Structural Evolution during Milling, Annealing, and Rapid Consolidation of Nanocrystalline Fe–10Cr–3Al Powder
Abstract
:1. Introduction
2. Materials and Experimental Procedure
2.1. Materials and Methods
2.2. Characterization Techniques
3. Results and Discussion
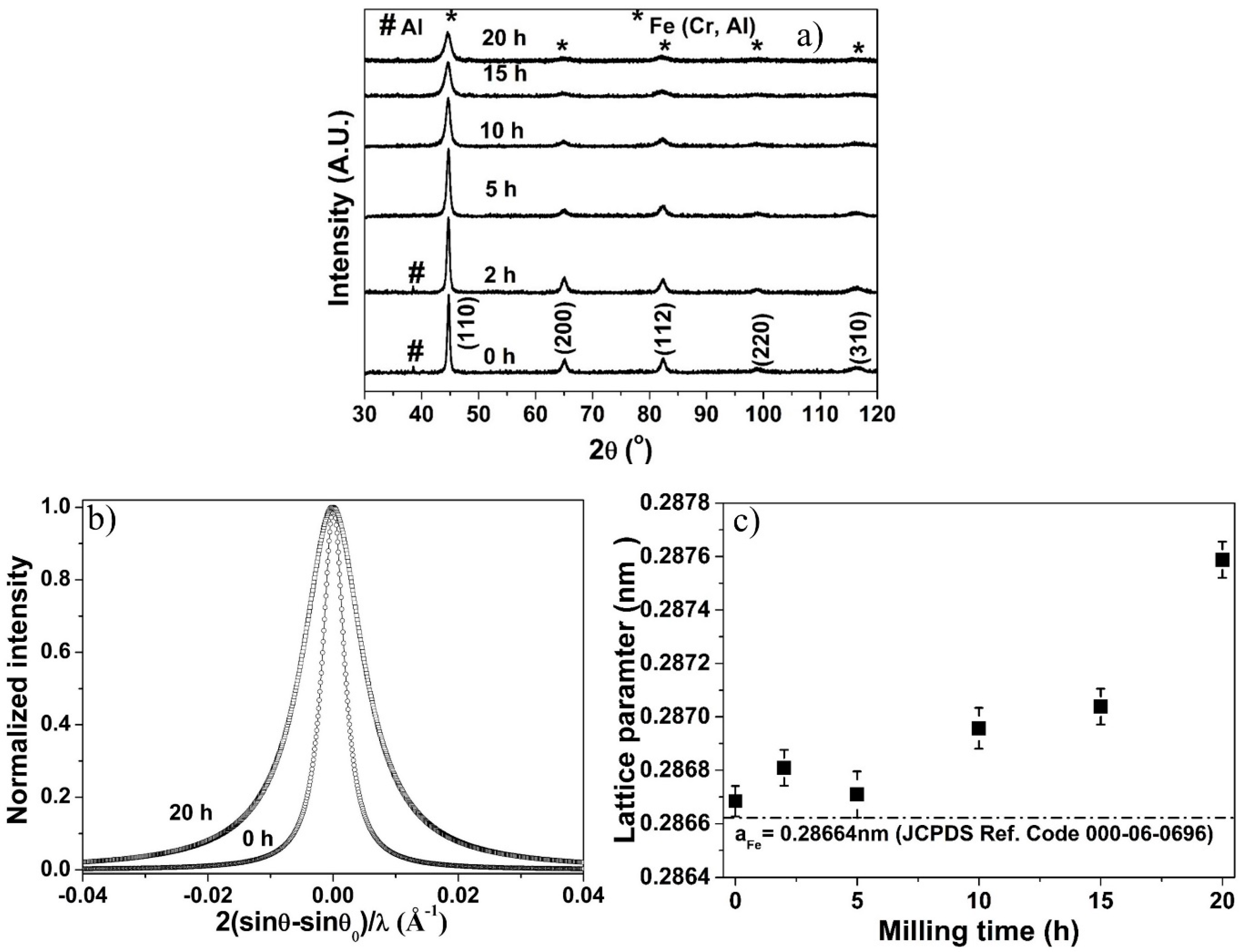
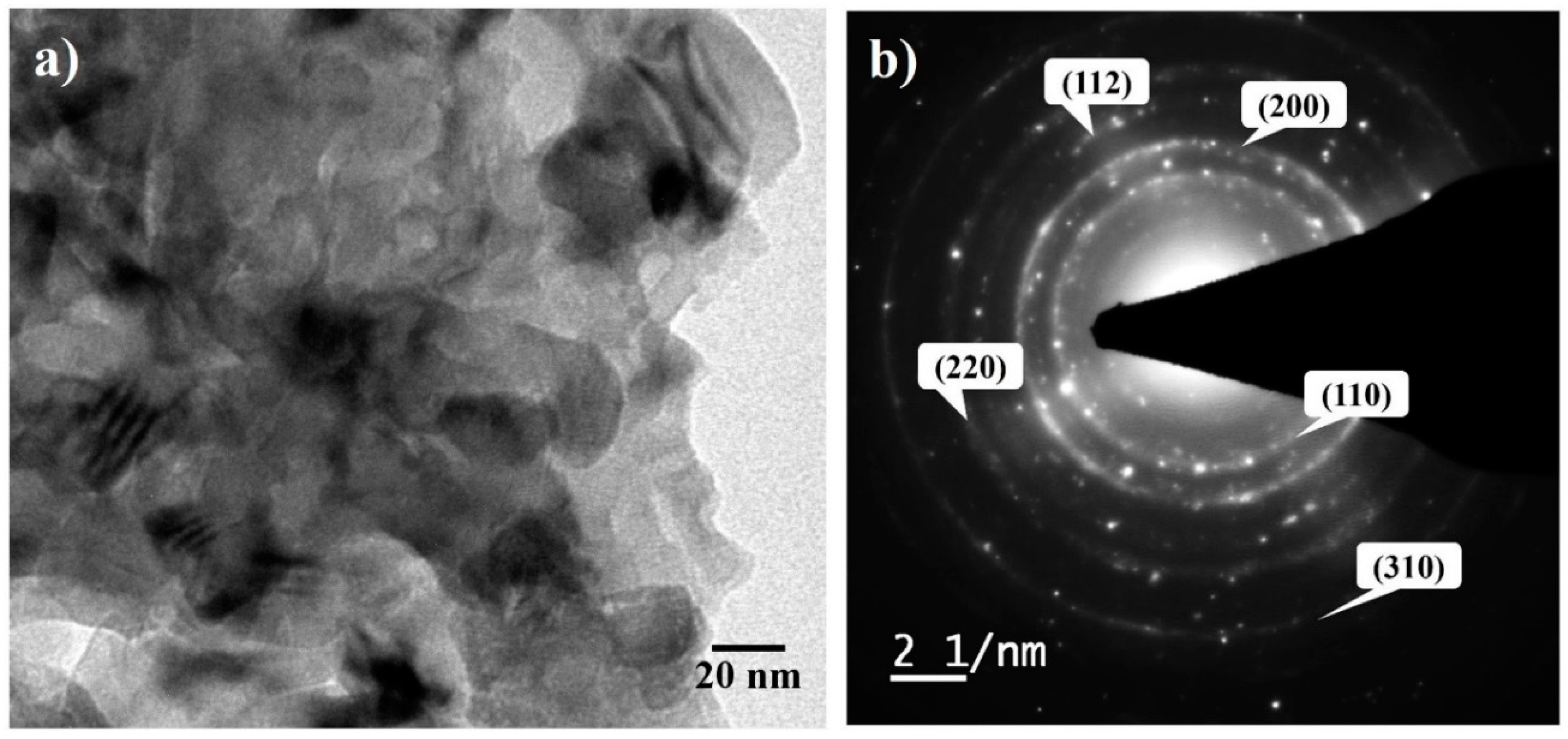
3.1. X-ray Diffraction of Milled Powder
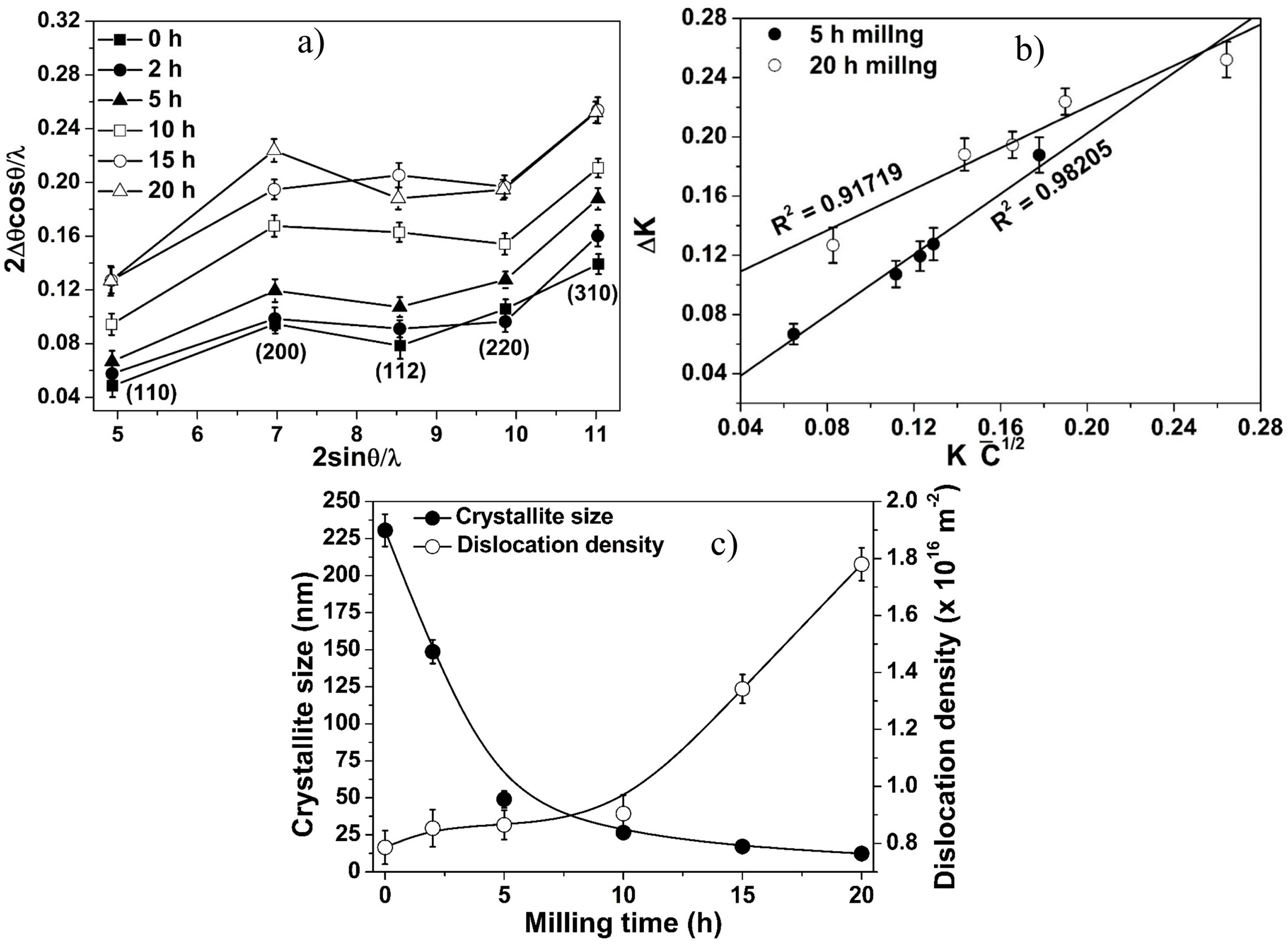
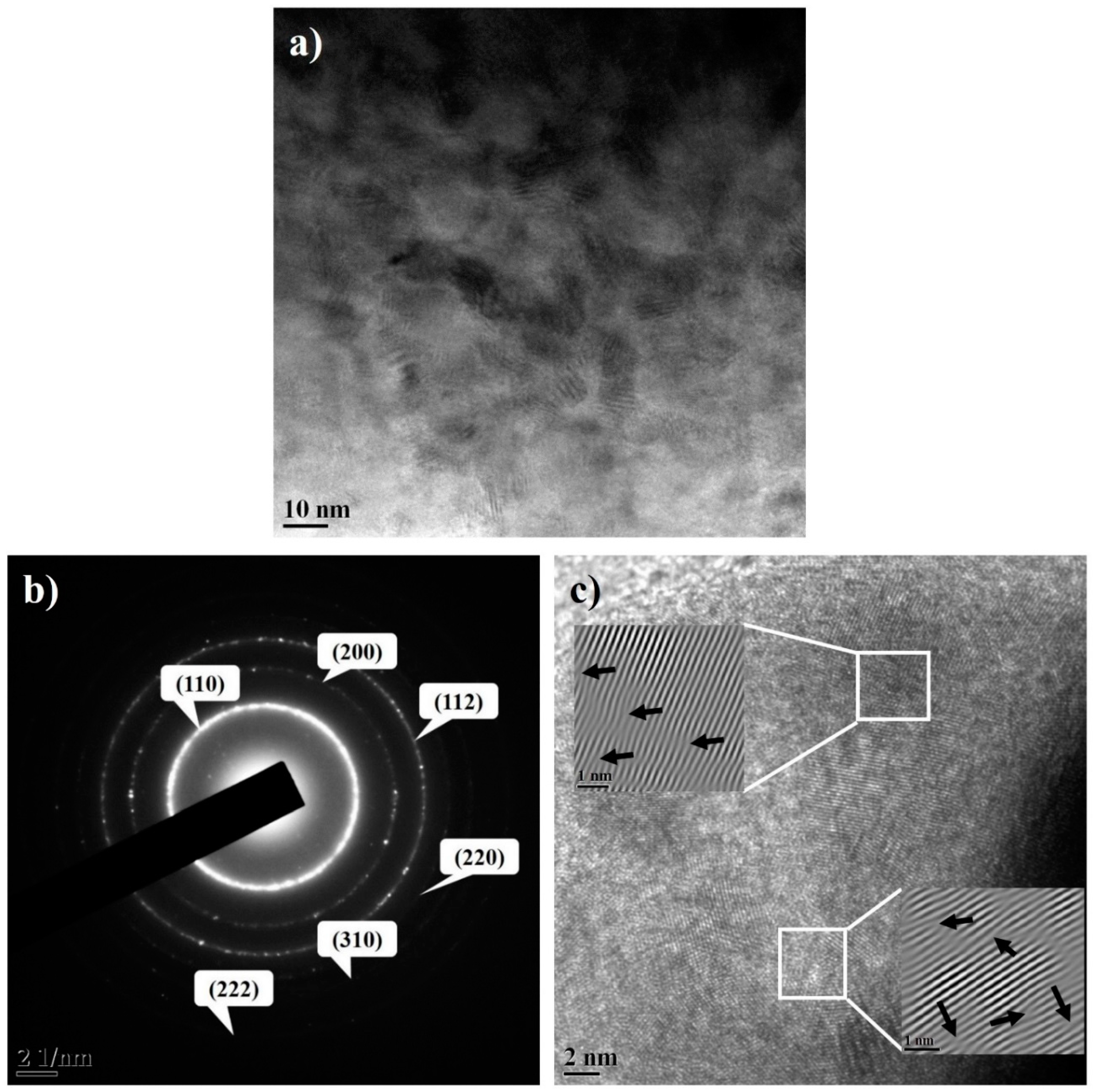
3.2. Structural Evolution during Milling
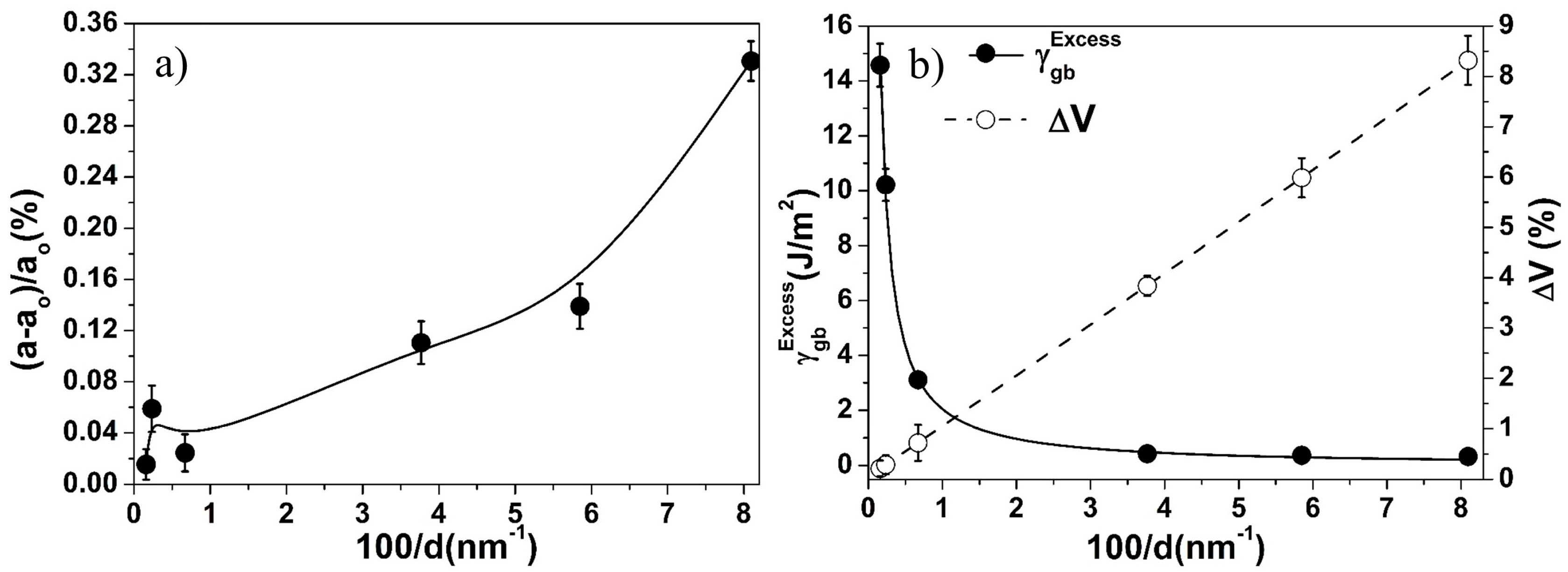
3.3. Effect of Crystallite Size on Lattice Parameter
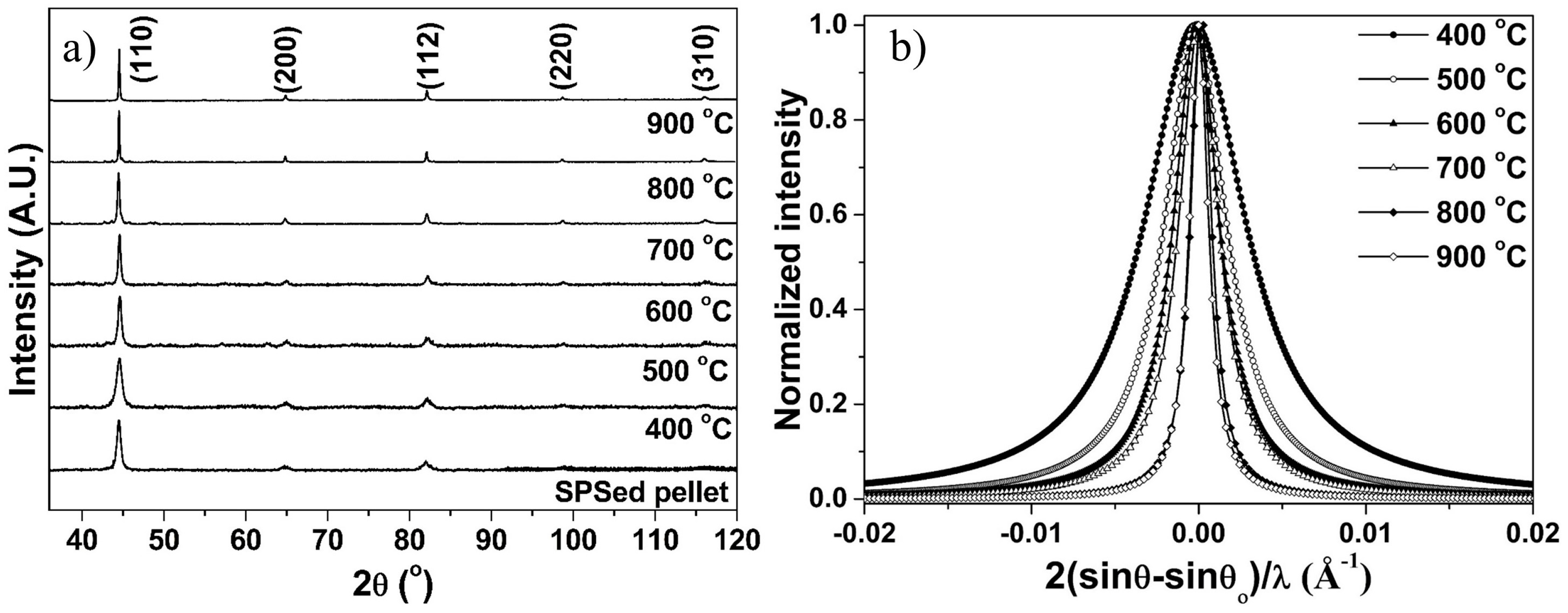
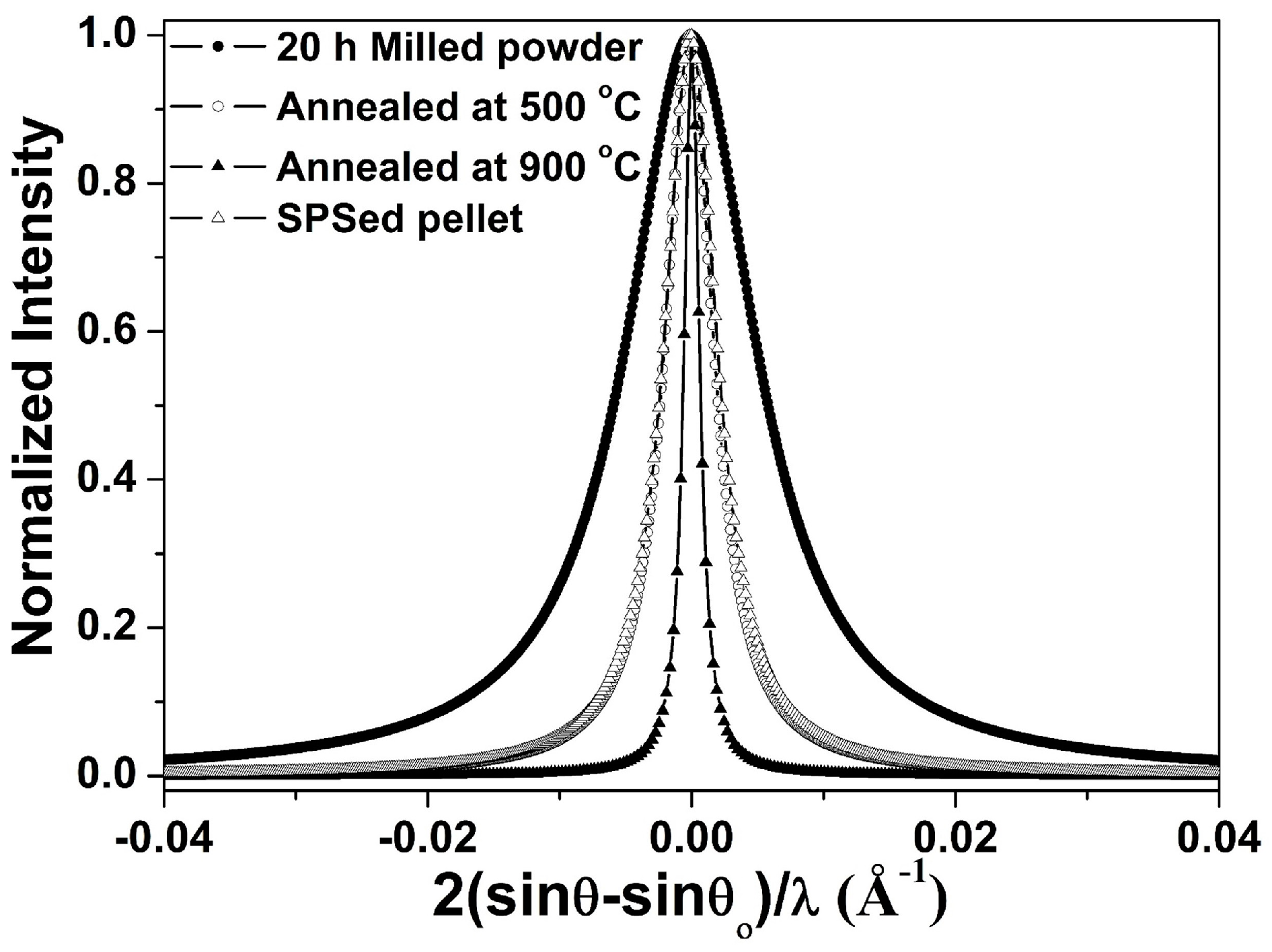
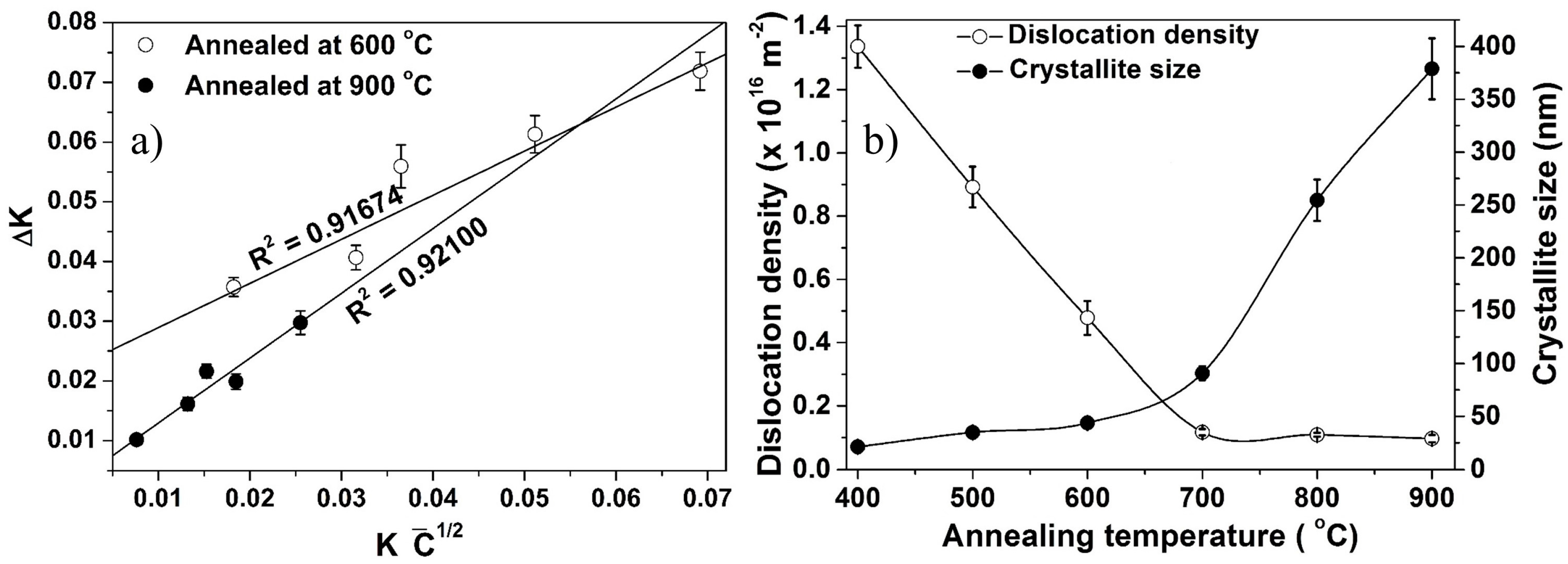
3.4. Structural Evolution during Annealing and Sintering
3.5. Activation Energy for Grain Growth
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.; Tang, W.; Wang, Y.; Liu, C.; Xu, G.; Zheng, Z. Structural evolutions of the Fe-40Al-5Cr powders during mechanical alloying and subsequent heat treatment. J. Alloys Compd. 2010, 506, 963–968. [Google Scholar] [CrossRef]
- Khatirkar, R.K.; Murty, B.S. Structural changes in iron powder during ball milling. Mater. Chem. Phys. 2010, 123, 247–253. [Google Scholar] [CrossRef]
- Shen, T.D.; Koch, C.C.; Tsui, T.Y.; Pharr, G.M. On the elastic moduli of nanocrystalline Fe, Cu, Ni, and Cu-Ni alloys prepared by mechanical milling/alloying. J. Mater. Res. 1995, 10, 2892–2896. [Google Scholar] [CrossRef]
- Rane, G.K.; Welzel, U.; Meka, S.R.; Mittemeijer, E.J. Non-monotonic lattice parameter variation with crystallite size in nanocrystalline solids. Acta Mater. 2013, 61, 4524–4533. [Google Scholar] [CrossRef]
- Malow, T.R.; Koch, C.C. Mechanical properties, ductility, and grain size of nanocrystalline iron produced by mechanical attrition. Metall. Mater. Trans. A 1998, 29, 2285–2295. [Google Scholar] [CrossRef]
- Malow, T.R.; Koch, C.C. Grain growth in nanocrystalline iron prepared by mechanical attrition. Acta Mater. 1997, 45, 2177–2186. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Gupta, R.K. Oxidation resistance of nanocrystalline vis-à-vis microcrystalline Fe–Cr alloys. Corros. Sci. 2009, 51, 316–321. [Google Scholar] [CrossRef]
- Moelle, C.H.; Fecht, H.J. Thermal stability of nanocrystalline iron prepared by mechanical attrition. Nanostruct. Mater. 1995, 6, 421–424. [Google Scholar] [CrossRef]
- Krill, C.E.; Helfen, L.; Michels, D.; Natter, H.; Fitch, A.; Masson, O.; Birringer, R. Size-dependent grain-growth kinetics observed in nanocrystalline Fe. Phys. Rev. Lett. 2001, 86, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.H.; Atzmon, M. Kinetics of microstructure evolution in nanocrystalline Fe powder during mechanical attrition. Acta Mater. 1999, 47, 1255–1261. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh Raman, R.K.; Koch, C.C. Grain growth behaviour and consolidation of ball-milled nanocrystalline Fe–10Cr alloy. Mater. Sci. Eng. A 2008, 494, 253–256. [Google Scholar] [CrossRef]
- Riontino, G.; Antonione, C.; Battezzati, L. Kinetics of abnormal grain growth in pure iron. J. Mater. Sci. 1979, 14, 86–90. [Google Scholar] [CrossRef]
- Cheng, S.; Ma, E.; Wang, Y.M.; Kecskes, L.J.; Youssef, K.M.; Koch, C.C.; Trociewitz, U.P.; Han, K. Tensile properties of in situ consolidated nanocrystalline Cu. Acta Mater. 2005, 53, 1521–1533. [Google Scholar] [CrossRef]
- Solliard, C.; Flueli, M. Surfaces stress and size effect on the lattice parameter in small particles of gold and platinum. Surf. Sci. 1985, 156, 487–494. [Google Scholar] [CrossRef]
- Rawers, J.; Krabbe, R.; Duttlinger, N. Nanostructure characterization of mechanical alloyed and consolidated iron alloys. Mater. Sci. Eng. A 1997, 230, 139–145. [Google Scholar] [CrossRef]
- Menapace, C.; Lonardelli, I.; Tait, M.; Molinari, A. Nanostructured/ultrafine multiphase steel with enhanced ductility obtained by mechanical alloying and spark plasma sintering of powders. Mater. Sci. Eng. A 2009, 517, 1–7. [Google Scholar] [CrossRef]
- Fabrègue, D.; Piallat, J.; Maire, E.; Jorand, Y.; Massardier-Jourdan, V. Nanograined size pure iron elaborated by means of spark plasma sintering. Mater. Sci. Forum 2010, 638-642, 1691–1696. [Google Scholar] [CrossRef]
- Oh-ishi, K.; Zhang, H.W.; Ohkubo, T.; Hono, K. Microstructure characterization of bulk nanocrystalline Fe-0.8C alloy produced by mechanical milling and spark plasma sintering. Mater. Sci. Eng. A 2007, 456, 20–27. [Google Scholar] [CrossRef]
- Karimpoor, A.A.; Erb, U.; Aust, K.T.; Palumbo, G. High strength nanocrystalline cobalt with high tensile ductility. Scr. Mater. 2003, 49, 651–656. [Google Scholar] [CrossRef]
- Eckert, J.; Holzer, J.C.; Krill, C.E.; Johnson, W.L. Structural and thermodynamic properties of nanocrystalline fcc metals prepared by mechanical attrition. J. Mater. Res. 1992, 7, 1751–1761. [Google Scholar] [CrossRef]
- Qin, W.; Nagase, T.; Umakoshi, Y.; Szpunar, J.A. Lattice distortion and its effects on physical properties of nanostructured materials. J. Phys. Condens. Matter 2007, 19, 236217. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Chawake, N.; Murty, B.S. Grain size dependent non-monotonic lattice parameter variation in nanocrystalline W: The role of non-equilibrium grain boundary structure. Scr. Mater. 2015, 98, 20–23. [Google Scholar] [CrossRef]
- German, R.M. Powder Metallurgy Science, 2nd ed.; Metal Powder Industries Federation: Princeton, NJ, USA, 1994. [Google Scholar]
- Zhao, Y.H.; Sheng, H.W.; Lu, K. Microstructure evolution and thermal properties in nanocrystalline Fe during mechanical attrition. Acta Mater. 2001, 49, 365–375. [Google Scholar] [CrossRef]
- Jiang, H.G.; Hu, H.M.; Lavernia, E.J. Synthesis of Fe-rich Fe–Al nanocrystalline solid solutions using ball milling. J. Mater. Res. 2011, 14, 1760–1770. [Google Scholar] [CrossRef]
- Mahesh, B.V.; Singh Raman, R.K. Role of nanostructure in electrochemical corrosion and high temperature oxidation: A review. Metall. Mater. Trans. A 2014, 45, 5799–5822. [Google Scholar] [CrossRef]
- Kumar, R.; Joardar, J.; Singh Raman, R.K.; Raja, V.S.; Joshi, S.V.; Parida, S. Effect of chromium and aluminum addition on anisotropic and microstructural characteristics of ball milled nanocrystalline iron. J. Alloys Compd. 2016, 671, 164–169. [Google Scholar] [CrossRef]
- Benjamin, J.S.; Volin, T.E. The mechanism of mechanical alloying. Metall. Trans. 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- Siegel, R.W. Mechanical properties of nanophase materials. Mater. Sci. Forum 1997, 235–238, 851–860. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Froes, F.H.; Korth, G.E. Compaction and characterization of mechanically alloyed nanocrystalline titanium aluminides. Metall. Mater. Trans. A 1997, 28, 293–302. [Google Scholar] [CrossRef]
- Gupta, R.K.; Fabijanic, D.; Zhang, R.; Birbilis, N. Corrosion behaviour and hardness of in situ consolidated nanostructured Al and Al-Cr alloys produced via high-energy ball milling. Corros. Sci. 2015, 98, 643–650. [Google Scholar] [CrossRef]
- Airiskallio, E.; Nurmi, E.; Heinonen, M.H.; Väyrynen, I.J.; Kokko, K.; Ropo, M.; Punkkinen, M.P.J.; Pitkänen, H.; Alatalo, M.; Kollár, J.; et al. High temperature oxidation of Fe–Al and Fe–Cr–Al alloys: The role of Cr as a chemically active element. Corros. Sci. 2010, 52, 3394–3404. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, X.L.; Sheng, L.; Teng, X. The effect of the third element Cr on oxidation behavior of Fe-xCr-10Al (at.%) alloys at 900 °C. Open Corros. J. 2009, 2, 37–44. [Google Scholar]
- Niu, Y.; Wang, S.; Gao, F.; Zhang, Z.G.; Gesmundo, F. The nature of the third-element effect in the oxidation of Fe-xCr-3 at.% Al alloys in 1 atm O2 at 1000 °C. Corros. Sci. 2008, 50, 345–356. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Gesmundo, F.; Hou, P.Y.; Niu, Y. Criteria for the formation of protective Al2O3 scales on Fe–Al and Fe–Cr–Al alloys. Corros. Sci. 2006, 48, 741–765. [Google Scholar] [CrossRef]
- Ida, T.; Ando, M.; Toraya, H. Extended pseudo-Voigt function for approximating the Voigt profile. J. Appl. Crystallogr. 2000, 33, 1311–1316. [Google Scholar]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminum and wolfrom. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Ungar, T.; Revesz, A.; Borbély, A. Dislocations and grain size in electrodeposited nanocrystalline Ni determined by the modified Williamson-Hall and Warren-Averbach procedures. J. Appl. Crystallogr. 1998, 31, 554–558. [Google Scholar] [CrossRef]
- Ungár, T.; Dragomir, I.; Révész, Á.; Borbély, A. The contrast factors of dislocations in cubic crystals: The dislocation model of strain anisotropy in practice. J. Appl. Crystallogr. 1999, 32, 992–1002. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Rane, G.K.; Welzel, U.; Mittemeijer, E.J. Grain growth studies on nanocrystalline Ni powder. Acta Mater. 2012, 60, 7011–7023. [Google Scholar] [CrossRef]
- Ungár, T.; Gubicza, J.; Ribárik, G.; Borbély, A. Crystallite size distribution and dislocation structure determined by diffraction profile analysis: Principles and practical application to cubic and hexagonal crystals. J. Appl. Crystallogr. 2001, 34, 298–310. [Google Scholar] [CrossRef]
- Tomaszewski, P.E. The uncertainty in the grain size calculation from X-ray diffraction data. Phase Transit. 2013, 86, 260–266. [Google Scholar] [CrossRef]
- Roesler, J.; Harders, H.; Baeker, M. Mechanical Behaviour of Engineering Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Dieter, G.E. Mechanical Metallurgy; SI Matrix; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Ledbetter, H.M.; Reed, R.P. Elastics properties of metals and alloys, I. iron, nickel and iron-nickel alloys. J. Phys. Chem. 1973, 2, 531–617. [Google Scholar] [CrossRef]
- Lu, K.; Zhao, Y.H. Experimental evidences of lattice distortion in nanocrystalline materials. Nanostruct. Mater. 1999, 12, 559–562. [Google Scholar] [CrossRef]
- Commarata, R.C.; Sieradzki, K. Surface and interface stresses. Annu. Rev. Mater. Sci. 1994, 24, 215–234. [Google Scholar] [CrossRef]
- Nazarov, A.A.; Romanov, A.E.; Valiev, R.Z. Random disclination ensembles in ultrafine-grained materials produced by severe plastic deformation. Scr. Mater. 1996, 34, 729–734. [Google Scholar] [CrossRef]
- Wagner, M. Structure and thermodynamic properties of nanocrystalline metals. Phys. Rev. B Condens. Matter Mater. Phys. 1992, 45, 635–640. [Google Scholar] [CrossRef]
- Chattopadhyay, P.P.; Nambissan, P.M.G.; Pabi, S.K.; Manna, I. Polymorphic BCC to FCC transformation of nanocrystalline niobium studied by positron annihilation. Phys. Rev. B 2001, 63, 054107. [Google Scholar] [CrossRef]
- Beck, P.A.; Kremer, J.C.; Demer, L.J.; Holzworth, M.L. Grain growth in high-purity aluminum and in an aluminum-magnesium alloy. Trans. AIME 1948, 175, 372–400. [Google Scholar]
- Rajan, K.; Rai, N.; Vadlamani, S.S.; Murty, B.S. Isothermal grain growth studies on nanostructured 9Cr-1Mo and 9Cr-1W ferritic steels containing nano-sized oxide dispersoids. Metall. Mater. Trans. A 2014, 45, 1684–1688. [Google Scholar] [CrossRef]

| Metal/Alloy | C11 (GPa) | C12 (GPa) | C44 (GPa) | Anisotropic Factor A = 2C44/(C11 − C12) | (hkl) | Elastic Modulus (GPa) |
|---|---|---|---|---|---|---|
| Fe | 237 | 141 | 116 | 2.42 | 110 | 220.58 |
| 200 | 131.81 | |||||
| 112 | 220.58 | |||||
| 220 | 220.58 | |||||
| 310 | 154.14 | |||||
| Fe–10Cr–3Al | 222.05 | 112.83 | 91.33 | 1.67 | 110 | 199.69 |
| 200 | 146.02 | |||||
| 112 | 199.69 | |||||
| 220 | 199.69 | |||||
| 310 | 161.66 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Bakshi, S.R.; Joardar, J.; Parida, S.; Raja, V.S.; Singh Raman, R.K. Structural Evolution during Milling, Annealing, and Rapid Consolidation of Nanocrystalline Fe–10Cr–3Al Powder. Materials 2017, 10, 272. https://doi.org/10.3390/ma10030272
Kumar R, Bakshi SR, Joardar J, Parida S, Raja VS, Singh Raman RK. Structural Evolution during Milling, Annealing, and Rapid Consolidation of Nanocrystalline Fe–10Cr–3Al Powder. Materials. 2017; 10(3):272. https://doi.org/10.3390/ma10030272
Chicago/Turabian StyleKumar, Rajiv, S. R. Bakshi, Joydip Joardar, S. Parida, V. S. Raja, and R. K. Singh Raman. 2017. "Structural Evolution during Milling, Annealing, and Rapid Consolidation of Nanocrystalline Fe–10Cr–3Al Powder" Materials 10, no. 3: 272. https://doi.org/10.3390/ma10030272






