The Effect of Different Storage Media on Color Stability of Self-Adhesive Composite Resin Cements for up to One Year
Abstract
:1. Introduction
- all SACRCs showed no impact of storage medium and aging level on sorption,
- all SACRCs showed no impact of storage medium and aging level on ∆E, and
- ∆E/Discoloration rates can be completely removed after final polishing after 365 days storage.
2. Material and Methods
2.1. Specimens’ Fabrication
- (a)
- Red wine/RW: Rioja Cepa Lebrel Joven (Spain) 2013 (pH = 3.8);
- (b)
- Curry solution/CU: 40 g curry powder was boiled up with 1 liter of water for 10 min and is filtrated through a fine tea strainer (Ostmann, Dissen a.T.W., Germany) (pH = 5.9);
- (c)
- Cress solution/CR: 174 g tamped fresh cress was boiled up in 1 liter of water for 10 min and is afterwards filtrated through a fine tea strainer (pH = 6.0);
- (d)
- Distilled water/DW: Aqua Bidest. Kerndl (Weissenfeld, Germany) (pH = 6.7).
2.2. Sorption Measurements
2.3. Discoloration Measurements
2.4. Statistical Analyses
3. Results
3.1. Impact of Sorption on SACRCs Material/Storage Medium/Aging Level
3.2. Impact of Discoloration on SACRCs/Storage Medium/Aging Level
3.3. Impact of Final Polishing on SACRCs/Storage Medium
4. Discussion
5. Conclusions
- SARCs showed a significant increase in sorption and discoloration rates within total aging time.
- Significant differences between all SARCs and media tested occurred for sorption and discoloration rates.
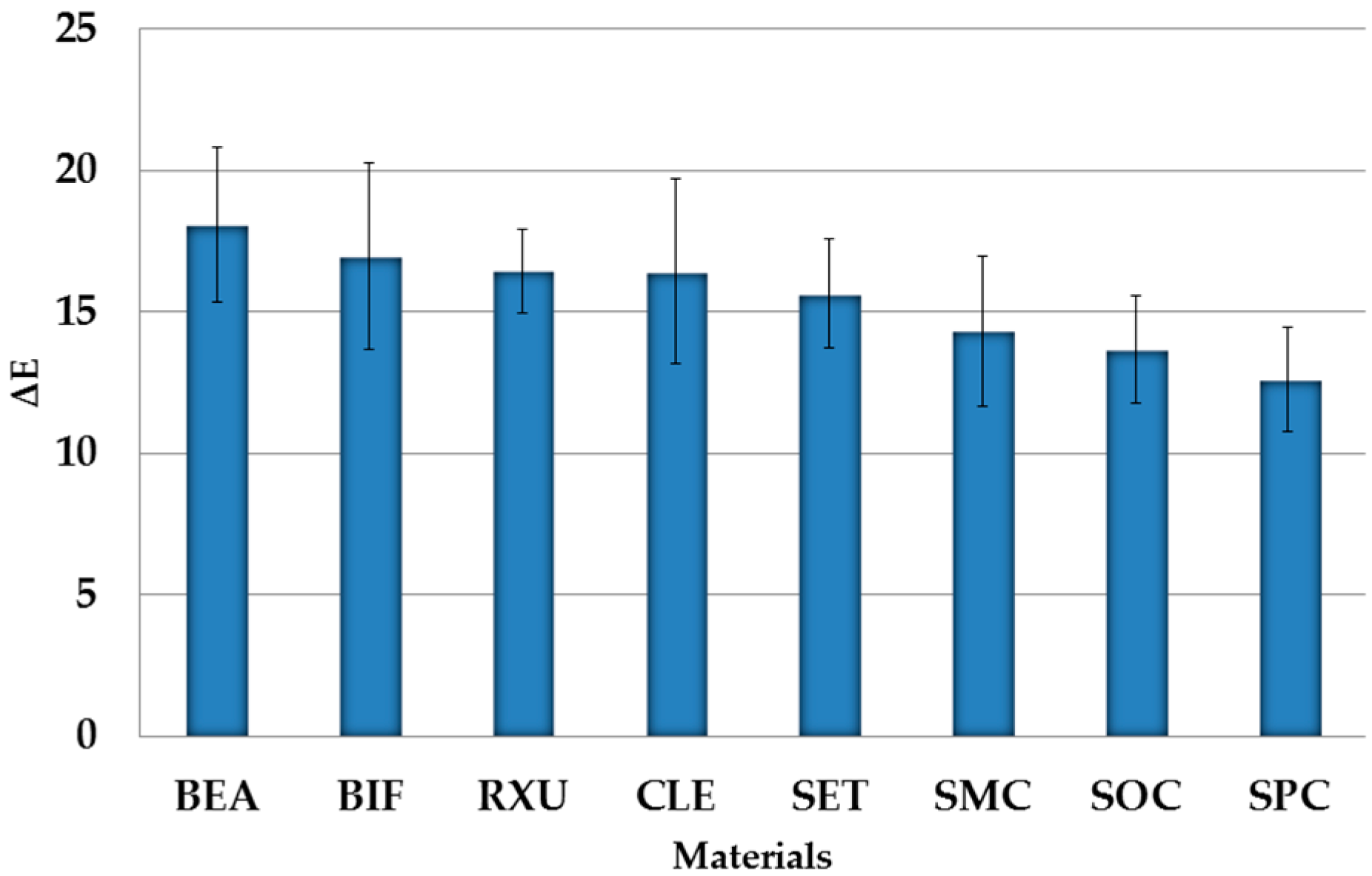
- Significantly lowest discoloration rates were measured for CLE, followed by SOC, BIF, and RXU; the highest were analyzed for SMC, followed by SET, SPC, and BEA.
- Highest discoloration rates were analyzed for red wine, followed by curry-solution, cress-solution, and distilled water.
- Discoloration was mostly extrinsic, but could not be removed completely by polishing procedures.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BEA | BeautyCem |
| BIF | Bifix SE |
| CLE | Clearfil SA Cement Automix |
| RXU | RelyX Unicem 2 Automix |
| SET | SET (Cement) |
| SMC | SmartCem 2 |
| SOC | SoloCem |
| SPC | SpeedCEM |
| CU | curry-solution |
| CR | cress-solution |
| DW | distilled water |
| RW | red wine |
| WA | water sorption |
| SACRC | self-adhesive composite resin cement |
| UDMA | urethandimethacrylate |
| HEMA | hydroxyethylmethacrylate |
| Bis-GMA | bisphenol-A-diglycidylmethacrylate |
| DMA | dimethacrylate |
| BP | benzoylperoxide |
| SG | silicate glass |
| ZiS | zirconium silicate |
| YTF | ytterbium trifluoride |
| DBP | dibenzoyl peroxide |
| GDMA | glycerol-dimethacrylate |
| HPMA | hydroxypropylmethacrylate |
| TEGDMA | triethyleneglycol-dimethacrylate |
| META | methacryloyloxyethyl-trimellitate-anhydride |
| Ba-Al | barium-aluminium |
| SiO2 | silicon dioxide |
| EBPADMA | ethoxylated bisphenol A dimethacrylate |
| PENTA | dipentaerythritol pentaacrylate monophosphate |
| ΔE | color difference |
References
- Calais, J.G.; Soderholm, K.J. Influence of filler type and water exposure on flexural strength of experimental composite resins. J. Dent. Res. 1988, 67, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, K.J. Degradation of glass filler in experimental composites. J. Dent. Res. 1981, 60, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Tyas, M.J.; Burrow, M.F. Subsurface degradation of resin-based composites. Dent. Mater. 2007, 23, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Stansbury, J.W.; Burke, F.J. Self-adhesive resin cements—chemistry, properties and clinical considerations. J. Oral. Rehab. 2011, 38, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Munchow, E.A.; Ferreira, A.C.; Machado, R.M.; Ramos, T.S.; Rodrigues-Junior, S.A.; Zanchi, C.H. Effect of acidic solutions on the surface degradation of a micro-hybrid composite resin. Braz. Dent. J. 2014, 25, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Qvist, V.; Thylstrup, A.; Mjör, I.A. Restorative treatment pattern and longivity of resin restorations in Denmark. Acta. Odontol. Scand. 1986, 44, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Canay, S.; Hersek, N.; Tulunoglu, I.; Uzun, G. Evaluation of colour and hardness changes of soft lining materials in food colorant solutions. J. Oral. Rehabil. 1999, 26, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, A.; Wimmer, T.; Schmidlin, P.R.; Scherer, H.; Loffler, P.; Roos, M.; Stawarczyk, B. Physicomechanical characterization of polyetheretherketone and current esthetic dental CAD/CAM polymers after aging in different storage media. J. Prosthet. Dent. 2015, 115, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Petropoulou, A.; Vrochari, A.D.; Hellwig, E.; Stampf, S.; Polydorou, O. Water sorption and water of self-etching and self-adhesive resin cements. J. Prosthet. Dent. 2015, 114, 674–679. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Vargas, M.; Van Landuyt, K.; Hikita, K.; Lambrechts, P.; Van Meerbeek, B. Bonding of an auto-adhesive luting material to enamel and dentin. Dent. Mater. 2004, 20, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Hitz, T.; Stawarczyk, B.; Fischer, J.; Hammerle, C.H.; Sailer, I. Are self-adhesive resin cements a valid alternative to conventional resin cements? A laboratory study of the long-term bond strength. Dent. Mater. 2012, 28, 1183–1190. [Google Scholar] [PubMed]
- Abo-Hamar, S.E.; Hiller, K.A.; Jung, H.; Federlin, M.; Friedl, K.H.; Schmalz, G. Bond strength of a new universal self-adhesive resin luting cement to dentin and enamel. Clin. Oral. Investig. 2005, 9, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zorzin, J.; Petschelt, A.; Ebert, J.; Lohbauer, U. pH neutralization and influence on mechanical strength in self-adhesive resin luting agents. Dent. Mater. 2012, 28, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hashimoto, M.; Wadgaonkar, B.; Svizero, N.; Carvalho, R.M.; Yiu, C.; Rueggeberg, F.A.; Foulger, S.; Saito, T.; Nishitani, Y.; et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 2005, 26, 6449–6459. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, L.A.; Kleverlaan, C.J.; Feilzer, A.J. Clinical success and survival of indirect resin composite crowns: Results of a 3-year prospective study. Dent. Mater. 2012, 28, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, Z.A.; Razzoog, M.E.; Yaman, P. Color stability of restorative resins. Quintessence Int. 1991, 22, 733–737. [Google Scholar] [PubMed]
- Samra, A.P.; Pereira, S.K.; Delgado, L.C.; Borges, C.P. Color stability evaluation of aesthetic restorative materials. Braz. Oral Res. 2008, 22, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kalachandra, S.; Turner, D.T. Water sorption of polymethacrylate networks: Bis-GMA/TEGDM copolymers. J. Biomed. Mater. Res. A 1987, 21, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mese, A.; Burrow, M.F.; Tyas, M.J. Sorption and solubility of luting cements in different solutions. Dent. Mater. J. 2008, 27, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Vrochari, A.D.; Eliades, G.; Hellwig, E.; Wrbas, K.T. Curing efficiency of four self-etching, self-adhesive resin cements. Dent. Mater. 2009, 25, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Marghalani, H.Y. Sorption and solubility characteristics of self-adhesive resin cements. Dent. Mater. 2012, 28, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Roeder, L.; Lu, H.; Vogel, K.; Powers, J.M. Effect of finishing and polishing procedures on surface roughness, gloss and color of resin-based composites. Am. J. Dent. 2004, 17, 262–266. [Google Scholar]
- Kalachandra, S. Influence of fillers on the water sorption of composites. Dent. Mater. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Imamura, S.; Takahashi, H.; Hayakawa, I.; Loyaga-Rendon, P.G.; Minakuchi, S. Effect of filler type and polishing on the discoloration of composite resin artificial teeth. Dent. Mater. J. 2008, 27, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Ertas, E.; Guler, A.U.; Yucel, A.C.; Koprulu, H.; Guler, E. Color stability of resin composites after immersion in different drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Rutkunas, V.; Sabaliauskas, V.; Mizutani, H. Effects of different food colorants and polishing techniques on color stability of provisional prosthetic materials. Dent. Mater. J. 2010, 29, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Turkun, L.S.; Turkun, M. Effect of bleaching and repolishing procedures on coffee and tea stain removal from three anterior composite veneering materials. J. Esthet. Restor. Dent. 2004, 16, 290–301. [Google Scholar] [CrossRef]
- Nasim, I.; Neelakantan, P.; Sujeer, R.; Subbarao, C.V. Color stability of microfilled, microhybrid and nanocomposite resins—An in vitro study. J. Dent. 2010, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Awad, D.; Stawarczyk, B.; Liebermann, A.; Ilie, N. Translucency of esthetic dental restorative CAD/CAM materials and composite resins with respect to thickness and surface roughness. J. Prosthet. Dent. 2015, 113, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Furuse, A.Y.; Gordon, K.; Rodrigues, F.P.; Silikas, N.; Watts, D.C. Colour-stability and gloss-retention of silorane and dimethacrylate composites with accelerated aging. J. Dent. 2008, 36, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Sener, B.; Trottmann, A.; Roos, M.; Ozcan, M.; Hammerle, C.H. Discoloration of manually fabricated resins and industrially fabricated CAD/CAM blocks versus glass-ceramic: Effect of storage media, duration, and subsequent polishing. Dent. Mater. J. 2012, 31, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ruyter, I.E.; Nilner, K.; Moller, B. Color stability of dental composite resin materials for crown and bridge veneers. Dent. Mater. 1987, 3, 246–251. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Egli, R.; Roos, M.; Ozcan, M.; Hammerle, C.H. The impact of in vitro aging on the mechanical and optical properties of indirect veneering composite resins. J. Prosthet. Dent. 2011, 106, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Stawarczyk, B.; Liebermann, A.; Eichberger, M.; Guth, J.F. Evaluation of mechanical and optical behavior of current esthetic dental restorative CAD/CAM composites. J. Mech. Behav. Biomed. Mater. 2015, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guignone, B.C.; Silva, L.K.; Soares, R.V.; Akaki, E.; Goiato, M.C.; Pithon, M.M.; Oliveira, D.D. Color stability of ceramic brackets immersed in potentially staining solutions. Dental Press J. Orthod. 2015, 20, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Guth, J.F.; Zuch, T.; Zwinge, S.; Engels, J.; Stimmelmayr, M.; Edelhoff, D. Optical properties of manually and CAD/CAM-fabricated polymers. Dent. Mater. J. 2013, 32, 865–871. [Google Scholar] [CrossRef] [PubMed]

| SACRC | Abbrev. | Manufacturer | Lot. No. | Composition |
|---|---|---|---|---|
| BeautyCem SA | BEA | Shofu Inc., Kyoto, Japan | 061201 | PASTE A: UDMA, Fluoroboroalumina-SG, SG, Reaction initiators PASTE B: UDMA, 2-HEMA, Carboxylic acid monomer, Phosphonic acid monomer, ZiS, initiator and others |
| Bifix SE | BIF | Voco, Cuxhaven, Germany | 1329157 | BASE: UDMA, GDMA, initatiors, catalyst CATALYST: UDMA, acidic adhesive monomer, Bis-GMA, GDMA, HPMA, BP, 70 wt %/61 vol % |
| Clearfil SA Cement Automix | CLE | Kuraray Medical Inc., Sakazu, Kurashiki, Okayama, Japan | 058AAA | PASTE A: MDP, Bis-GMA, TEGDMA, DMA, Ba-Al fluoro-SG, SiO2, BP, initiators PASTE B: Bis-GMA, DMA, Ba-Al fluoro-SG, SiO2, pigments, 66 wt %/45 vol % fillers |
| RelyX Unicem 2 Automix | RXU | 3M, Seefeld, Germany | 522135 | methacrylated phosphoric esters, methacrylated monomer, DMA fillers, silanated fillers. 72 wt %/54 vol % fillers |
| seT | SET | SDI, Koeln, Germany | S13061003 | 35 wt % methacrylate ester; 65 wt % inorganic filler |
| SmartCem 2 | SMC | Dentsply Detrey, Konstanz, Germany | 130430 | UDMA, EBPADMA urethane resin, di- and tri-functional diluents, PENTA, 69 wt %/46 vol % fillers |
| SoloCem | SOC | Coltène/Whaledent, Altstaetten, Switzerland | F28793 | UDMA, TEGDMA, 4-META, 2-HEMA, DBP; BP |
| SpeedCEM | SPC | Ivoclar Vivadent, Schaan, Liechtenstein | S40661 | DMA, YTF, co-polymer, glass filler 40 vol %, SiO2, adhesive monomer, initiators, stabilizers and pigments |
| Material | 0d | 7d | 28d | 90d | 180d | 365d |
|---|---|---|---|---|---|---|
| Distilled water | ||||||
| BEA | 0.337 ± 0.008 | 0.342 ± 0.007 | 0.346 ± 0.007 | 0.347 ± 0.007 | 0.348 ± 0.007 | 0.348 ± 0.007 |
| BIF | 0.366 ± 0.011 | 0.368 ± 0.011 | 0.375 ± 0.011 | 0.375 ± 0.011 | 0.376 ± 0.011 | 0.375 ± 0.011 |
| CLE | 0.353 ± 0.011 | 0.357 ± 0.011 | 0.358 ± 0.011 | 0.360 ± 0.011 | 0.360 ± 0.011 | 0.361 ± 0.011 |
| RXU | * 0.357 ± 0.008 | * 0.363 ± 0.008 | * 0.364 ± 0.008 | * 0.367 ± 0.008 | * 0.368 ± 0.008 | 0.367 ± 0.008 |
| SET | 0.356 ± 0.012 | 0.368 ± 0.013 | 0.368 ± 0.013 | 0.369 ± 0.013 | 0.369 ± 0.013 | 0.369 ± 0.012 |
| SMC | 0.388 ± 0.011 | 0.394 ± 0.011 | 0.396 ± 0.011 | 0.396 ± 0.011 | 0.395 ± 0.011 | 0.395 ± 0.011 |
| SOC | 0.431 ± 0.013 | 0.439 ± 0.014 | 0.441 ± 0.014 | 0.442 ± 0.014 | 0.443 ± 0.014 | 0.443 ± 0.014 |
| SPC | 0.411 ± 0.015 | 0.416 ± 0.015 | 0.417 ± 0.015 | 0.417 ± 0.015 | 0.417 ± 0.015 | 0.417 ± 0.015 |
| Red wine | ||||||
| BEA | 0.334 ± 0.011 | 0.339 ± 0.010 | 0.342 ± 0.010 | 0.344 ± 0.010 | 0.345 ± 0.011 | 0.351 ± 0.011 |
| BIF | 0.368 ± 0.012 | 0.371 ± 0.012 | 0.376 ± 0.013 | 0.378 ± 0.012 | 0.378 ± 0.012 | 0.394 ± 0.015 |
| CLE | 0.357 ± 0.009 | 0.361 ± 0.009 | 0.361 ± 0.010 | 0.365 ± 0.009 | 0.367 ± 0.100 | 0.373 ± 0.011 |
| RXU | 0.359 ± 0.008 | 0.363 ± 0.008 | 0.365 ± 0.008 | 0.367 ± 0.008 | 0.369 ± 0.007 | 0.382 ± 0.013 |
| SET | * 0.350 ± 0.016 | 0.361 ± 0.016 | * 0.362 ± 0.016 | 0.362 ± 0.016 | 0.360 ± 0.015 | 0.363 ± 0.016 |
| SMC | 0.383 ± 0.008 | 0.391 ± 0.008 | 0.392 ± 0.008 | 0.393 ± 0.008 | 0.394 ± 0.008 | 0.406 ± 0.013 |
| SOC | 0.440 ± 0.016 | 0.447 ± 0.016 | 0.450 ± 0.0160 | 0.451 ± 0.016 | 0.452 ± 0.016 | * 0.467 ± 0.025 |
| SPC | 0.412 ± 0.016 | 0.417 ± 0.016 | 0.417 ± 0.016 | 0.419 ± 0.016 | 0.423 ± 0.016 | 0.433 ± 0.016 |
| Curry solution | ||||||
| BEA | 0.337 ± 0.009 | 0.342 ± 0.009 | 0.346 ± 0.010 | 0.347 ± 0.010 | 0.347 ± 0.010 | 0.347 ± 0.010 |
| BIF | 0.370 ± 0.010 | * 0.371 ± 0.010 | 0.379 ± 0.010 | * 0.380 ± 0.011 | * 0.380 ± 0.010 | * 0.380 ± 0.011 |
| CLE | 0.358 ± 0.007 | 0.362 ± 0.007 | * 0.364 ± 0.008 | * 0.367 ± 0.007 | * 0.367 ± 0.007 | * 0.367 ± 0.007 |
| RXU | 0.358 ± 0.009 | 0.362 ± 0.009 | 0.367 ± 0.009 | 0.368 ± 0.009 | 0.368 ± 0.009 | 0.369 ± 0.009 |
| SET | 0.357 ± 0.009 | 0.369 ± 0.010 | 0.370 ± 0.01 | 0.370 ± 0.010 | 0.369 ± 0.010 | 0.369 ± 0.010 |
| SMC | 0.388 ± 0.012 | 0.394 ± 0.012 | 0.394 ± 0.012 | 0.394 ± 0.012 | 0.393 ± 0.012 | 0.393 ± 0.012 |
| SOC | 0.443 ± 0.018 | 0.450 ± 0.018 | 0.452 ± 0.019 | 0.453 ± 0.019 | 0.454 ± 0.019 | 0.454 ± 0.018 |
| SPC | 0.410 ± 0.017 | 0.416 ± 0.017 | 0.416 ± 0.017 | 0.417 ± 0.017 | 0.416 ± 0.017 | 0.418 ± 0.016 |
| Cress solution | ||||||
| BEA | 0.332 ± 0.008 | 0.338 ± 0.008 | 0.342 ± 0.008 | 0.342 ± 0.008 | 0.343 ± 0.008 | 0.344 ± 0.008 |
| BIF | 0.367 ± 0.010 | 0.370 ± 0.010 | 0.376 ± 0.011 | 0.376 ± 0.010 | 0.376 ± 0.010 | 0.377 ± 0.010 |
| CLE | 0.355 ± 0.009 | 0.360 ± 0.009 | 0.361 ± 0.010 | 0.362 ± 0.010 | 0.363 ± 0.009 | 0.364 ± 0.010 |
| RXU | 0.356 ± 0.007 | 0.360 ± 0.007 | 0.365 ± 0.008 | 0.366 ± 0.007 | 0.366 ± 0.007 | 0.369 ± 0.006 |
| SET | 0.360 ± 0.009 | 0.371 ± 0.010 | 0.371 ± 0.010 | 0.370 ± 0.010 | 0.370 ± 0.010 | 0.371 ± 0.010 |
| SMC | 0.390 ± 0.009 | 0.395 ± 0.004 | 0.395 ± 0.009 | 0.395 ± 0.009 | 0.395 ± 0.009 | 0.395 ± 0.009 |
| SOC | 0.448 ± 0.015 | 0.455 ± 0.015 | 0.456 ± 0.015 | 0.457 ± 0.015 | 0.458 ± 0.015 | 0.459 ± 0.015 |
| SPC | 0.408 ± 0.015 | 0.414 ± 0.015 | 0.414 ± 0.015 | 0.414 ± 0.015 | 0.414 ± 0.015 | 0.414 ± 0.015 |
| Material | Distilled Water | Red Wine | Curry Solution | Cress Solution | Sorption |
|---|---|---|---|---|---|
| BEA | 345,000 ± 8000 | 343,000 ± 12,000 | 344,000 ± 10,000 | 340,000 ± 9000 | 26.53 |
| BIF | 372,000 ± 11,000 | 377,000 ± 15,000 | 377,000 ± 11,000 | 374,000 ± 11,000 | 8.84 |
| CLE | 358,000 ± 11,000 | 364,000 ± 12,000 | 364,000 ± 8000 | 361,000 ±10,000 | 17.68 |
| RXU | 364,000 ± 9000 | 368,000 ± 12,000 | 365,000 ± 10,000 | 364,000 ± 8000 | 17.68 |
| SET | 366,000 ± 13,000 | 360,000 ± 16,000 | 368,000 ± 11,000 | 369,000 ± 10,000 | 39.79 |
| SMC | 394,000 ± 11,000 | 393,000 ± 11,000 | 393,000 ±12,000 | 394,000 ± 9000 | 26.53 |
| SOC | 440,000 ± 14,000 | 451,000 ± 19,000 | 451,000 ± 18,000 | 455,000 ± 15,000 | 35.37 |
| SPC | 416,000 ± 15,000 | 420,000 ± 17,000 | 451,000 ± 17,000 | 413,000 ± 15,000 | 26.53 |
| Storage Level (Days) | Material | Distilled Water | Red Wine | Curry Solution | Cress Solution |
|---|---|---|---|---|---|
| 7 | BEA | 1.66 ± 0.46 a | 6.7 ± 1.32 bc | 13.87 ± 3.65 b | 1.72 ± 0.64 a |
| BIF | * 4.73 ± 2.86 cd | * 7.60 ± 2.57 c | * 10.45 ± 1.93 ab | 6.77 ± 1.42 e | |
| CLE | 2.19 ± 1.00 ab | * 5.06 ± 2.03 ab | 5.67 ± 1.73 a | * 1.95 ± 1.36 ab | |
| RXU | 3.31 ± 0.74 bc | * 20.37 ± 3.07 e | 9.08 ± 2.45 ab | 2.91 ± 1.30 abc | |
| SET | 1.38 ± 0.78 a | * 12.98 ± 2.45 d | 22.55 ± 5.52 c | 3.21 ± 0.95 bc | |
| SMC | 4.51 ± 0.74 cd | 13.12 ± 2.79 d | 33.41 ± 11.44 d | 1.85 ± 0.39 a | |
| SOC | 5.69 ± 1.27 cd | 3.92 ± 0.90 a | 21.93 ± 2.62 c | 5.41 ± 1.81 d | |
| SPC | 0.84 ± 0.37 a | 6.50 ± 1.30 bc | 19.89 ± 5.36 c | 3.83 ± 1.06 c | |
| 28 | BEA | 1.79 ± 0.44 a | 22.61 ± 1.53 cd | 9.32 ± 1.58 a | * 5.42 ± 4.07 bc |
| BIF | * 3.30 ± 2.51 bc | 13.32 ± 2.14 a | 16.90 ± 4.88 b | 2.48 ± 1.39 a | |
| CLE | * 2.07 ± 1.03 ab | 10.58 ± 2.07 a | 6.38 ± 1.93 a | 2.53 ± 1.35 a | |
| RXU | * 4.20 ± 1.31 cd | 19.34 ± 2.58 bc | 18.56 ± 3.95 b | 3.16 ± 0.92 ab | |
| SET | 1.96 ± 0.53 ab | 33.71 ± 2.99 e | * 24.89 ± 4.84 c | 7.23 ± 2.46 cd | |
| SMC | 3.76 ± 0.58 cd | 23.43 ± 4.84 d | 47.55 ± 6.66 d | 2.77 ± 0.61 a | |
| SOC | 4.74 ± 1.28 d | 17.22 ± 6.33 b | 24.20 ± 2.38 c | 4.02 ± 1.22 ab | |
| SPC | 1.65 ± 0.48 a | 33.36 ± 2.25 e | 21.14 ± 2.55 bc | 9.17 ± 2.55 d | |
| 90 | BEA | 2.61 ± 0.50 b | 40.24 ± 5.08 c | 31.67 ± 4.84 d | 3.65 ± 1.09 ab |
| BIF | * 6.37 ± 2.55 d | 21.68 ± 4.31 a | 30.09 ± 6.80 d | 3.69 ± 1.43 ab | |
| CLE | 2.85 ± 1.48 b | 25.54 ± 3.00 ab | 12.14 ± 2.61 a | 2.50 ± 1.31 a | |
| RXU | 0.98 ± 0.55 a | 30.96 ± 5.21 b | 22.97 ± 5.19 bc | 12.57 ± 1.04 d | |
| SET | 4.59 ± 1.41 c | 58.09 ± 3.63 d | 23.54 ± 4.43 c | 9.81 ± 1.84 c | |
| SMC | 6.61 ± 1.03 d | 42.85 ± 6.41 c | 30.73 ± 7.74 d | 3.80 ± 1.07 ab | |
| SOC | 3.84 ± 0.84 bc | 39.19 ± 8.08 c | 18.77 ± 3.07 bc | 4.79 ± 1.10 b | |
| SPC | 5.35 ± 1.20 cd | 58.81 ± 4.10 d | 17.54 ± 1.46 ab | 11.15 ± 1.51 cd | |
| 180 | BEA | 5.51 ± 0.74 bc | 63.00 ± 6.13 d | 41.29 ± 9.81 d | 6.97 ± 0.92 c |
| BIF | * 10.49 ± 2.9 d | 43.91 ± 6.95 b | 26.45 ± 5.35 c | 4.67 ± 1.52 ab | |
| CLE | 4.81 ± 1.30 ab | 58.69 ± 6.88 cd | 15.67 ± 4.89 a | 3.20 ± 1.35 a | |
| RXU | * 6.08 ± 1.84 bc | 56.50 ± 7.07 c | 18.65 ± 1.41 ab | * 18.19 ± 2.27 e | |
| SET | 3.21 ± 0.95 a | * 76.30 ± 2.93 e | * 21.34 ± 3.33 abc | 12.64 ± 2.00 d | |
| SMC | 8.87 ± 1.43 d | 88.04 ± 2.00 f | 26.37 ± 5.99 c | * 5.42 ± 1.93 bc | |
| SOC | 4.37 ± 1.00 ab | 37.53 ± 5.70 a | 16.53 ± 2.36 a | 5.71 ± 0.77 bc | |
| SPC | 6.97 ± 1.72 c | 76.80 ± 1.22 e | 22.85 ± 1.61 bc | 13.29 ± 1.96 d | |
| 365 | BEA | 12.34 ± 1.57 c | 91.82 ± 0.63 d | 31.26 ± 4.50 c | 14.73 ± 1.77 b |
| BIF | 17.85 ± 3.16 d | * 74.97 ± 3.09 b | * 22.76 ± 4.80 b | 8.32 ± 1.20 a | |
| CLE | 8.66 ± 1.33 b | * 78.62 ± 2.17 bc | 15.57 ± 3.84 a | 8.08 ± 1.60 a | |
| RXU | 13.00 ± 1.69 c | 78.97 ± 1.41 c | 20.68 ± 1.33 b | 18.78 ± 1.97 c | |
| SET | * 4.40 ± 0.77 a | * 78.18 ± 1.29 bc | * 20.28 ± 3.95 b | 14.34 ± 2.13 b | |
| SMC | 12.31 ± 1.43 c | 91.14 ± 0.51 d | 28.05 ± 5.03 c | 7.01 ± 2.03 a | |
| SOC | 6.41 ± 1.39 a | 64.17 ± 8.51 a | 16.12 ± 1.84 a | 6.47 ± 1.68 a | |
| SPC | 8.95 ± 1.94 b | 77.92 ± 0.78 bc | 21.21 ± 1.74 b | 16.84 ± 2.05 c |
| Material | Distilled Water | Red Wine | Curry Solution | Cress Solution |
|---|---|---|---|---|
| BEA | * 7.64 ± 2.86 | * 32.23 ± 3.33 | 14.85 ± 12.13 | 5.36 ± 0.72 |
| BIF | 17.22 ± 3.08 | * 25.23 ± 2.95 | * 17.84 ± 6.10 | * 7.54 ± 1.07 |
| CLE | 11.17 ± 1.07 | 27.32 ± 4.52 | * 18.24 ± 5.84 | 8.94 ± 1.63 |
| RXU | 14.25 ± 1.88 | 19.16 ± 1.35 | 14.37 ± 1.19 | 18.01 ± 1.47 |
| SET | 6.47 ± 1.36 | 30.74 ± 2.24 | * 14.49 ± 2.50 | * 10.86 ± 1.58 |
| SMC | 12.10 ± 2.33 | 20.85 ± 3.06 | * 17.57 ± 4.34 | 6.77 ± 0.95 |
| SOC | 9.52 ± 1.33 | 23.97 ± 2.28 | 14.39 ± 2.42 | 6.81 ± 1.51 |
| SPC | 7.58 ± 2.19 | 21.70 ± 2.28 | 12.31 ± 1.58 | 8.87 ± 1.36 |
| Material | Distilled Water | Red Wine | Curry Solution | Cress Solution |
|---|---|---|---|---|
| BEA | * 4.70 ± 0.84 | * 59.58 ± 0.87 | * 16.41 ± 3.34 | * 9.37 ± 0.49 |
| BIF | 0.63 ± 1.14 | * 49.73 ± 1.10 | * 4.92 ± 2.01 | 0.78 ± 0.42 |
| CLE | * −2.51 ± 0.44 | * 51.30 ± 1.29 | −2.67 ± 1.80 | −0.86 ± 0.59 |
| RXU | −1.25 ± 0.65 | * 59.81 ± 0.50 | * 6.31 ± 0.46 | 0.77 ± 0.63 |
| SET | * −2,07 ± 0.40 | * 47.44 ± 0.67 | * 5.79 ± 1.21 | * 3.48 ± 0.69 |
| SMC | 0.21 ± 0.71 | * 70.29 ± 0.80 | * 10.48 ± 1.72 | 0.24 ± 0.58 |
| SOC | * −3.10 ± 0.50 | * 40.20 ± 2.27 | * 1.73 ± 0.79 | −0.34 ± 0.58 |
| SPC | 1.37 ± 0.76 | * 56.22 ± 0.62 | * 8.89 ± 0.61 | * 7.97 ± 0.64 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebermann, A.; Roos, M.; Stawarczyk, B. The Effect of Different Storage Media on Color Stability of Self-Adhesive Composite Resin Cements for up to One Year. Materials 2017, 10, 300. https://doi.org/10.3390/ma10030300
Liebermann A, Roos M, Stawarczyk B. The Effect of Different Storage Media on Color Stability of Self-Adhesive Composite Resin Cements for up to One Year. Materials. 2017; 10(3):300. https://doi.org/10.3390/ma10030300
Chicago/Turabian StyleLiebermann, Anja, Malgorzata Roos, and Bogna Stawarczyk. 2017. "The Effect of Different Storage Media on Color Stability of Self-Adhesive Composite Resin Cements for up to One Year" Materials 10, no. 3: 300. https://doi.org/10.3390/ma10030300







