H, Hao T-H, Hu G-H, Shi D, Huang D, Jiang T, Zhang Q-C. Preparation and Characterization of Polyurethanes with Cross-Linked Siloxane in the Side Chain by Sol-Gel Reactions. Materials. 2017; 10(3):247. https://doi.org/10.3390/ma10030247
Chicago/Turabian StyleZhao, Hui, Tong-Hui Hao, Guo-Hua Hu, Dean Shi, Da Huang, Tao Jiang, and Qun-Chao Zhang. 2017. "Preparation and Characterization of Polyurethanes with Cross-Linked Siloxane in the Side Chain by Sol-Gel Reactions" Materials 10, no. 3: 247. https://doi.org/10.3390/ma10030247