Wettability and Contact Time on a Biomimetic Superhydrophobic Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biological Sample
2.2. Fabrication of Superhydrophobic Surfaces
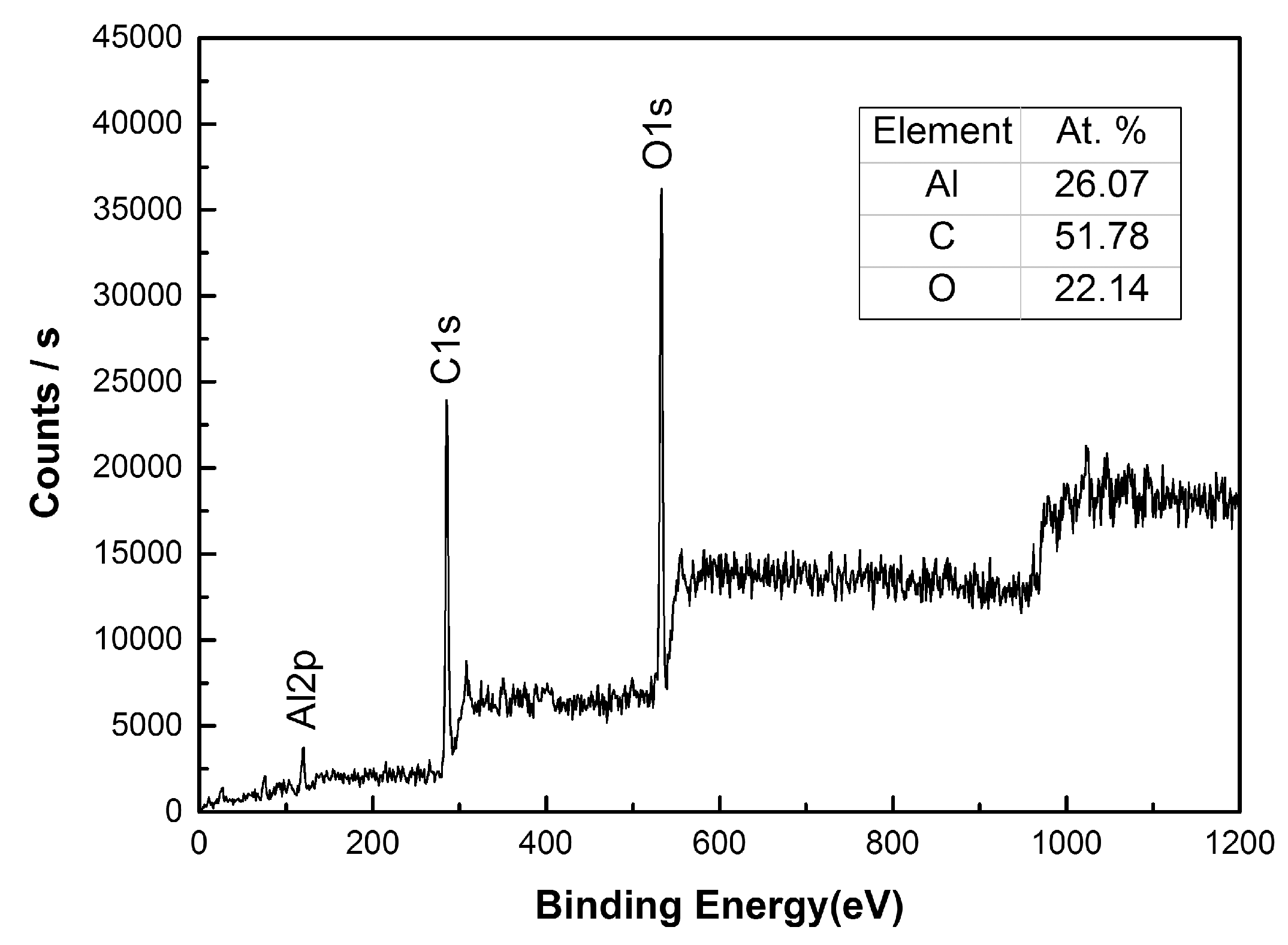
2.3. Sample Characterization
3. Results and Discussion
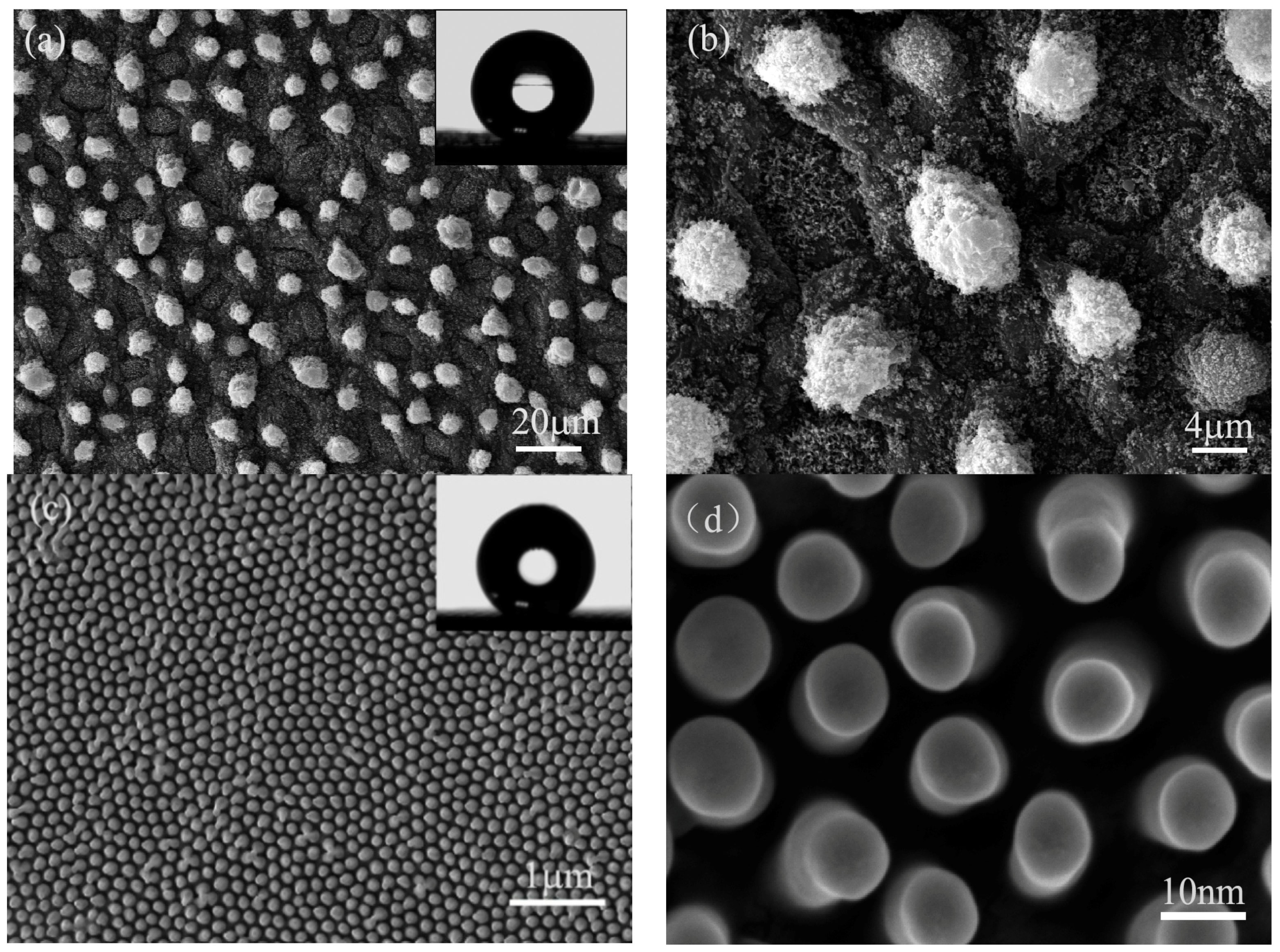
3.1. Wettability and Microstructure of Lotus Leaf and Cicada Wing
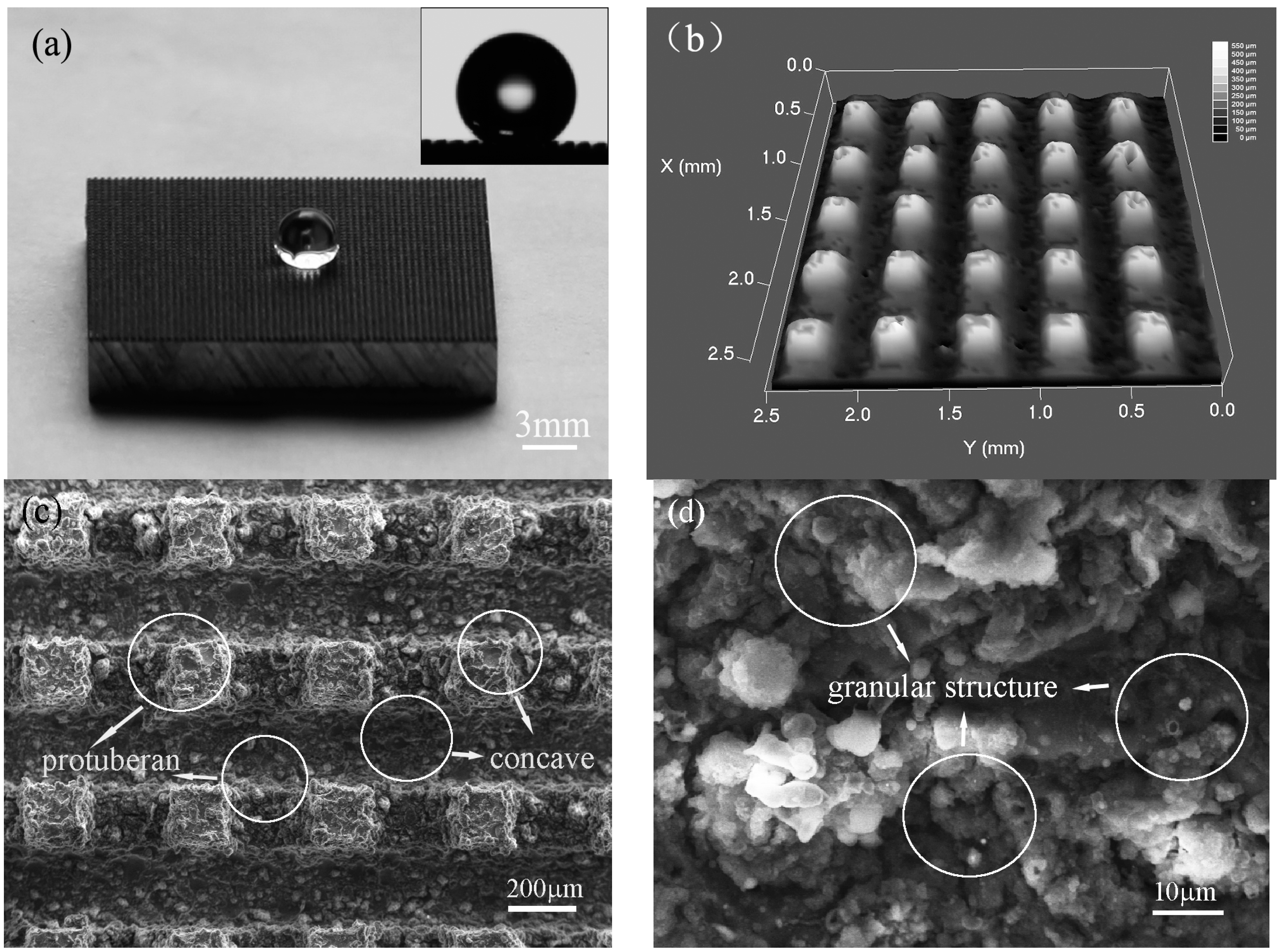
3.2. Microstructure and Wettability of Artificial Superhydrophobic Surface
3.3. Superhydrophobic Mechanism of Artificial Surface
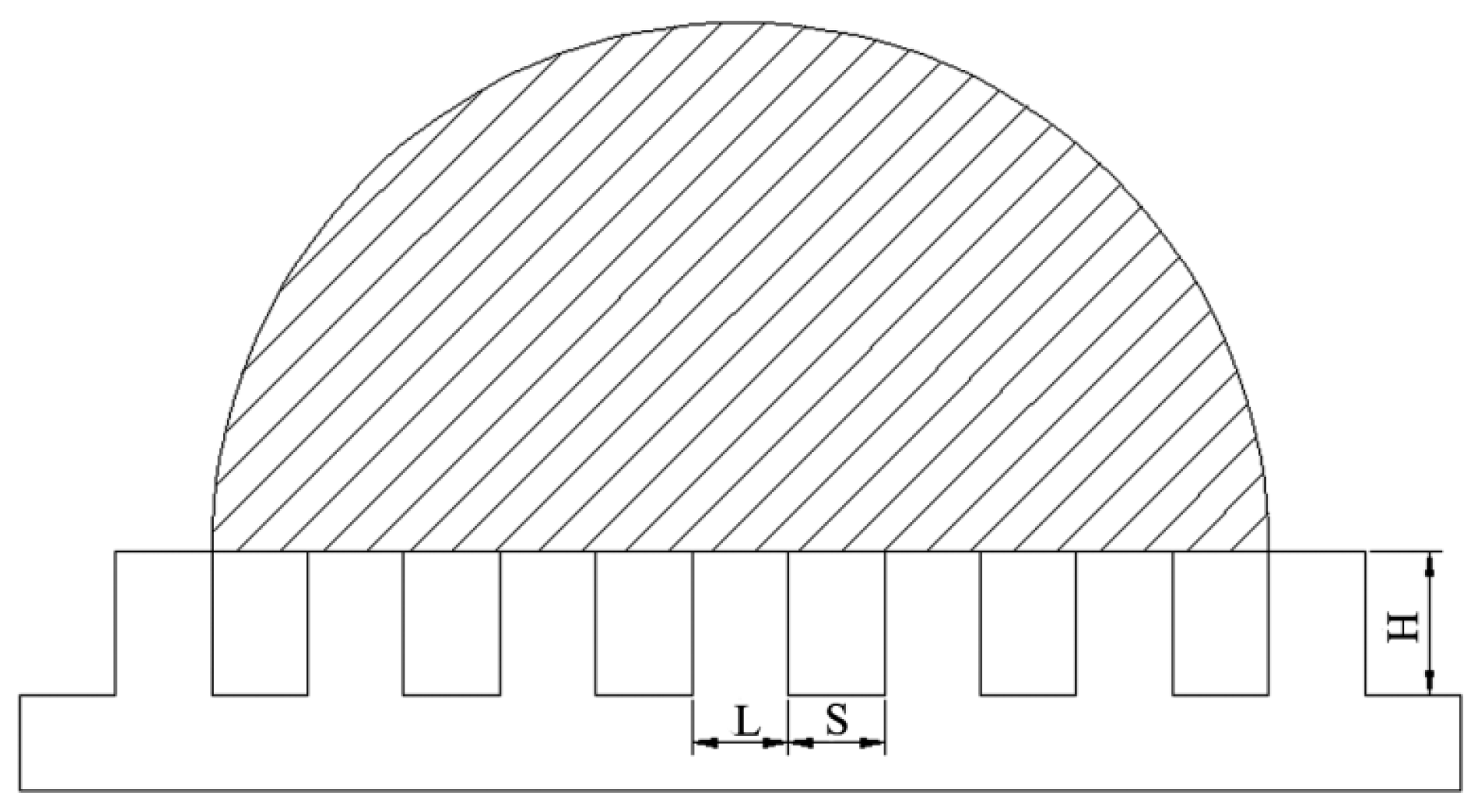
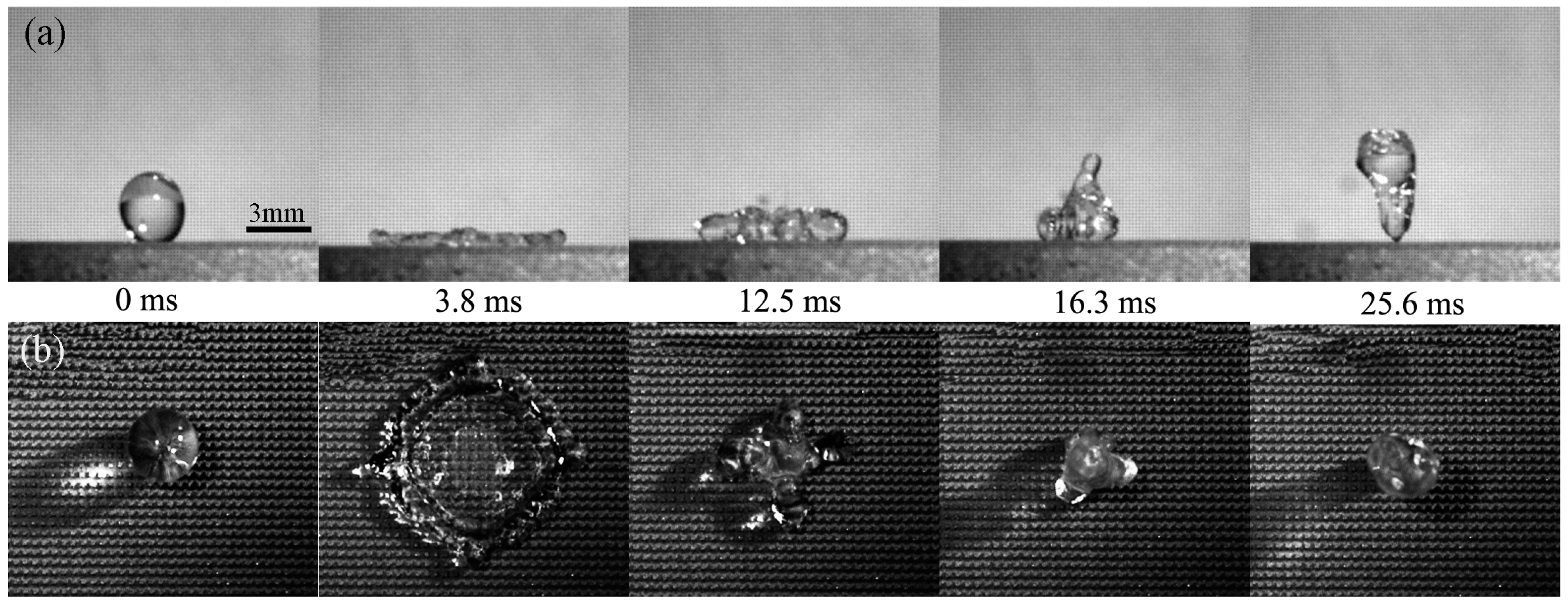
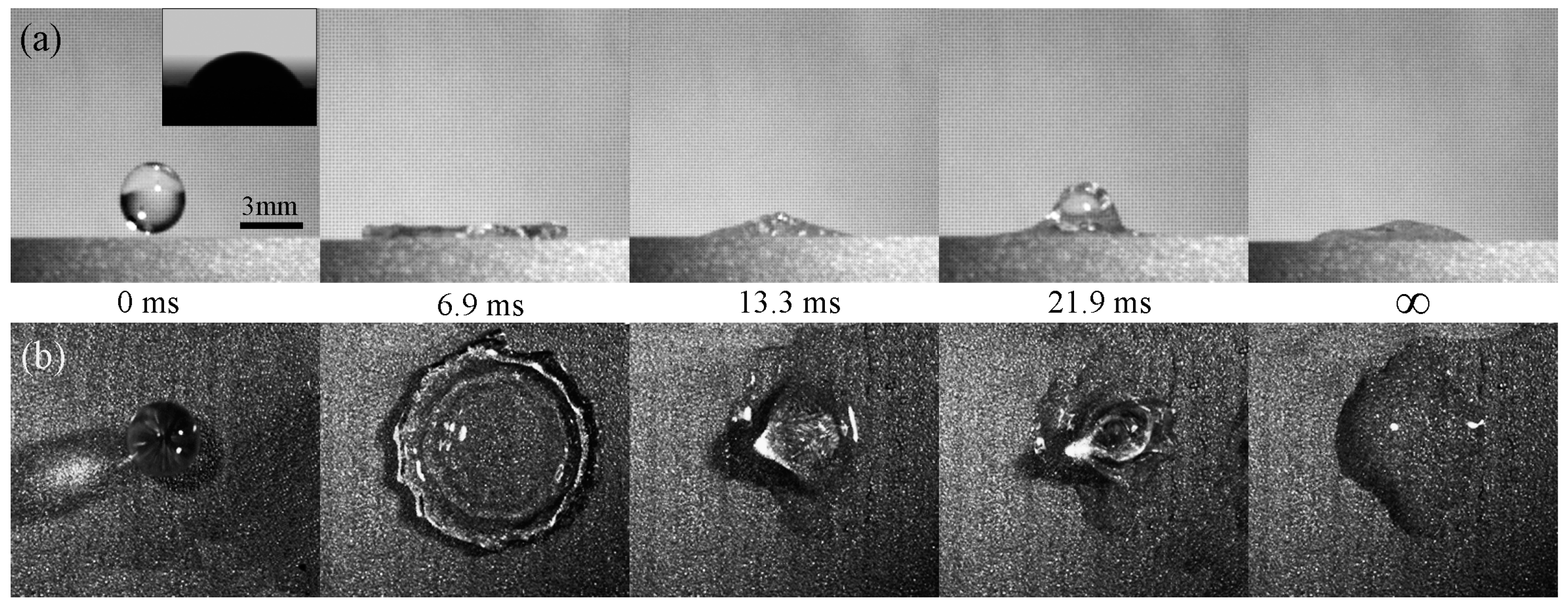
3.4. Contact Time on the Artificial Surface and How to Reduce the Contact Time
4. Conclusions
- Lotus leaf and cicada wing have unique superhydrophobic properties. The apparent contact angle of lotus leaf and cicada wing is about 157° ± 1° and 151° ± 1°, respectively. The surfaces of the lotus leaf and cicada wing both possess an array microstructure, which is a very important factor for a natural superhydrophobic surface.
- Inspired by the array microstructure on the surfaces of the lotus leaf and cicada wing, the array microstructure had been successfully constructed by HS-WEDM on the surfaces of the 7075 aluminum alloy without any chemical treatment. The apparent contact angle of the artificial surface is about 153° ± 1°, with a contact angle hysteresis less than 5°, showing a good superhydrophobic property. During the machining process, surfaces of the aluminum alloy not only possess the array structure followed by micro-/nano-scale microstructure, but also generate new metal oxides and carbides. The fabrication of the superhydrophobic surface is simple, low-cost, and environmentally friendly.
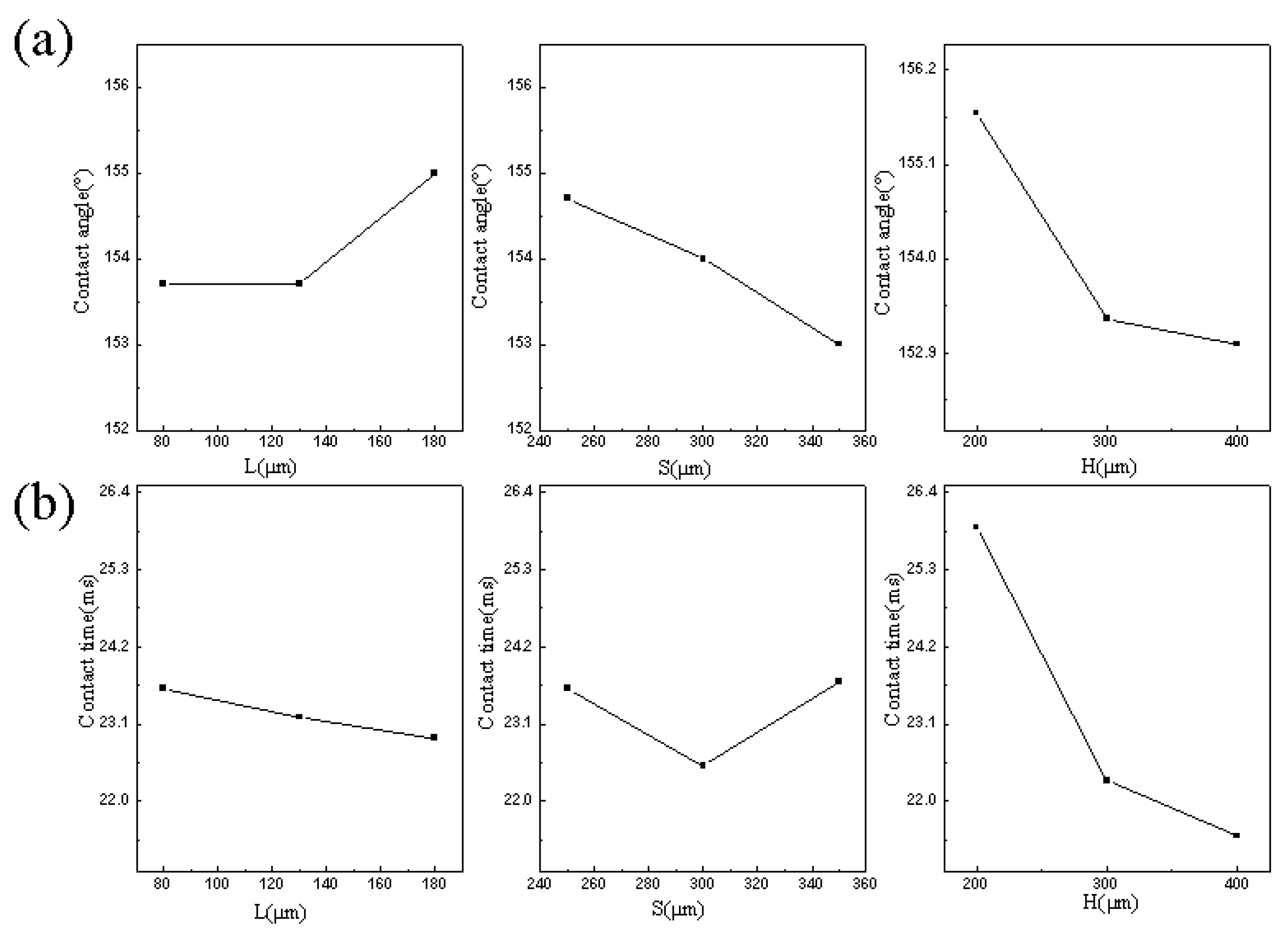
- Different array microstructures have different effects on the wettability and contact time of the artificial superhydrophobic surface. The length (L), interval (S), and height (H) of the array microstructure are the main influential factors on the wettability and contact time. Changing these factors can control the wettability and the contact time. The order of importance of these factors is H > S > L for increasing the apparent contact angle and reducing the contact time, which would have a potential application in the surface processing and preparation in anti-icing and self-cleaning.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chunglok, A.; Muensit, N.; Daengngam, C. Extreme Wetting-Resistant Multiscale Nano-/Microstructured Surfaces for Viscoelastic Liquid Repellence. J. Nanomater. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.Y. Wetting of Real Surfaces; De Gruyter: Berlin, Germany, 2013; Volume 44, pp. 406–409. [Google Scholar]
- Bae, W.G.; Song, K.Y.; Rahmawan, Y.; Chu, C.N.; Kim, D.; Chung do, K.; Suh, K.Y. One-step process for superhydrophobic metallic surfaces by wire electrical discharge machining. ACS Appl. Mater. Interfaces 2012, 4, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Gennes, P.G.D.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena; Springer: Berlin, Germany, 2004; p. 1700. [Google Scholar]
- Jiang, L.; Zhao, Y.; Zhai, J. A lotus-leaf-like superhydrophobic surface: A porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. 2004, 43, 4338–4341. [Google Scholar] [CrossRef] [PubMed]
- Barthwal, S.; Kim, Y.S.; Lim, S.H. Mechanically robust superamphiphobic aluminum surface with nanopore-embedded microtexture. Langmuir ACS J. Surf. Colloids 2013, 29, 11966–11974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Li, S.; Wang, Y.; Han, Z.; Ren, L. One-step method for fabrication of biomimetic superhydrophobic surface on aluminum alloy. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 125–131. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Feng, X.J.; Jiang, L. Design and Creation of Superwetting/Antiwetting Surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Han, X. Fabrication of CuO hierarchical flower-like structures with biomimetic superamphiphobic, self-cleaning and corrosion resistance properties. Chem. Eng. J. 2016, 283, 1443–1454. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Lu, Y.; Pan, S.; Wang, K.; Deng, Y.; Shi, Y. Design and Fabrication of the Lyophobic Slippery Surface and Its Application in Anti-Icing. J. Phys. Chem. C 2016, 120, 11054–11059. [Google Scholar] [CrossRef]
- Ramachandran, R.; Kozhukhova, M.; Sobolev, K.; Nosonovsky, M. Anti-Icing Superhydrophobic Surfaces: Controlling Entropic Molecular Interactions to Design Novel Icephobic Concrete. Entropy 2016, 18, 132. [Google Scholar] [CrossRef]
- Bayer, I.S.; Davis, A.J.; Loth, E.; Steele, A. Water jet resistant superhydrophobic carbonaceous films by flame synthesis and tribocharging. Mater. Today Commun. 2015, 3, 57–68. [Google Scholar] [CrossRef]
- Bucher, T.M.; Emami, B.; Tafreshi, H.V.; Gad-el-Hak, M.; Tepper, G.C. Modeling resistance of nanofibrous superhydrophobic coatings to hydrostatic pressures: The role of microstructure. Phys. Fluids 2012, 24, 022109. [Google Scholar] [CrossRef]
- Zhan, N.; Li, Y.; Zhang, C.; Song, Y.; Wang, H.; Sun, L.; Yang, Q.; Hong, X. A novel multinozzle electrospinning process for preparing superhydrophobic PS films with controllable bead-on-string/microfiber morphology. J. Colloid Interface Sci. 2010, 345, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Jabarullah, N.H.; Verrelli, E.; Mauldin, C.; Navarro, L.A.; Golden, J.H.; Madianos, L.M.; Kemp, N.T. Superhydrophobic SAM Modified Electrodes for Enhanced Current Limiting Properties in Intrinsic Conducting Polymer Surge Protection Devices. Langmuir ACS J. Surf. Colloids 2015, 31, 6253–6264. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Seo, J.; Han, H.; Kang, S.; Kim, H.; Lee, T. Bio-Inspired Extreme Wetting Surfaces for Biomedical Applications. Materials 2016, 9, 116. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic transparent and superhydrophobic coatings: From nature and beyond nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, H.; Tang, J.; Gong, H.; Liu, Y.; Wang, Z.; Shi, P.; Zhang, J.; Chen, X. A novel preparation of polystyrene film with a superhydrophobic surface using a template method. J. Phys. D Appl. Phys. 2007, 40, 3485–3489. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Chaniel, G.; Taitelbaum, H.; Bormashenko, Y. Robust technique allowing manufacturing superoleophobic surfaces. Appl. Surf. Sci. 2013, 270, 98–103. [Google Scholar] [CrossRef]
- González Lazo, M.; Katrantzis, I.; Dalle Vacche, S.; Karasu, F.; Leterrier, Y. A Facile in Situ and UV Printing Process for Bioinspired Self-Cleaning Surfaces. Materials 2016, 9, 738. [Google Scholar] [CrossRef]
- Su, C.; Li, Y.; Dai, Y.; Gao, F.; Tang, K.; Cao, H. Fabrication of three-dimensional superhydrophobic membranes with high porosity via simultaneous electrospraying and electrospinning. Mater. Lett. 2016, 170, 67–71. [Google Scholar] [CrossRef]
- Kothary, P.; Dou, X.; Fang, Y.; Gu, Z.; Leo, S.Y.; Jiang, P. Superhydrophobic hierarchical arrays fabricated by a scalable colloidal lithography approach. J. Colloid Interface Sci. 2017, 487, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, M.; Chen, G.; Chen, Q.; Tian, J. Robust fabrication of fluorine-free superhydrophobic steel mesh for efficient oil/water separation. J. Mater. Sci. 2016, 52, 2549–2559. [Google Scholar] [CrossRef]
- Feng, L.; Yang, M.; Shi, X.; Liu, Y.; Wang, Y.; Qiang, X. Copper-based superhydrophobic materials with long-term durability, stability, regenerability, and self-cleaning property. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 39–47. [Google Scholar] [CrossRef]
- Bird, J.C.; Dhiman, R.; Kwon, H.M.; Varanasi, K.K. Reducing the contact time of a bouncing drop. Nature 2013, 503, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Fang, J.; Wang, L.; Liu, W. Fabrication of superhydrophobic copper by wet chemical reaction. Thin Solid Films 2007, 515, 7190–7194. [Google Scholar] [CrossRef]
- Liang, Y.H.; Peng, J.; Li, X.J.; Xu, J.K.; Zhang, Z.H.; Ren, L.Q. From natural to biomimetic: The superhydrophobicity and the contact time. Microsc. Res. Tech. 2016, 79, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Superhydrophobic surfaces and emerging applications: Non-adhesion, energy, green engineering. Curr. Opin. Colloid Interface Sci. 2009, 14, 270–280. [Google Scholar] [CrossRef]
| Element | Al (%) | Si (%) | Fe (%) | Cu (%) | Mn (%) | Mg (%) | Cr (%) | Zn (%) | Ti (%) |
|---|---|---|---|---|---|---|---|---|---|
| content | 89.2 | 0.1 | 0.2 | 1.3 | 0.2 | 2.7 | 0.2 | 6 | 0.1 |
| Sample No. | L Side Length of Protrusion (μm) | S Interval of Protrusion (μm) | H Height of Protrusion (μm) | yi APCA (°) | y′i Contact Time (ms) |
|---|---|---|---|---|---|
| 1 | (L1)80 | (S1)250 | (H1)200 | 155 | 25.3 |
| 2 | (L1)80 | (S2)300 | (H2)300 | 153 | 22.7 |
| 3 | (L1)80 | (S3)350 | (H3)400 | 152 | 22.8 |
| 4 | (L2)130 | (S1)250 | (H3)400 | 154 | 22.7 |
| 5 | (L2)130 | (S2)300 | (H1)200 | 153 | 25.6 |
| 6 | (L2)130 | (S3)350 | (H2)300 | 151 | 21.4 |
| 7 | (L3)180 | (S1)250 | (H2)300 | 156 | 22.8 |
| 8 | (L3)180 | (S2)300 | (H3)400 | 153 | 19.1 |
| 9 | (L3)180 | (S3)350 | (H1)200 | 156 | 26.8 |
| Indices | Item | L | S | H |
|---|---|---|---|---|
| APCA (°) | yj1 | 461 | 464 | 467 |
| yj2 | 461 | 462 | 460 | |
| yj3 | 465 | 459 | 459 | |
| j1 | 153.7 | 154.7 | 155.7 | |
| j2 | 153.7 | 154 | 153.3 | |
| j3 | 155 | 153 | 153 | |
| Rj | 1.3 | 1.7 | 2.7 | |
| L | L3 | S1 | H1 | |
| F | SHL | |||
| S | L3S1H1 | |||
| Contact time (ms) | y’j1 | 70.8 | 70.8 | 77.7 |
| y’j2 | 69.7 | 67.4 | 66.9 | |
| y’j3 | 68.7 | 71 | 64.6 | |
| ′j1 | 23.6 | 23.6 | 25.9 | |
| ’j2 | 23.2 | 22.5 | 22.3 | |
| ’j3 | 22.9 | 23.7 | 21.5 | |
| R’j | 0.7 | 1.2 | 4.4 | |
| L’ | L3 | S2 | H3 | |
| F’ | HSL | |||
| S’ | L3S2H3 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Peng, J.; Li, X.; Huang, J.; Qiu, R.; Zhang, Z.; Ren, L. Wettability and Contact Time on a Biomimetic Superhydrophobic Surface. Materials 2017, 10, 254. https://doi.org/10.3390/ma10030254
Liang Y, Peng J, Li X, Huang J, Qiu R, Zhang Z, Ren L. Wettability and Contact Time on a Biomimetic Superhydrophobic Surface. Materials. 2017; 10(3):254. https://doi.org/10.3390/ma10030254
Chicago/Turabian StyleLiang, Yunhong, Jian Peng, Xiujuan Li, Jubin Huang, Rongxian Qiu, Zhihui Zhang, and Luquan Ren. 2017. "Wettability and Contact Time on a Biomimetic Superhydrophobic Surface" Materials 10, no. 3: 254. https://doi.org/10.3390/ma10030254






