The Effect of Heat Treatment on the Sensitized Corrosion of the 5383-H116 Al-Mg Alloy
Abstract
:1. Introduction
2. Literature and Theory
2.1. Al-Mg Alloy Sensitization Characteristics
2.2. β-Phase Precipitation Characteristics and Effects on Corrosion
2.2.1. Influence of Mg Content on β-Phase Precipitation
2.2.2. Influence of Sensitization Temperature and Time on β-Phase Precipitation
3. Experiment Setup
3.1. Heat Treatment Stage
3.2. Corrosion Testing
3.3. Measuring Electrical Conductivity
4. Results and Discussion
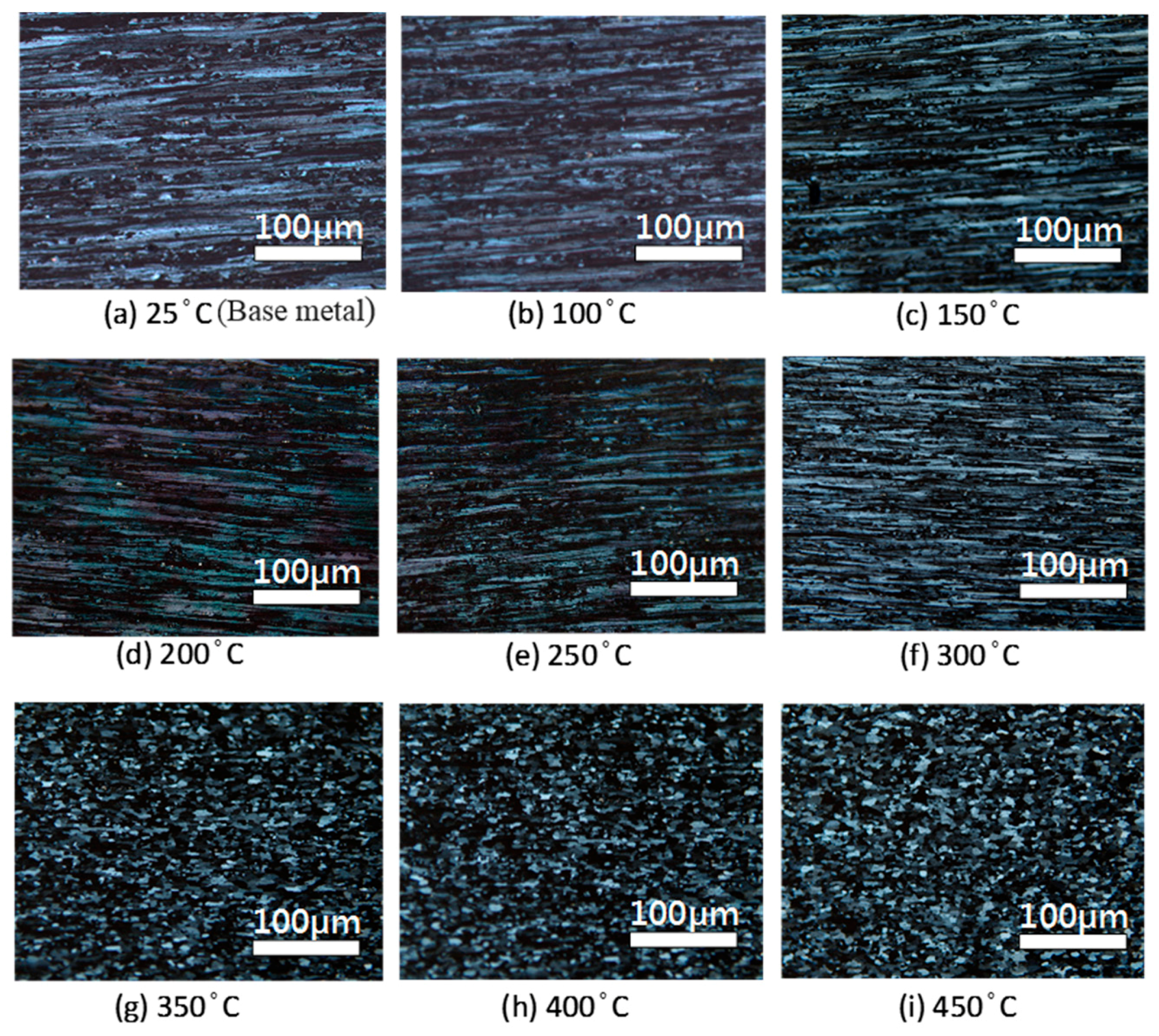
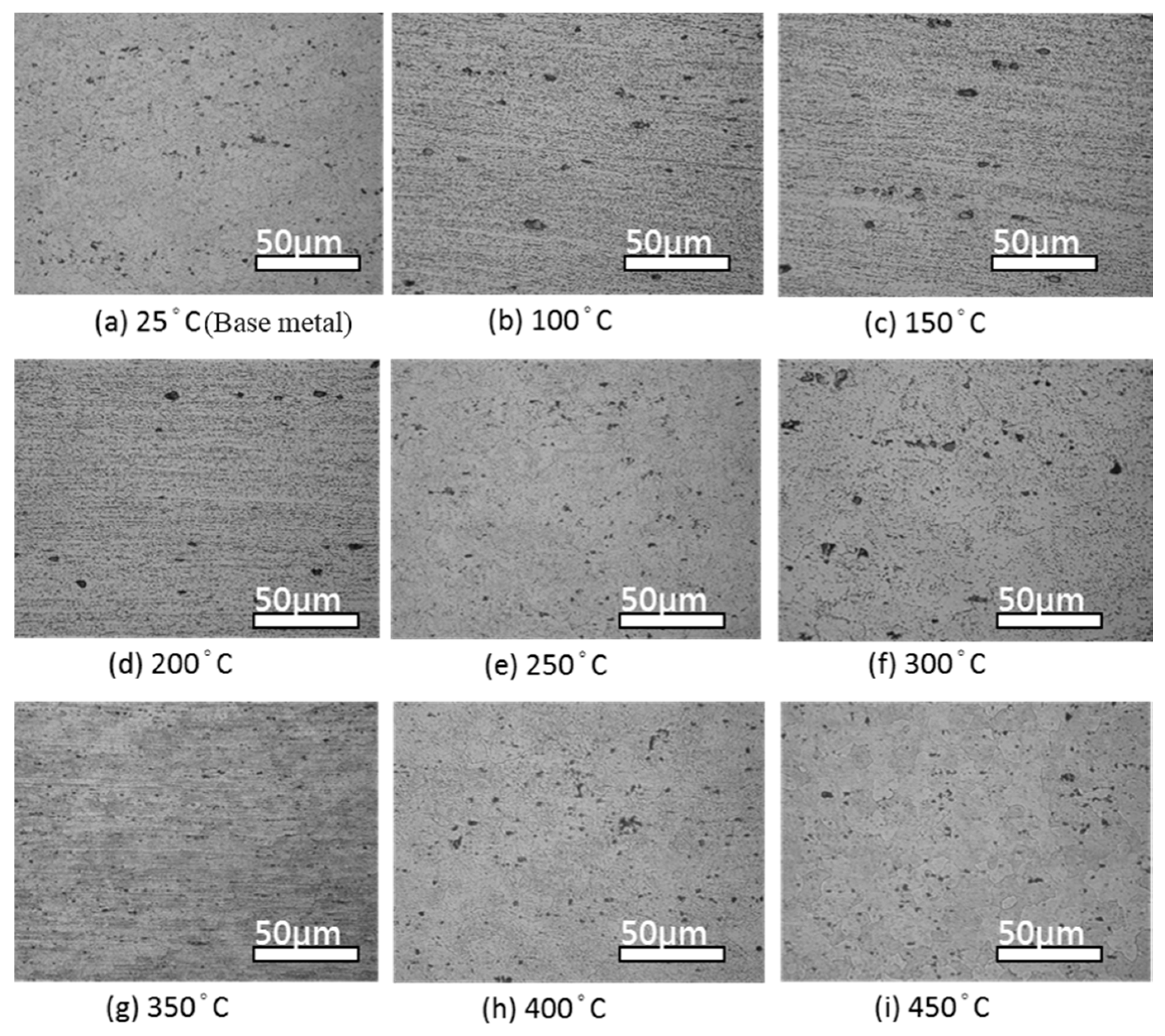
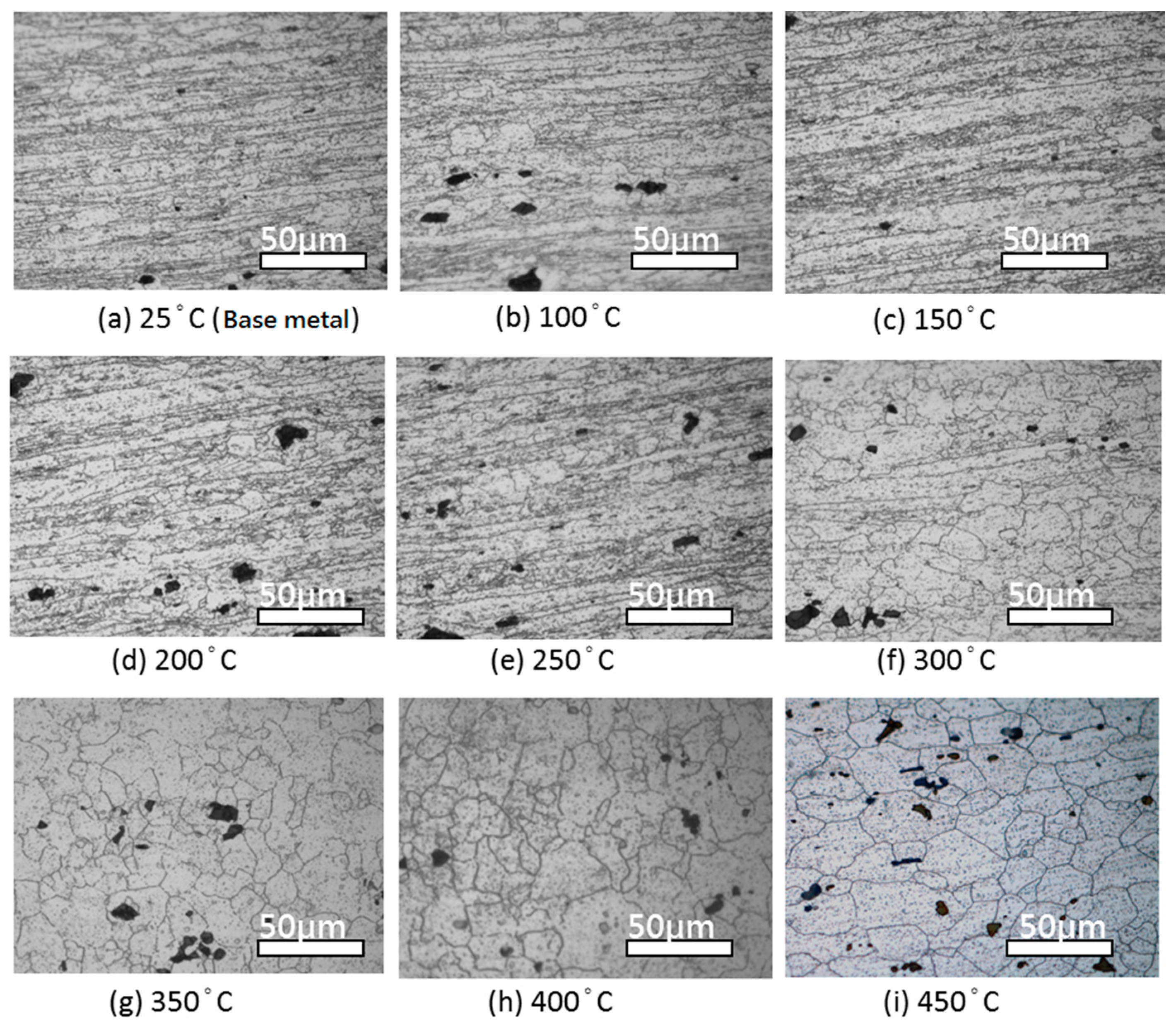
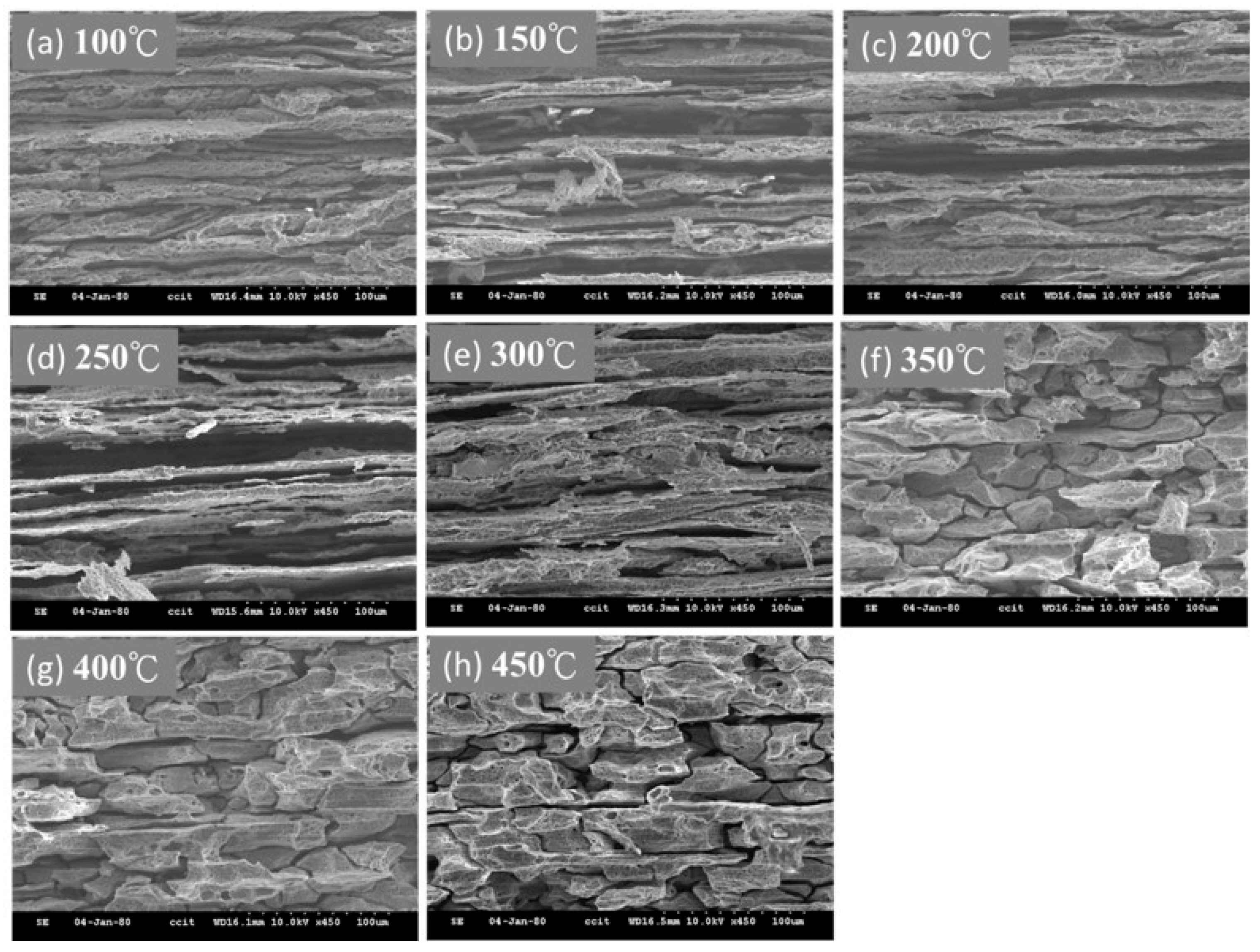
4.1. Microstructure Changes Resulting from the Heat Treatment
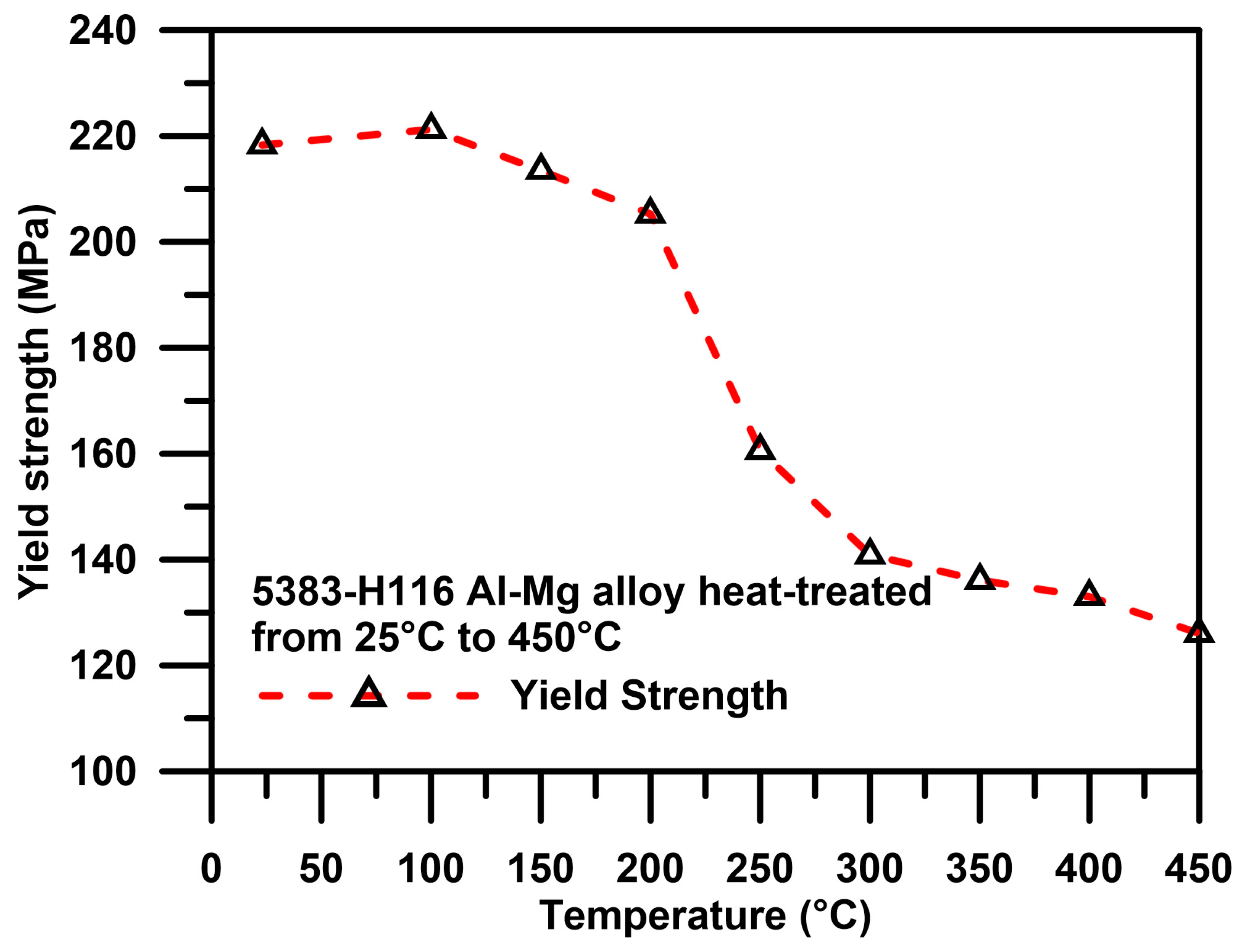
4.2. Influence of Sensitization on Microstructure and Mechanical Properties
4.3. Influence of Heat Treatment and Sensitization Treatment on Intergranular Corrosion Sensitivity
4.4. Changes in Electrical Conductivity during the Sensitization Process
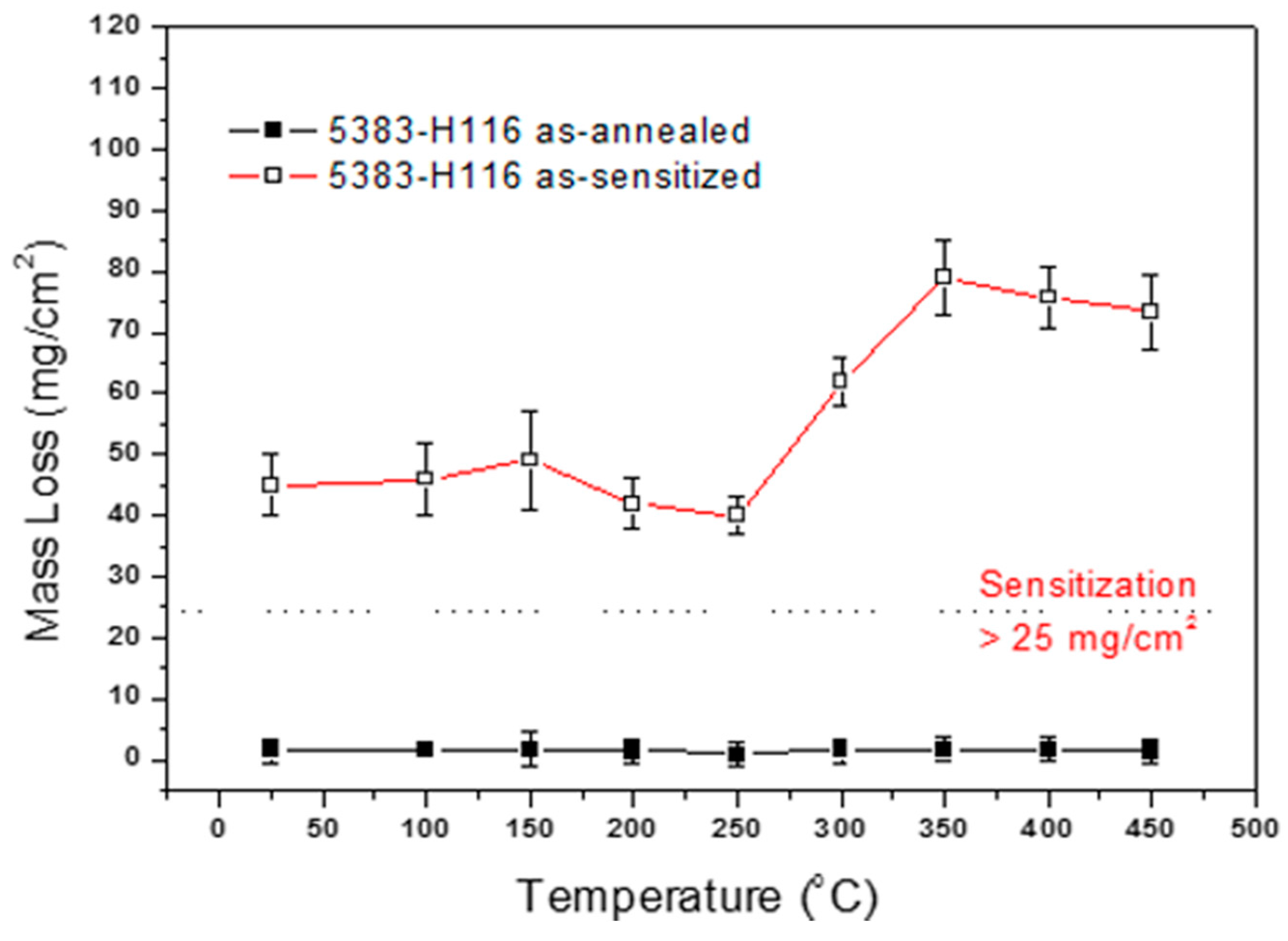
4.5. NAMLT Corrosion
5. Conclusions
- The heat treatment temperature is an important factor affecting 5383-H116 Al-Mg alloy microstructure changes. When the treatment temperature exceeds 300 °C, the structure begins to recrystallize, which decreases the mechanical strength.
- The DoS of the recrystallized structure is bigger than that of the recovery structure; therefore, in the same sensitized environment, the recrystallized structure will be more susceptible to intergranular corrosion than the recovery structure.
- The β-phase precipitation helps increase the electrical conductivity, especially in the recovery structure due to the higher dislocation density. In addition, the electrical conductivity after sensitization is markedly increased and can be employed as indirect evidence of the variation of sensitization.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanders, R.E., Jr.; Hollinshead, P.; Simielli, E.A. Industrial development of non-heat treatable aluminum alloys. Mater. Forum 2004, 28, 53–64. [Google Scholar]
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; ASM International: Metals Park, OH, USA, 1999. [Google Scholar]
- Sielski, R.A. SSC-452 Aluminum Structure Design and Fabrication Guide; Ship Structure Committee: Washington, DC, USA, 2007. [Google Scholar]
- Sensharma, P.; Collette, M.; Harrington, J. SSC-4 Effect of Welded Properties on Aluminum Structures; Ship Structure Committee: Washington, DC, USA, 2010. [Google Scholar]
- Toros, S.; Ozturk, F. Modeling uniaxial, temperature and strain rate dependent behavior of Al-Mg alloys. Comput. Mater. Sci. 2010, 49, 333–339. [Google Scholar] [CrossRef]
- Liu, J.; Tan, M.J.; Jarfors, A.E.W.; Aue-u-lan, Y.; Castagne, S. Formability in AA5083 and AA6061 alloys for light weight applications. Mater. Des. 2010, 31, S66–S70. [Google Scholar] [CrossRef]
- Picu, R.C.; Vincze, G.; Ozturk, F.; Gracio, J.J.; Barlat, F.; Maniatty, A.M. Strain rate sensitivity of the commercial aluminum alloy AA5182-O. Mater. Sci. Eng. A 2005, 390, 334–343. [Google Scholar] [CrossRef]
- Popović, M.; Romhanji, E. Characterization of microstructural changes in an Al-6.8 wt. % Mg alloy by electrical resistivity measurements. Mater. Sci. Eng. A 2008, 492, 460–467. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- ASTM B928/B928M-09. Standard Specification for High Magnesium Aluminium-Alloy Sheet and Plate for Marine Service; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM G67-13. Standard Test Method for Visual Assessment of Exfoliation Corrosion Susceptibility of 5XXX Series Aluminum Alloys; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Gupta, R.K.; Zhang, R.; Davies, C.H.J.; Birbilis, N. Influence of Mg content on the sensitization and corrosion of Al-xMg(-Mn) alloys. Corrosion 2013, 69, 1081–1087. [Google Scholar] [CrossRef]
- Dix, E.H., Jr.; Anderson, W.A.; Shumaker, M.B. Influence of service temperature on the resistance of wrought aluminum-magnesium alloys to corrosion. Corrosion 1959, 15, 19–26. [Google Scholar] [CrossRef]
- Czyryca, E.J.; Hack, H.P.; David, W. Corrosion of Aluminum Alloys in Exfoliation-Resistant Tempers Exposed to Marine Environments for 2 Years; David W. Taylor Naval Ship Research and Development Center: Bethesda, MD, USA, 1974. [Google Scholar]
- Searles, J.L.; Gouma, P.I.; Buchheit, R.G. Stress corrosion cracking of sensitized AA5083 (Al-4.5Mg-1.0Mn). Metall. Mater. Trans. A 2001, 32, 2859–2867. [Google Scholar] [CrossRef]
- Oguocha, I.N.A.; Adigun, O.J.; Yannacopoulos, S. Effect of sensitization heat treatment on properties of Al-Mg alloy AA5083-H116. J. Mater. Sci. 2008, 43, 4208–4214. [Google Scholar] [CrossRef]
- Jones, R.H.; Baer, D.R.; Danielson, M.J.; Vetrano, J.S. Role of Mg in the stress corrosion cracking of an Al-Mg alloy. Metall. Mater. Trans. A 2001, 32, 1699–1711. [Google Scholar] [CrossRef]
- Lim, M.L.C.; Scully, J.R.; Kelly, R.G. Intergranular Corrosion Penetration in an Al-Mg Alloy as a Function of Electrochemical and Metallurgical Conditions. Corrosion 2013, 69, 35–47. [Google Scholar] [CrossRef]
- Mulazimoglu, M.H.; Drew, R.A.L.; Gruzelski, J.E. Electrical conductivity of aluminium-rich Al-Si-Mg alloys. J. Mater. Sci. Lett. 1989, 8, 297–300. [Google Scholar] [CrossRef]

| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| 5383-H116 | 0.07 | 0.21 | 0.09 | 0.81 | 4.70 | 0.08 | 0.08 | 0.02 | remainder |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-K.; Wang, S.-H.; Chen, R.-Y.; Hsieh, T.-S.; Tsai, L.; Chiang, C.-C. The Effect of Heat Treatment on the Sensitized Corrosion of the 5383-H116 Al-Mg Alloy. Materials 2017, 10, 275. https://doi.org/10.3390/ma10030275
Lin Y-K, Wang S-H, Chen R-Y, Hsieh T-S, Tsai L, Chiang C-C. The Effect of Heat Treatment on the Sensitized Corrosion of the 5383-H116 Al-Mg Alloy. Materials. 2017; 10(3):275. https://doi.org/10.3390/ma10030275
Chicago/Turabian StyleLin, Ying-Kai, Shing-Hai Wang, Ren-Yu Chen, Tso-Sheng Hsieh, Liren Tsai, and Chia-Chin Chiang. 2017. "The Effect of Heat Treatment on the Sensitized Corrosion of the 5383-H116 Al-Mg Alloy" Materials 10, no. 3: 275. https://doi.org/10.3390/ma10030275






