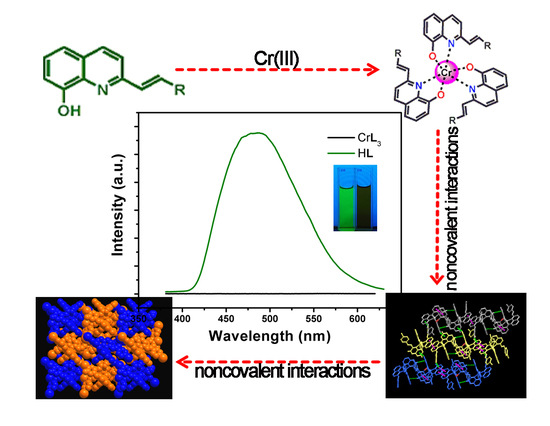
Two 8-Hydroxyquinolinate Based Supramolecular Coordination Compounds: Synthesis, Structures and Spectral Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
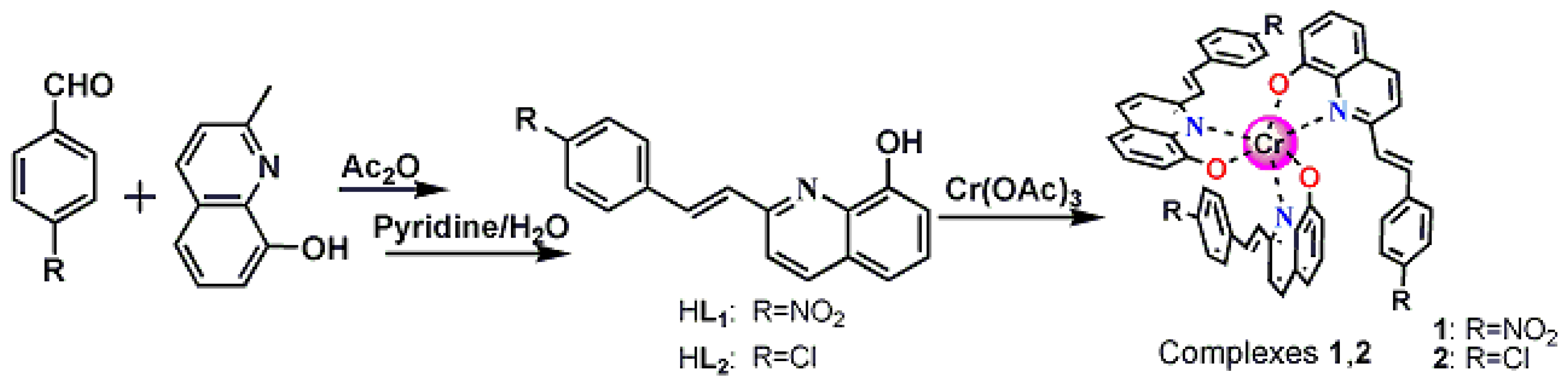
2.2. Synthesis of 1
2.3. Synthesis of 2
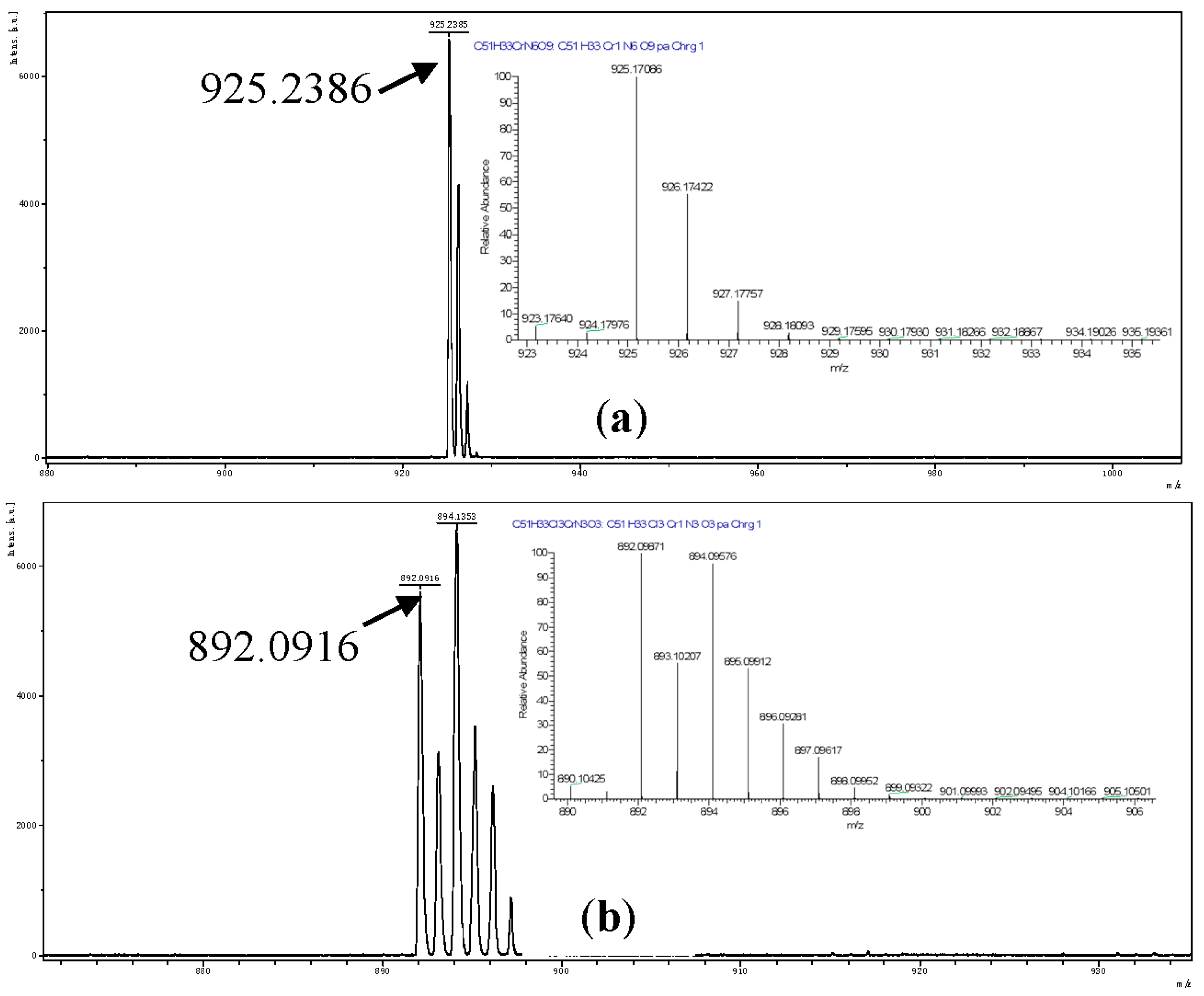
2.4. X-ray Crystallography
3. Results and Discussion
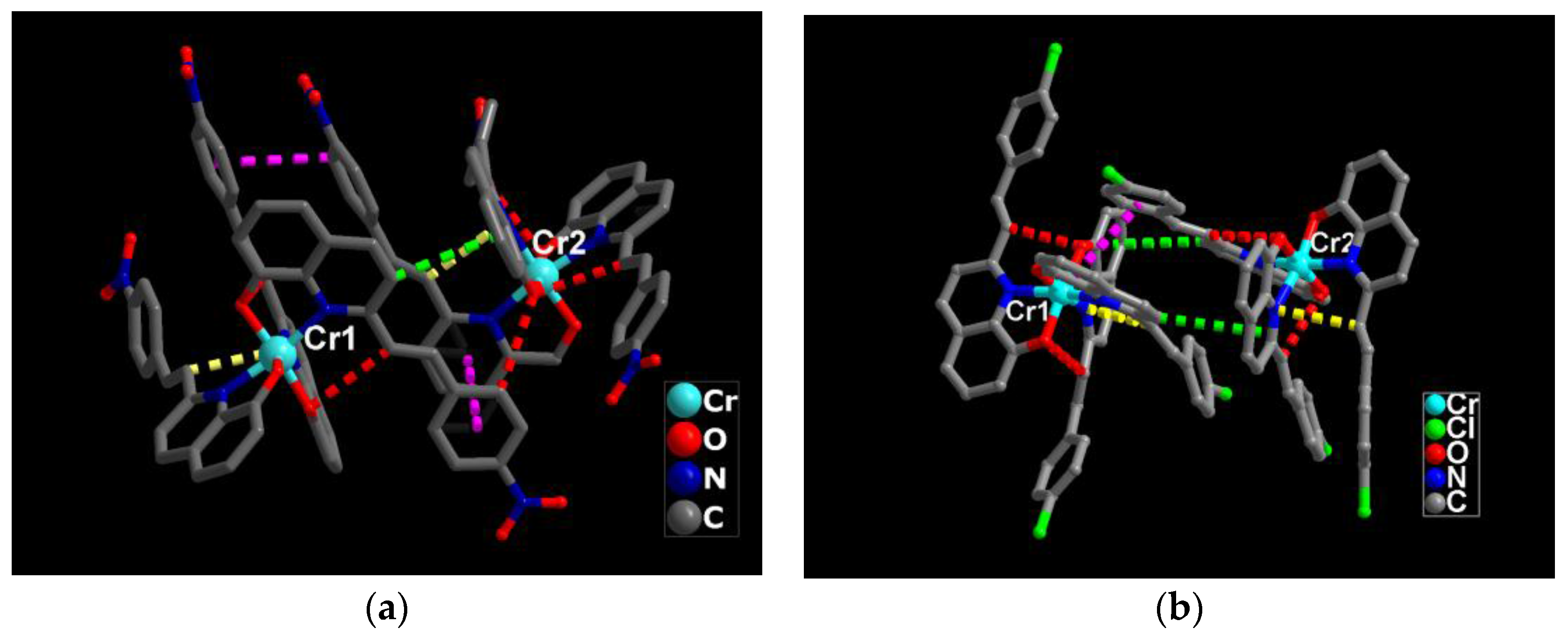
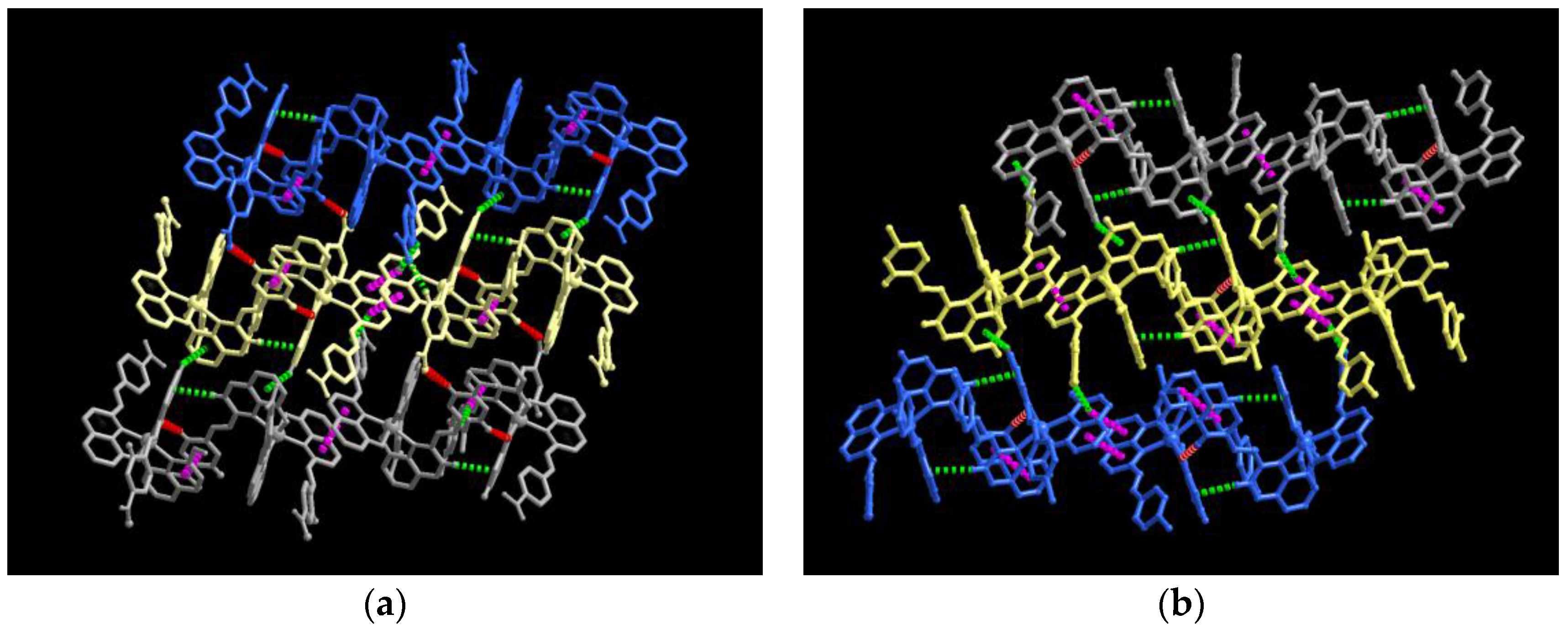
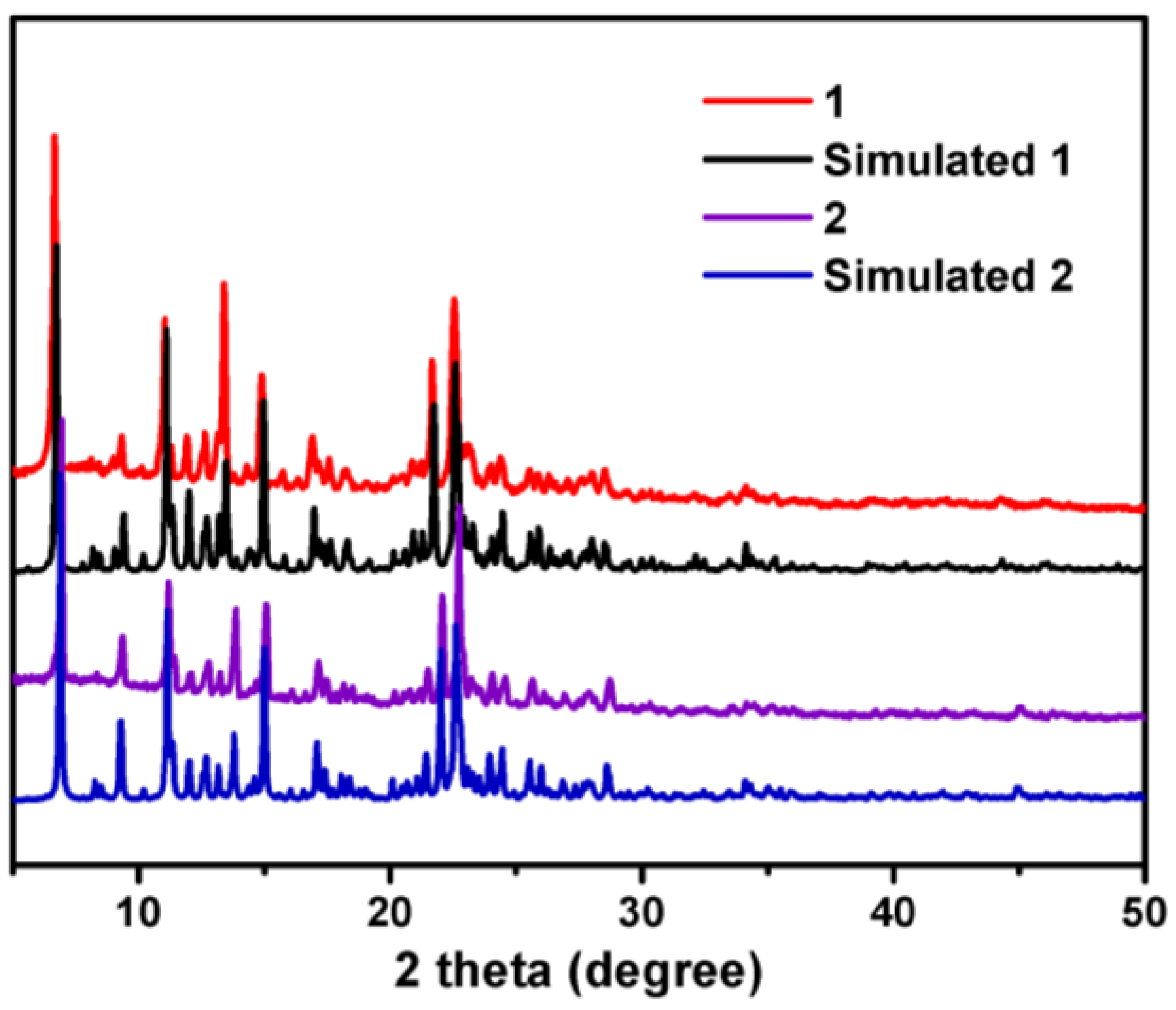
3.1. Single Crystal Structures
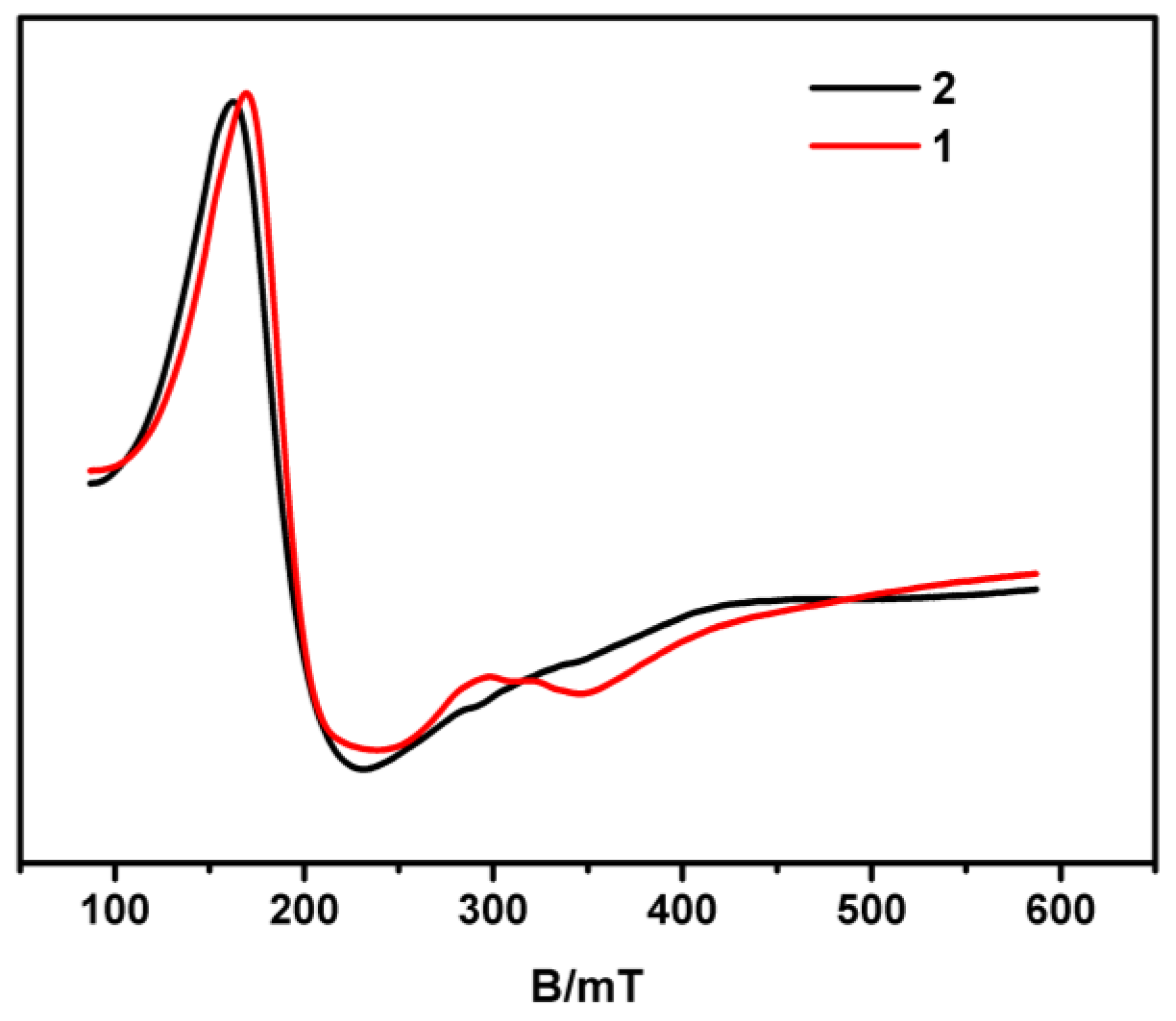
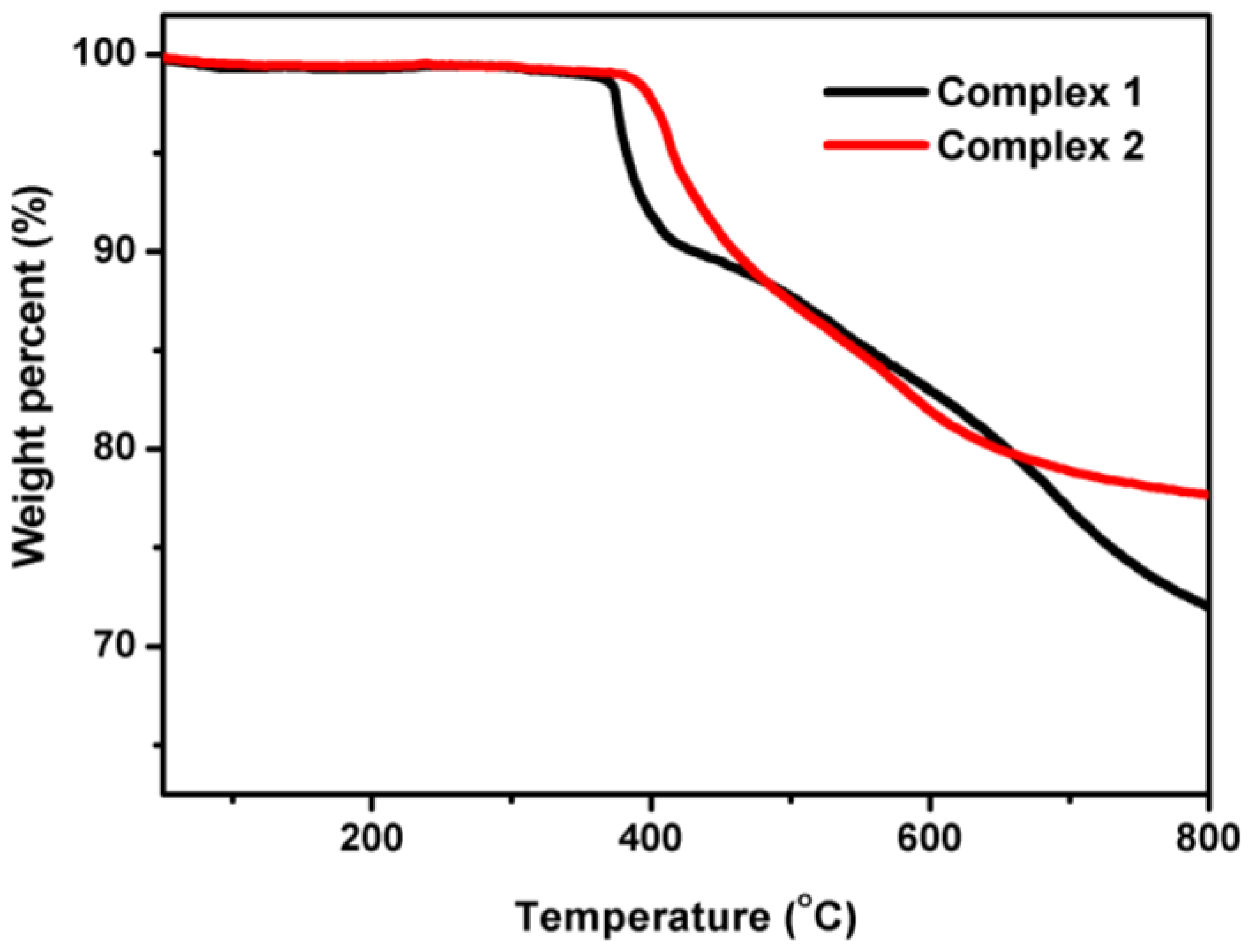
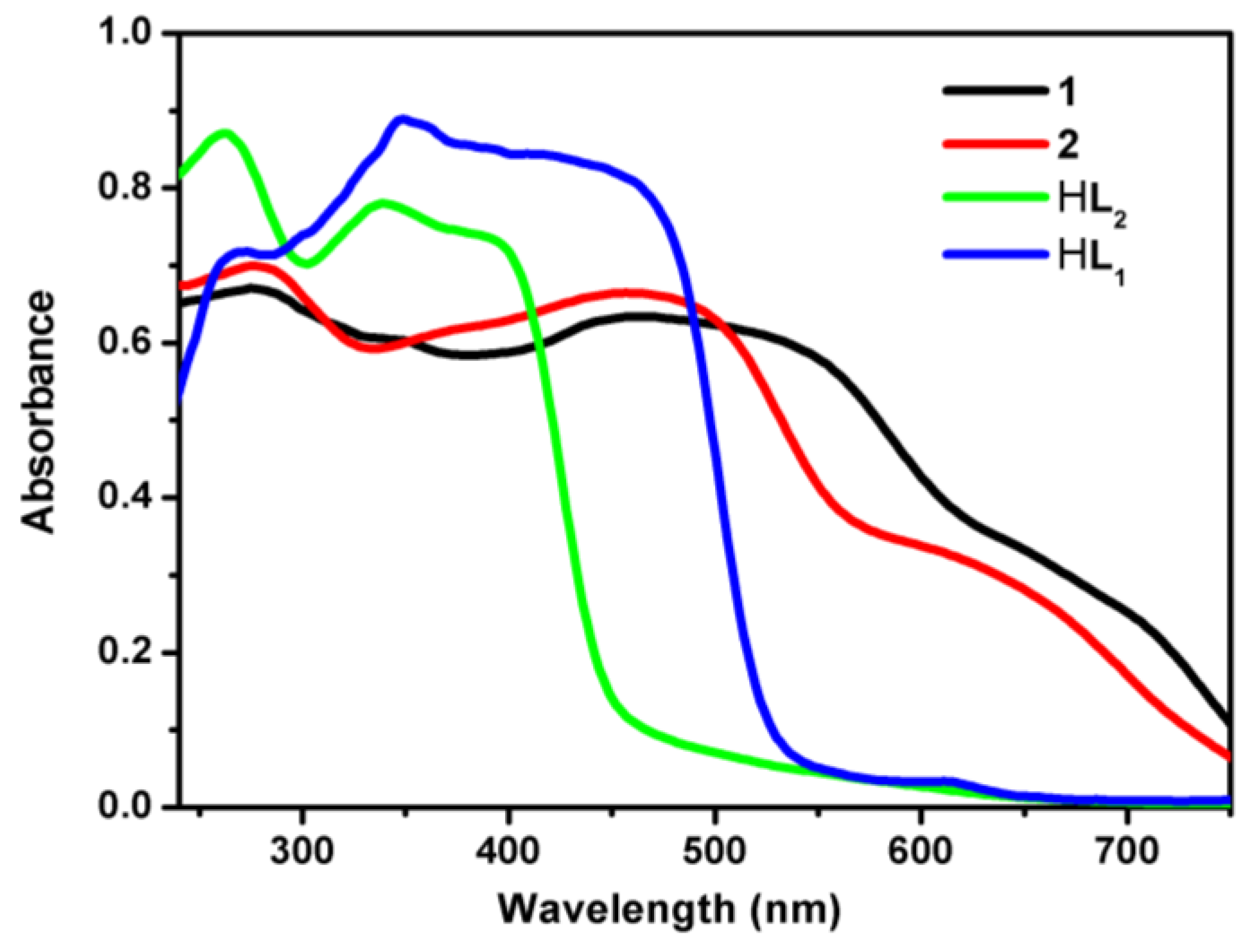
3.2. The stability of 1 and 2
3.3. The IR spectra of 1 and 2
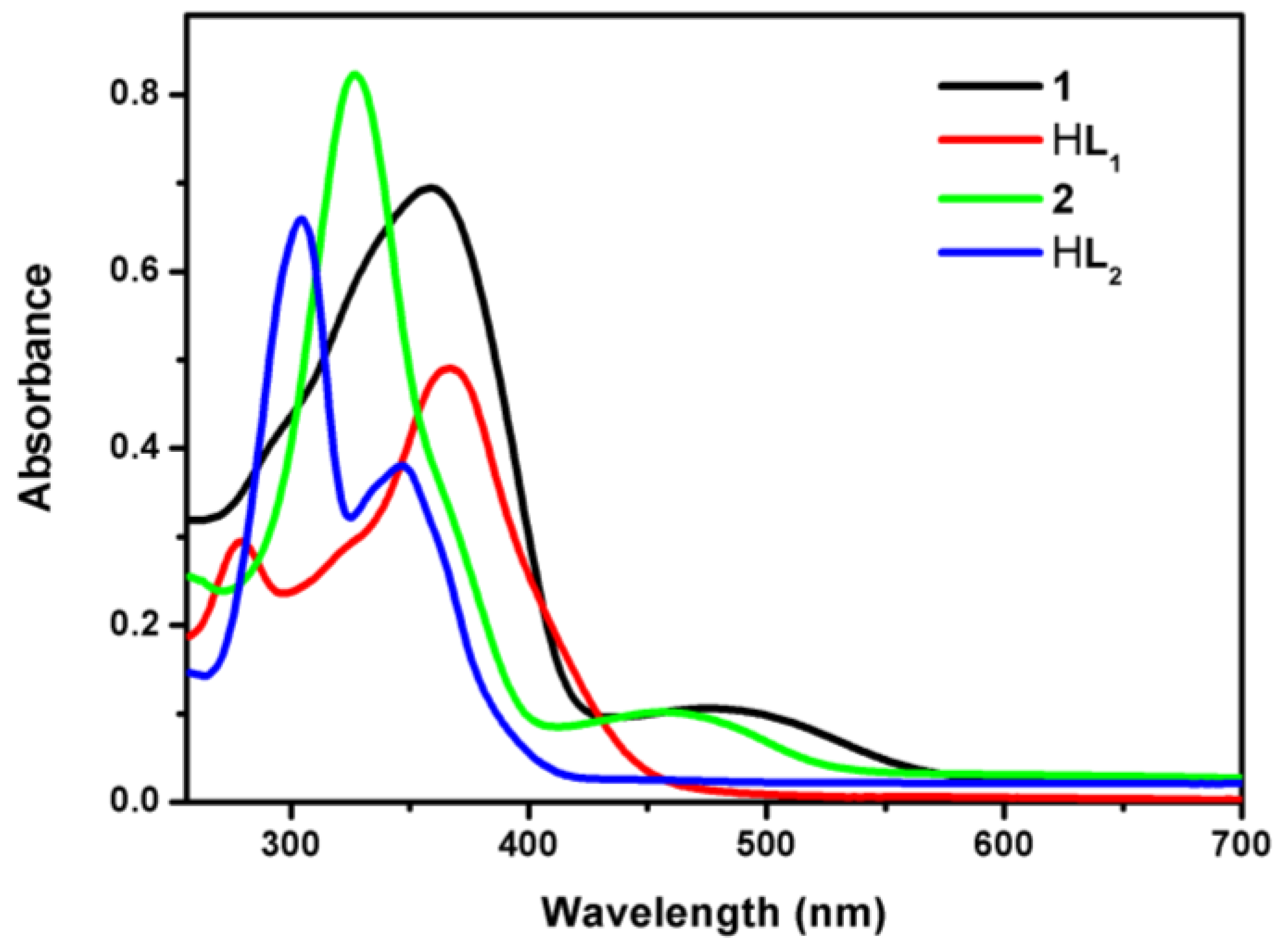
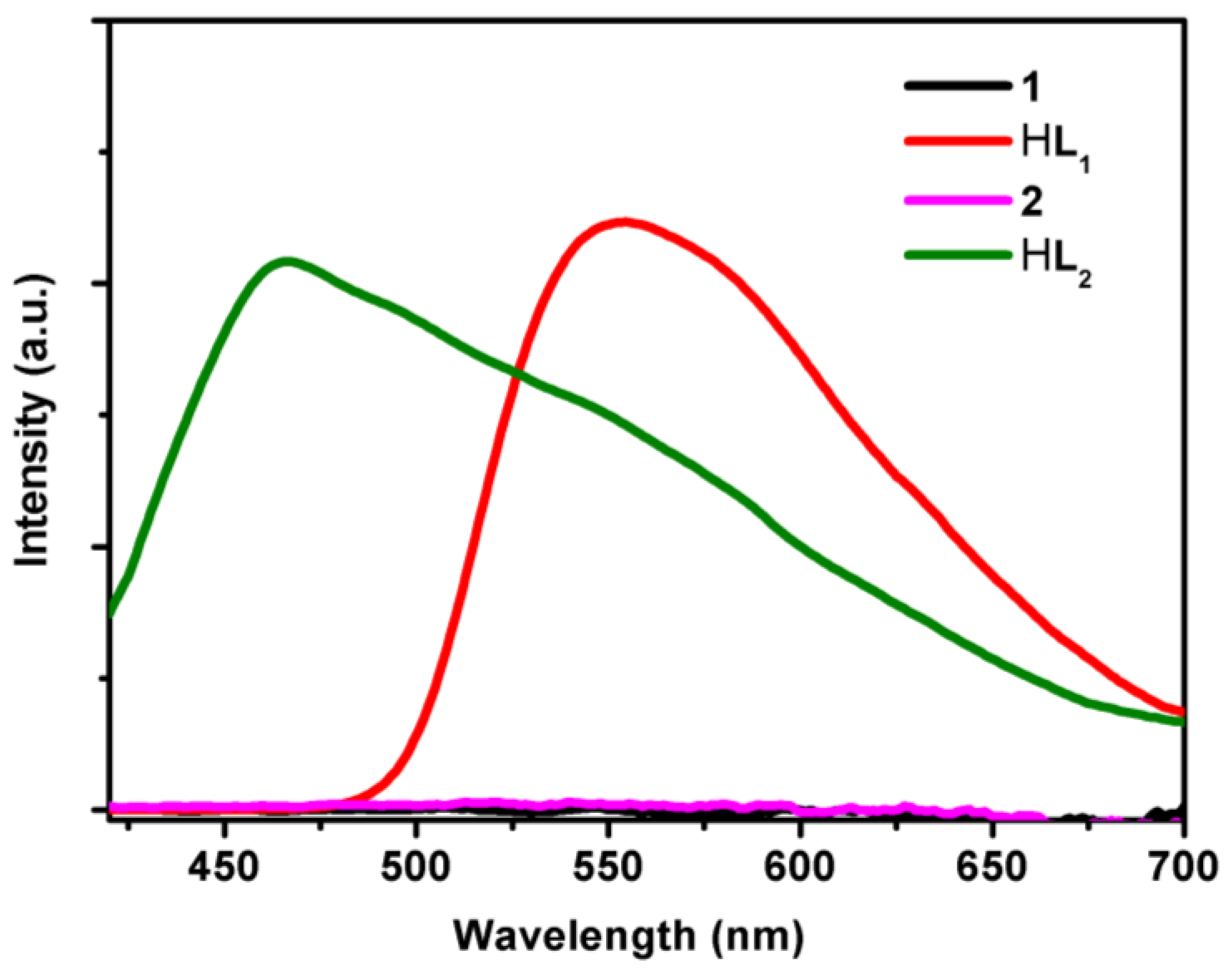
3.4. UV/Visible Absorption and Fluorescence Emission Spectra Studies
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Song, Y.N.; Xu, H.; Chen, W.M.; Zhan, P.; Liu, X.Y. 8-hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. Med. Chem. Comm. 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Garrison, A.T.; Abouelhassan, Y.; Yang, H.; Yousaf, H.H.; Nguyen, T.J.; Huigens Iii, R.W. Microwave-enhanced friedlander synthesis for the rapid assembly of halogenated quinolines with antibacterial and biofilm eradication activities against drug resistant and tolerant bacteria. Med.Chem.Comm. 2017. [Google Scholar] [CrossRef]
- Cheng, J.A.; Chen, C.H.; Liao, C.H. Solution-processible small molecular organic light-emitting diode material and devices based on the substituted aluminum quinolate. Chem. Mater. 2004, 16, 2862–2868. [Google Scholar] [CrossRef]
- Filik, H.; Hayvali, M.; Kilic, E.; Apak, R.; Aksu, D.; Yanaz, Z.; Cengel, T. Development of an optical fibre reflectance sensor for p-aminophenol detection based on immobilised bis-8-hydroxyquinoline. Talanta 2008, 77, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Memari, Z.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Highly sensitive gold nanoparticles-based optical sensing of DNA hybridization using bis(8-hydroxyquinoline-5-solphonate)cerium(III) chloride as a novel fluorescence probe. J. Pharm. Biomed. 2016, 118, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Sun, L.Y.; Li, X.Y.; Tian, Y.L.; Yuan, G.Z. Five 8-hydroxyquinolinate-based coordination polymers with tunable structures and photoluminescent properties for sensing nitroaromatics. Dalton Trans. 2015, 44, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, K.; Hemamalini, M.; Francis, S.; Muthiah, P.T.; Vijay, T.; Row, T.N.G.; Bocelli, G.; Cantoni, A. Supramolecular organization in tetra aqua (µ-8-hydroxyquinoline-5-sulfonate) barium (II) and Ag···I interactions in a pseudopolymorphic form of (7-iodo-8-hydroxyquinoline-5-sulfonate) silver (I) monohydrate. J. Chem. Crystallogr. 2010, 40, 316–322. [Google Scholar] [CrossRef]
- Yuan, G.; Huo, Y.; Nie, X.; Jiang, H.; Liu, B.; Fang, X.; Zhao, F. Controllable supramolecular structures and luminescent properties of unique trimeric Zn(II) 8-hydroxyquinolinates tuned by functional substituents. Dalton Trans. 2013, 42, 2921–2929. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Lu, J.; Lu, T.; Fang, X.; Ouyang, X.; Zhang, L.; Yuan, G. Comparative studies on oled performances of chloro and fluoro substituted Zn(II) 8-hydroxyquinolinates. New J. Chem. 2015, 39, 333–341. [Google Scholar] [CrossRef]
- Yuan, G.; Rong, L.; Qiao, X.; Ma, L.; Wei, X. Anion-controlled structures and luminescent properties of three Cd(II) complexes assembled by a 2-substituted 8-hydroxyquinoline ligand. Cryst. Eng. Comm. 2013, 15, 7307–7314. [Google Scholar] [CrossRef]
- Xu, H.-B.; Zhong, Y.-T.; Zhang, W.-X.; Chen, Z.-N.; Chen, X.-M. Syntheses, structures and photophysical properties of heterotrinuclear Zn2Ln clusters (Ln = Nd, Eu, Tb, Er, Yb). Dalton Trans. 2010, 39, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E. Understanding substituent effects in noncovalent interactions involving aromatic rings. Acc. Chem. Res. 2013, 46, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Jiang, F.; Xu, Y.; Yuan, D.; Chen, L.; Yan, C.; Hong, M. The aggregations and strong emissions of d8 and d10 metal–8-hydroxyquinaldine complexes. Cryst. Growth Des. 2008, 8, 2721–2728. [Google Scholar] [CrossRef]
- Yuan, G.; Rong, L.; Yue, C.; Wei, X. Supramolecular assembly of two two-folded helical structures based on 2-substituted 8-hydroxyquinoline complexes. Inorg. Chem. Commun. 2013, 33, 19–24. [Google Scholar] [CrossRef]
- Huo, Y.P.; Wang, C.Q.; Lu, J.G.; Hu, S.; Li, X.Y.; Zhang, L. A novel trimeric Zn (II) complex based on 8-hydroxyquinoline with trifluoromethylbenzene group: Synthesis, crystal structure, photophysical properties and DNA binding. J. Mol. Struct. 2015, 1098, 311–317. [Google Scholar] [CrossRef]
- Bakewell, C.; Fateh-Iravani, G.; Beh, D.W.; Myers, D.; Tabthong, S.; Hormnirun, P.; White, A.J.P.; Long, N.; Williams, C.K. Comparing a series of 8-quinolinolato complexes of aluminium, titanium and zinc as initiators for the ring-opening polymerization of rac-lactide. Dalton Trans. 2015, 44, 12326–12337. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.L.; Shan, X.H.; Li, X.Y.; Liu, J.; Zhang, L.Y.; Yuan, G.A. Self-assembly and luminescent properties of one novel tetranuclear Cd(II) complex based on 8-hydroxyquinolinate ligand. Inorg. Chem. Commun. 2014, 48, 131–135. [Google Scholar] [CrossRef]
- Baranov, E.V.; Fukin, G.K.; Balashova, T.V.; Pushkarev, A.P.; Grishin, I.D.; Bochkarev, M.N. 8-quinolinolate complexes of yttrium and ytterbium: Molecular arrangement and fragmentation under laser impact. Dalton Trans. 2013, 42, 15699–15705. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.-K.; Zhang, Y.-H.; Huang, J.; Liu, B.; Zheng, L.-M. Isostructural lanthanide oxalatophosphonates Ln(5pm8hqh3)(C2O4)1.5(H2O)·2H2O [Ln(III) = Eu, Gd, Tb, Dy] (5pm8hqh3 = 5-phosphonomethyl-8-hydroxyquinoline): Structures, magnetic and fluorescent properties. Rsc. Adv. 2012, 2, 6680–6685. [Google Scholar] [CrossRef]
- Wang, W.-M.; Qiao, W.-Z.; Zhang, H.-X.; Wang, S.-Y.; Nie, Y.-Y.; Chen, H.-M.; Liu, Z.; Gao, H.-L.; Cui, J.-Z.; Zhao, B. Structures and magnetic properties of several phenoxo-o bridged dinuclear lanthanide complexes: Dy derivatives displaying substituent dependent magnetic relaxation behavior. Dalton Trans. 2016, 45, 8182–8191. [Google Scholar] [CrossRef] [PubMed]
- Sapochak, L.S.; Benincasa, F.E.; Schofield, R.S.; Baker, J.L.; Riccio, K.K.C.; Fogarty, D.; Kohlmann, H.; Ferris, K.F.; Burrows, P.E. Electroluminescent Zinc(II) bis(8-hydroxyquinoline): Structural effects on electronic states and device performance. J. Am. Chem. Soc. 2002, 124, 6119–6125. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, P.S.; Ford, P.C. Metal centered ligand field excited states: Their roles in the design and performance of transition metal based photochemical molecular devices. Coordin. Chem. Rev. 2011, 255, 591–616. [Google Scholar] [CrossRef]
- Forster, L.S. Excited state relaxation of Cr(III) in oxygen environments. Coordin. Chem. Rev. 2004, 248, 261–272. [Google Scholar] [CrossRef]
- Omar, W.A.E.; Hormi, O.E.O. Synthesis of 4-(2-arylvinyl)-8-hydroxyquinolines via anhydrous heck coupling reaction and the pl properties of their al complexes. Tetrahedron 2009, 65, 4422–4428. [Google Scholar] [CrossRef]
- Barberis, V.P.; Mikroyannidis, J.A. Synthesis and optical properties of aluminum and zinc quinolates through styryl subsituent in 2-position. Synth. Met. 2006, 156, 865–871. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Zhang, X.; Tian, J.; Huang, G. The correlation between magnetism and structures for new solvent free iron (III) and chromium (III) homoleptic 8-quinolinato complexes. Inorg. Chim. Acta 2011, 366, 241–246. [Google Scholar] [CrossRef]
- Lima, C.; Taveira, R.J.S.; Costa, J.C.S.; Fernandes, A.M.; Melo, A.; Silva, A.M.S.; Santos, L. Understanding M-ligand bonding and mer-/fac-isomerism in tris(8-hydroxyquinolinate) metallic complexes. Phys. Chem. Chem. Phys. 2016, 18, 16555–16565. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gong, C.; Zeng, X.; Zhang, J.; Zhu, C.; Xie, J. Three multinuclear metal–organic coordination compounds based on 8-hydroxyquinoline derivative: Syntheses, structures and fluorescence properties. Polyhedron 2015, 102, 562–568. [Google Scholar] [CrossRef]
- Zangana, K.H.; Pineda, E.M.; Vitorica-Yrezabal, I.J.; McInnes, E.J.; Winpenny, R.E. Linking Cr3 triangles through phosphonates and lanthanides: Synthetic, structural, magnetic and epr studies. Dalton Trans. 2014, 43, 13242–13249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Suresh, R.; Giribabu, K.; Manigandan, R.; Munusamy, S.; Muthamizh, S.; Narayanan, V. Synthesis and characterization of chromium(III) schiff base complexes: Antimicrobial activity and its electrocatalytic sensing ability of catechol. Spectrochim. Acta A 2015, 139, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Spek, A. Single-crystal structure validation with the program platon. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, W.; Shan, X.; Li, X.; Liu, J.; Zhang, L.; Yuan, G. Self-assembly and luminescent properties of one novel tetranuclear Cd(II) complex based on 8-hydroxyquinolinate ligand. Inorg. Chem. Commun. 2014, 48, 131–135. [Google Scholar] [CrossRef]
- Freitas, A.R.; Silva, M.; Ramos, M.L.; Justino, L.L.G.; Fonseca, S.M.; Barsan, M.M.; Brett, C.M.A.; Silva, M.R.; Burrows, H.D. Synthesis, structure, and spectral and electrochemical properties of chromium(III) tris-(8-hydroxyquinolinate). Dalton Trans. 2015, 44, 11491–11503. [Google Scholar] [CrossRef] [PubMed]
- El-Megharbel, S.M.; Refat, M.S. Ligational behavior of clioquinol antifungal drug towards Ag(I), Hg(II), Cr(III) and Fe(III) metal ions: Synthesis, spectroscopic, thermal, morphological and antimicrobial studies. J. Mol. Struct. 2015, 1085, 222–234. [Google Scholar] [CrossRef]
- Wen, W. Gadolinium complex of schiff base as efficient suppression ratio for hydroxyl radical. Asian J. Chem. 2013, 25, 8307–8310. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Gao, B.J.; Hou, X.D. Twofold influence of nitro substituent on aromatic ring for photoluminescence properties of benzoic acid-functionalized polystyrene and Eu(III) complexes. Acta Phys. Chim. Sin. 2014, 30, 745–752. [Google Scholar]
- Praveen, L.; Reddy, M.L.P.; Varma, R.L. Dansyl-styrylquinoline conjugate as divalent iron sensor. Tetrahedron Lett. 2010, 51, 6626–6629. [Google Scholar] [CrossRef]
- Ramos, M.L.; Justino, L.L.G.; Fonseca, S.M.; Burrows, H.D. NMR, DFT and luminescence studies of the complexation of V(v) oxoions in solution with 8-hydroxyquinoline-5-sulfonate. New J. Chem. 2015, 39, 1488–1497. [Google Scholar] [CrossRef]
- Wang, D.-H.; Zhang, Y.; Sun, R.; Zhao, D.-Z. Dimethyl yellow-based colorimetric chemosensors for “naked eye” detection of Cr3+ in aqueous media via test papers. RSC Adv. 2016, 6, 4640–4646. [Google Scholar] [CrossRef]



| Identification Code | 1 | 2 |
|---|---|---|
| Empirical formula | C102H66Cr2N12O18 | C102H66Cl6Cr2N6O6 |
| Formula weight | 1851.67 | 1788.31 |
| Temperature (K) | 296(2) K | 296(2) K |
| Crystal system | triclinic | triclinic |
| Space group | P-1 | P-1 |
| Unit cell dimensions | a = 13.1901(16) Å | a = 12.884(3) Å |
| b = 16.133(2) Å | b = 16.175(3) Å | |
| c = 20.623(3) Å | c = 20.794(4) Å | |
| α = 77.999(2)° | α = 77.349(3)° | |
| β = 86.580(2)° | β = 87.155(2)° | |
| γ = 85.524(2)° | γ = 84.676(3)° | |
| Volume (Å3), Z | 4275.2(1), 2 | 4208.1(2), 2 |
| Density (calculated) (mg/m3) | 1.438 | 1.411 |
| F(000) | 1908 | 1836 |
| Limiting indices | −17≤ h ≤ 16, −20 ≤ k ≤ 21, −18 ≤ l ≤ 26 | −15 ≤ h ≤ 15, −19 ≤ k ≤ 17, −21 ≤ l ≤24 |
| Reflections collected | 27000 | 21571 |
| Independent reflections | 19127 [R(int) = 0.0261] | 14500 [R(int) = 0.0271] |
| Completeness to theta | 25.00/98.2% | 25.00/97.7% |
| Data/restraints/parameters | 19127/0/1207 | 14500/3/1119 |
| Goodness-of-fit on F2 | 0.970 | 1.034 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0570, wR2 = 0.1379 | R1 = 0.0497, wR2 = 0.1224 |
| R indices (all data) | R1 = 0.1197, wR2 = 0.1707 | R1 = 0.0858, wR2 = 0.1418 |
| Largest diff. peak and hole (e/Å−3) | 0.339 and −0.302 | 0.405 and −0.307 |
| Complex 1 | Complex 2 | ||||||
|---|---|---|---|---|---|---|---|
| Bond | Dist. | Bond | Dist. | Bond | Dist. | Bond | Dist. |
| Cr(1)-O(9) | 1.9176(2) | Cr(1)-N(1) | 2.130(2) | Cr(1)-O(3) | 1.916(2) | Cr(1)-N(1) | 2.122(2) |
| Cr(1)-O(3) | 1.938(2) | Cr(1)-N(3) | 2.172(2) | Cr(1)-O(1) | 1.934(2) | Cr(1)-N(3) | 2.133(2) |
| Cr(1)-O(6) | 1.949(2) | Cr(1)-N(5) | 2.102(2) | Cr(1)-O(2) | 1.945(2) | Cr(1)-N(2) | 2.196(3) |
| Cr(2)-O(12) | 1.920(2) | Cr(2)-N(11) | 2.121(2) | Cr(2)-O(6) | 1.918(2) | Cr(2)-N(6) | 2.098(2) |
| Cr(2)-O(18) | 1.943(2) | Cr(2)-N(7) | 2.140(2) | Cr(2)-O(4) | 1.935(2) | Cr(2)-N(4) | 2.135(2) |
| Cr(2)-O(15) | 1.944(2) | Cr(2)-N(9) | 2.186(2) | Cr(2)-O(5) | 1.958(2) | Cr(2)-N(5) | 2.175(2) |
| 1 | 2 | Assignments |
|---|---|---|
| 3410 | 3408 | ν (trace H2O in the KBr discs) |
| 3058, 2926 | 3059, 2925 | ν (aromatic and vinylic C-H) |
| 1595 | 1593 | ν (C=N) |
| 1539~1575 | 1536~1573 | ν (C=C) |
| 1276, 1242, 1208 | 1275, 1243, 1205 | ν (C-O) |
| 548 | 552 | ν (Cr-O) |
| 497 | 501 | ν (Cr-N) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Wang, Y.; Mao, Q.; Li, F.; Li, Y.; Chen, C. Two 8-Hydroxyquinolinate Based Supramolecular Coordination Compounds: Synthesis, Structures and Spectral Properties. Materials 2017, 10, 313. https://doi.org/10.3390/ma10030313
Zhu C, Wang Y, Mao Q, Li F, Li Y, Chen C. Two 8-Hydroxyquinolinate Based Supramolecular Coordination Compounds: Synthesis, Structures and Spectral Properties. Materials. 2017; 10(3):313. https://doi.org/10.3390/ma10030313
Chicago/Turabian StyleZhu, Chengfeng, Yunfei Wang, Qingqing Mao, Fang Li, Yougui Li, and Changle Chen. 2017. "Two 8-Hydroxyquinolinate Based Supramolecular Coordination Compounds: Synthesis, Structures and Spectral Properties" Materials 10, no. 3: 313. https://doi.org/10.3390/ma10030313