The Passive Film Growth Mechanism of New Corrosion-Resistant Steel Rebar in Simulated Concrete Pore Solution: Nanometer Structure and Electrochemical Study
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Electrochemical Measurements
2.3. Surface Analysis
3. Results
3.1. Electrochemical Results
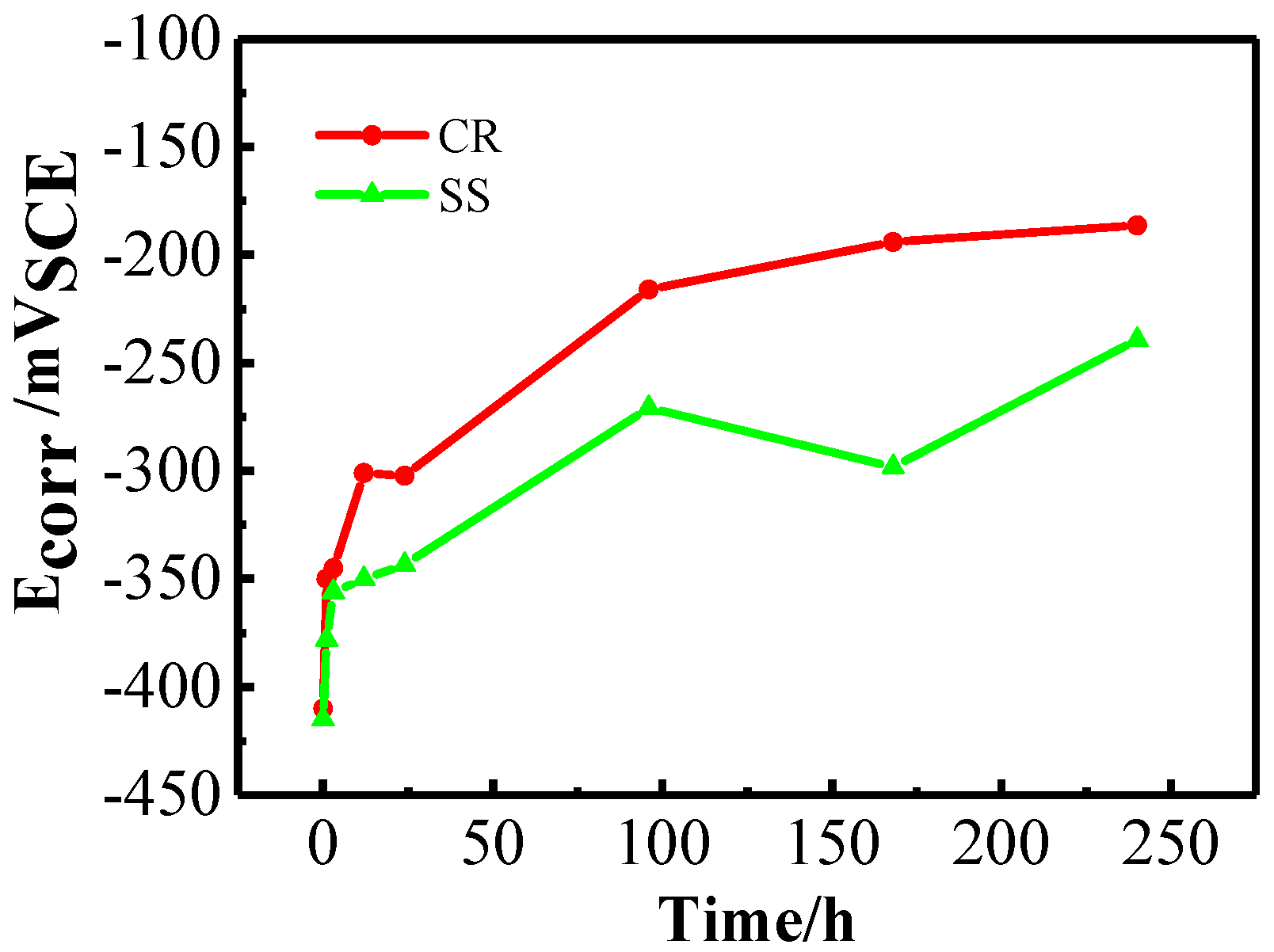
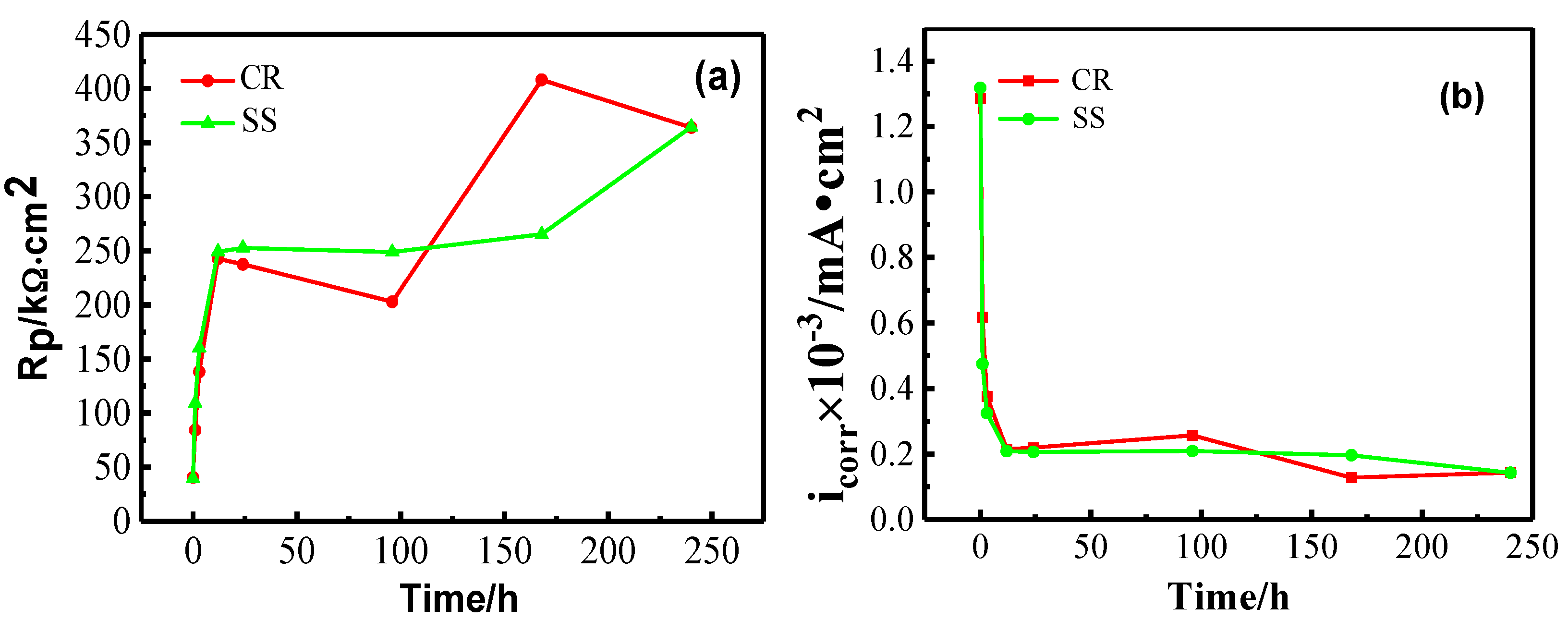
3.1.1. Open Circuit Potential (OCP) and Linear Polarization Resistance (LPR) Measurements
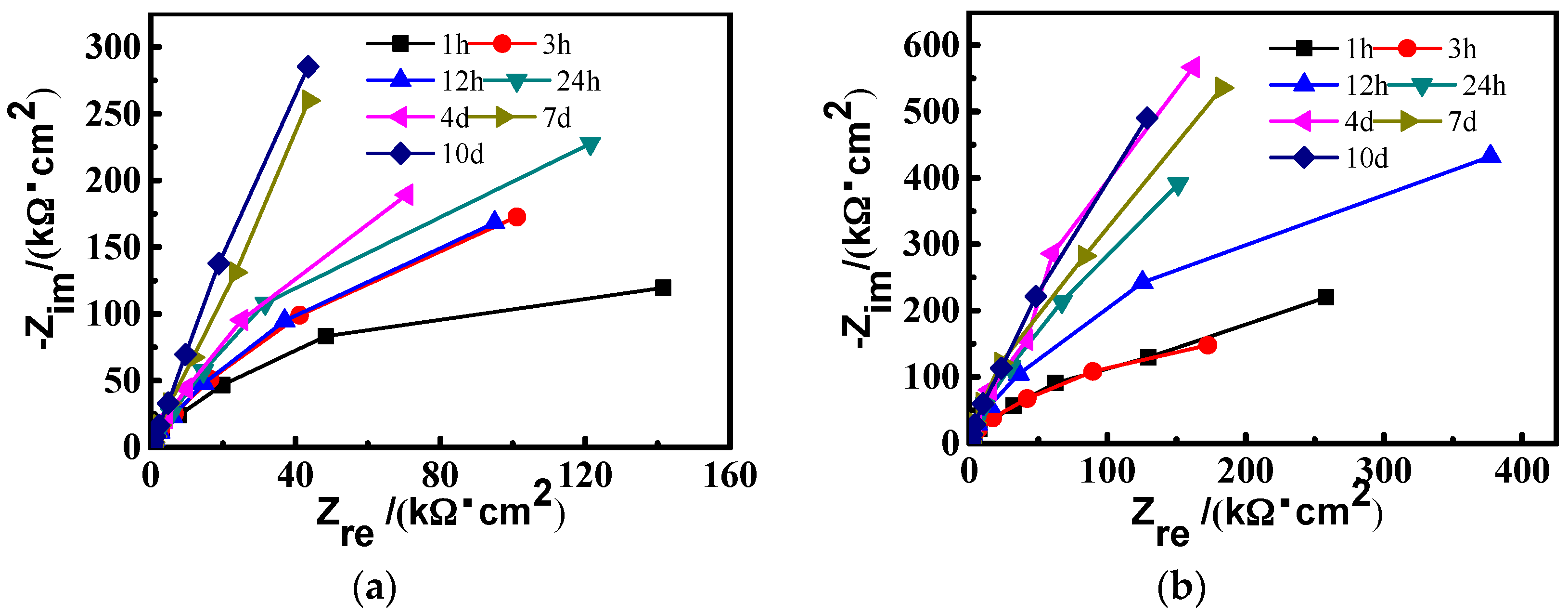
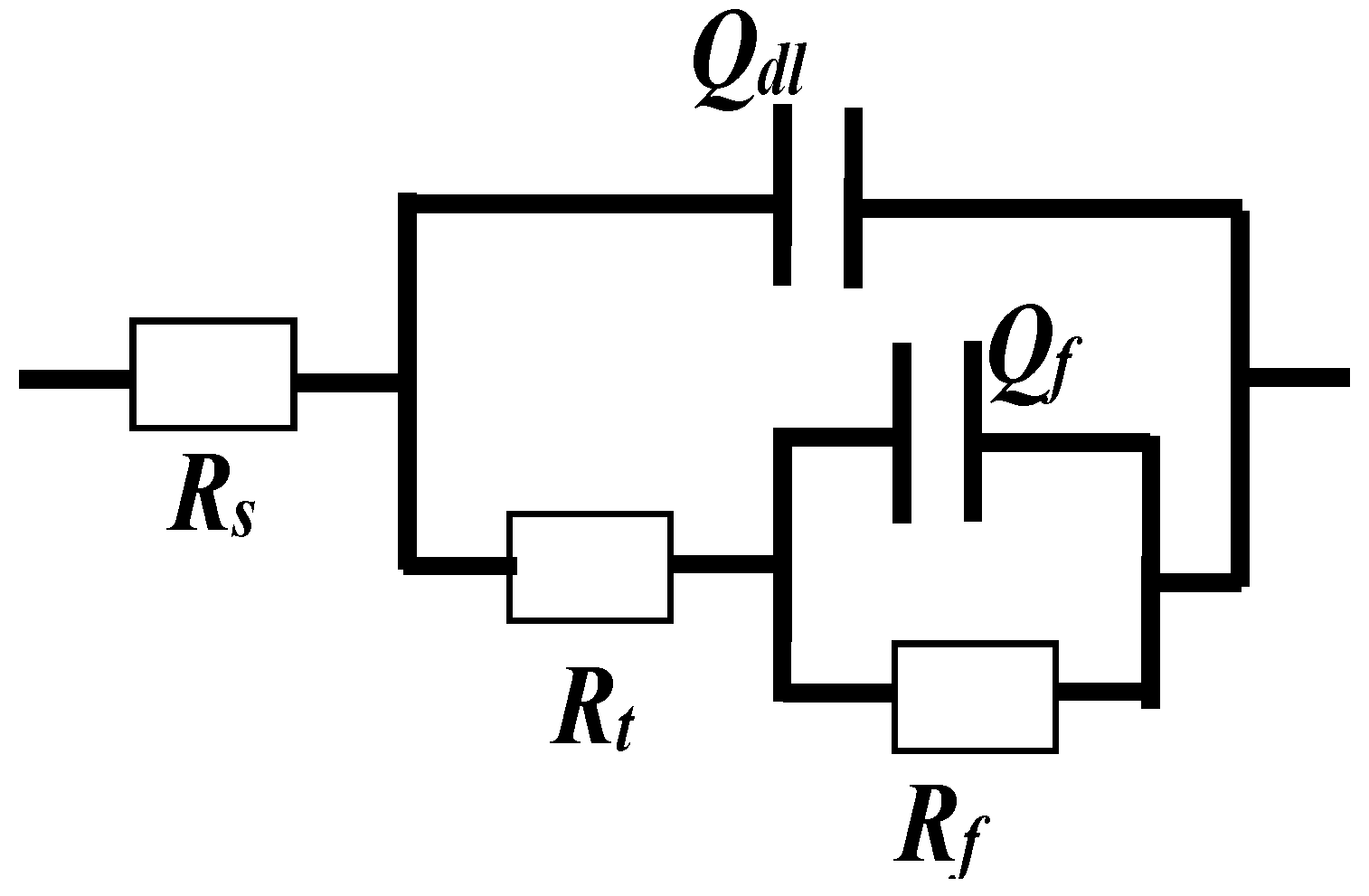
3.1.2. Electrochemical Impedance Spectroscopy (EIS) Measurements
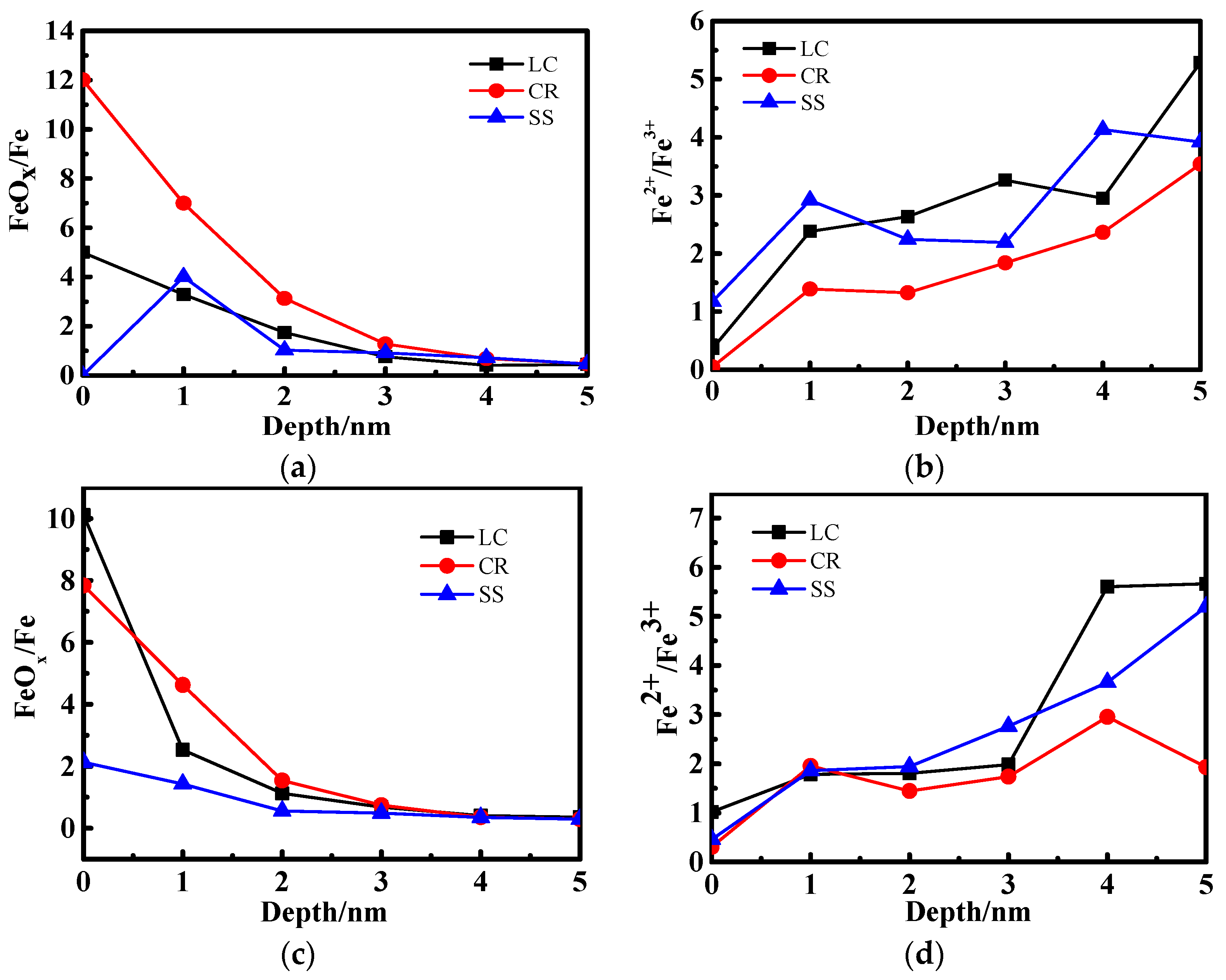
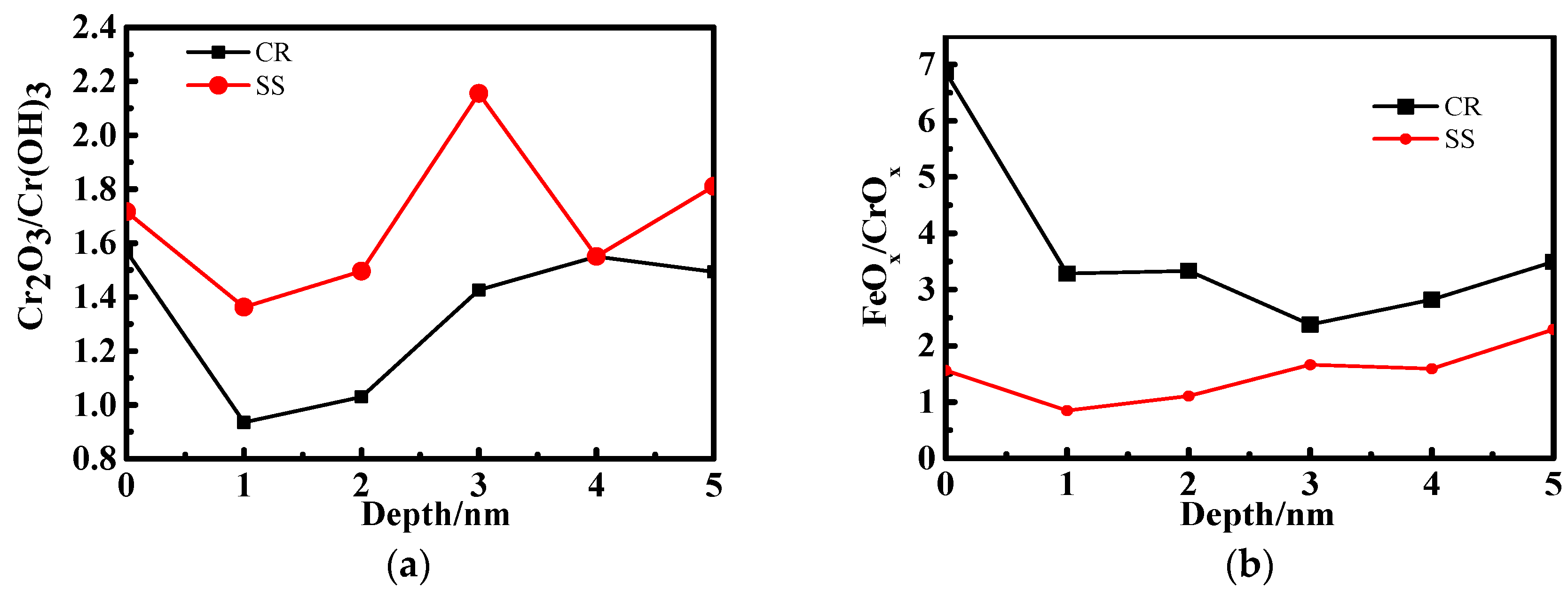
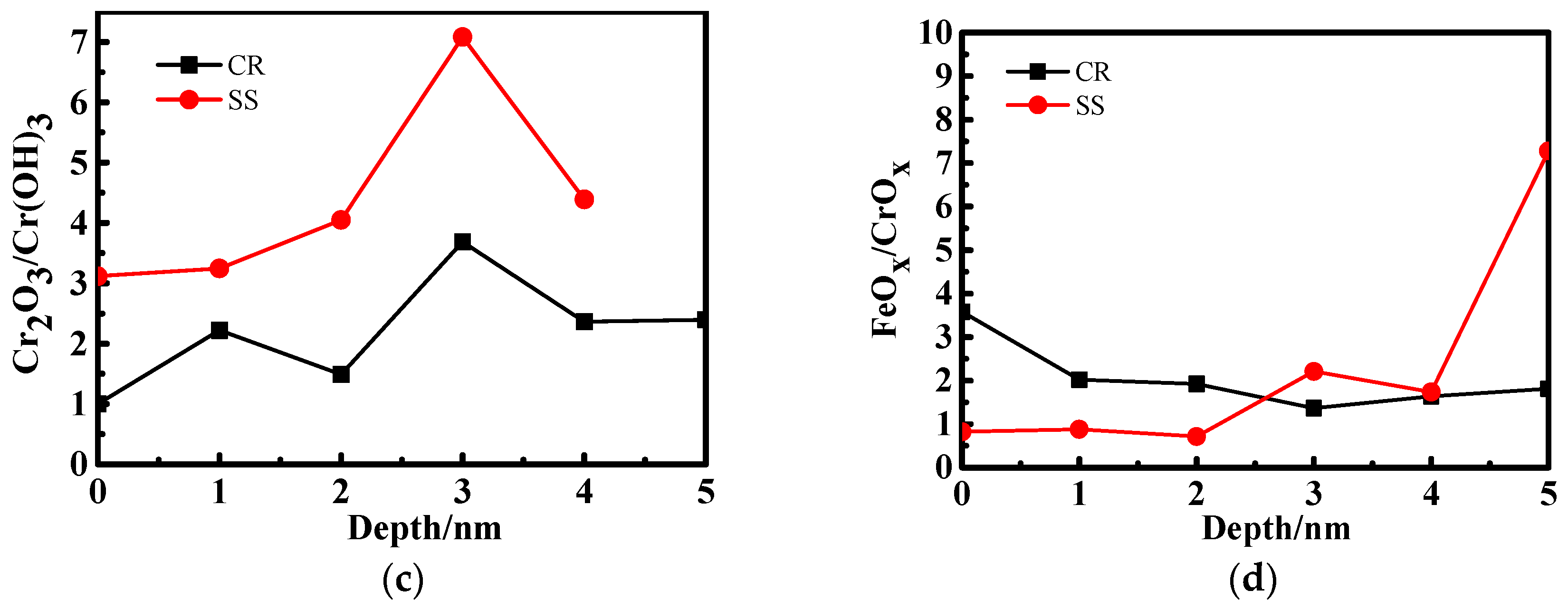
3.2. X-ray Photoelectron Spectrometer (XPS) Depth Profiling
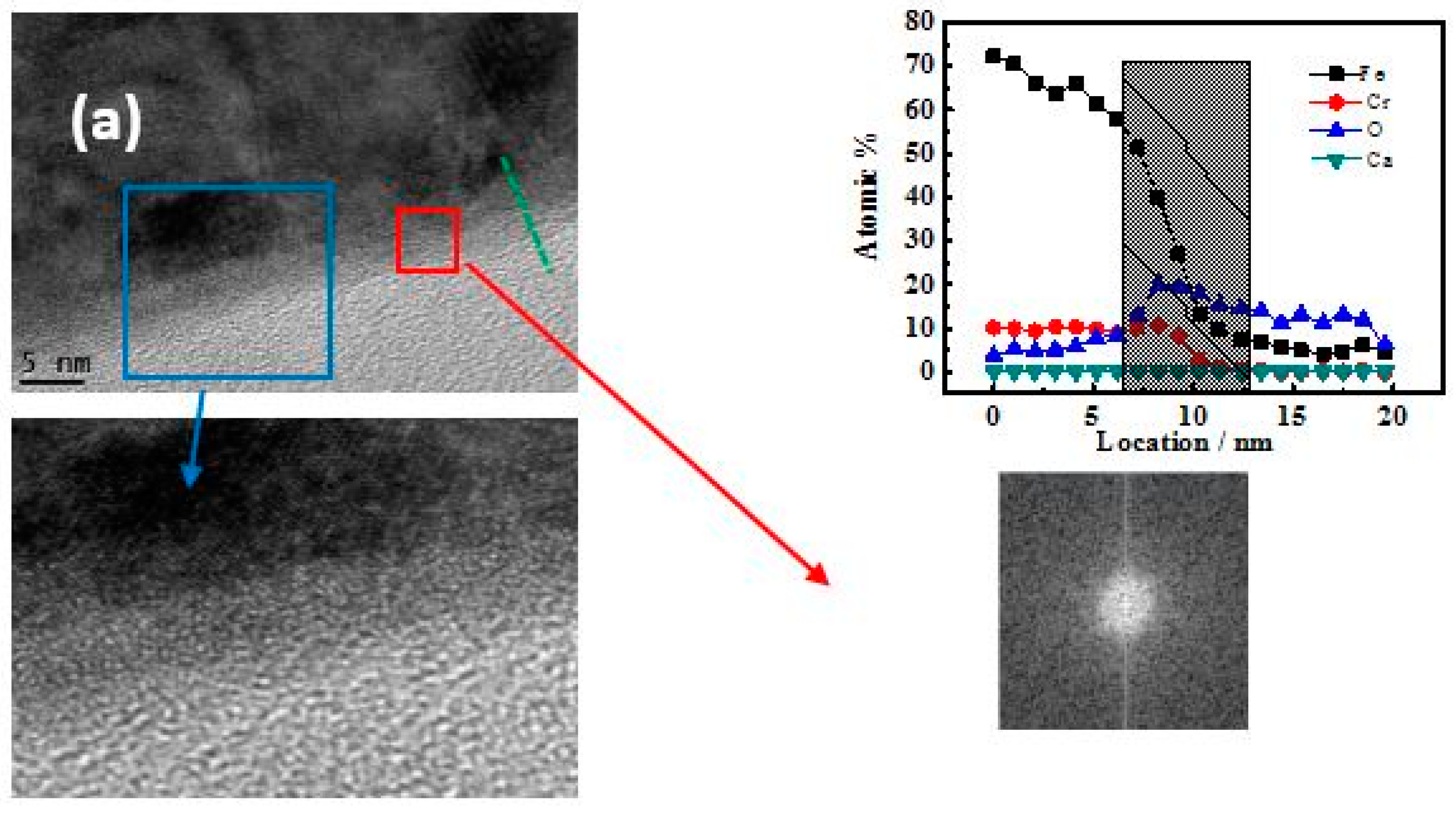
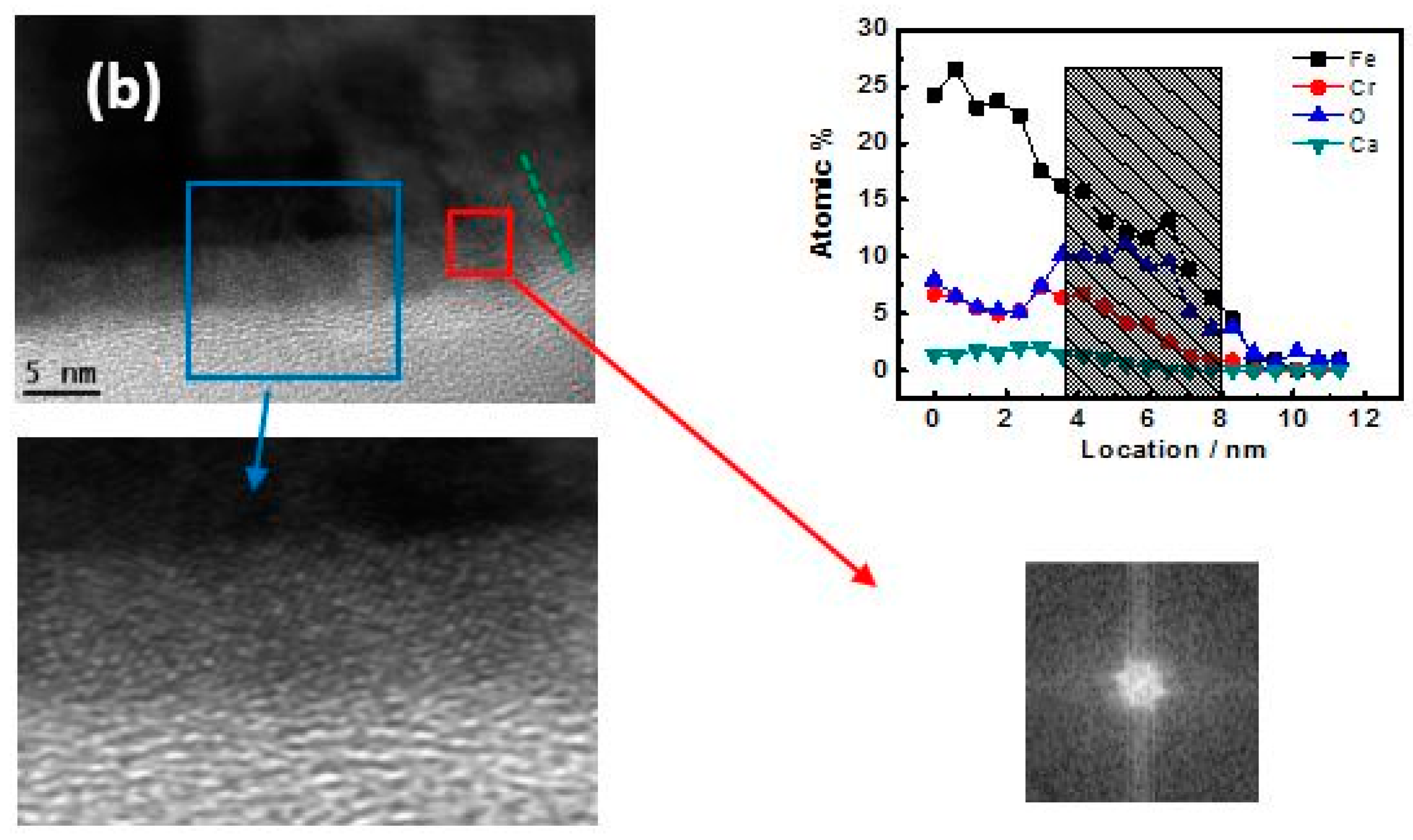
3.3. TEM-Longitudinal Section Image Analysis
3.4. Mott-Schottky (M-S) Analysis
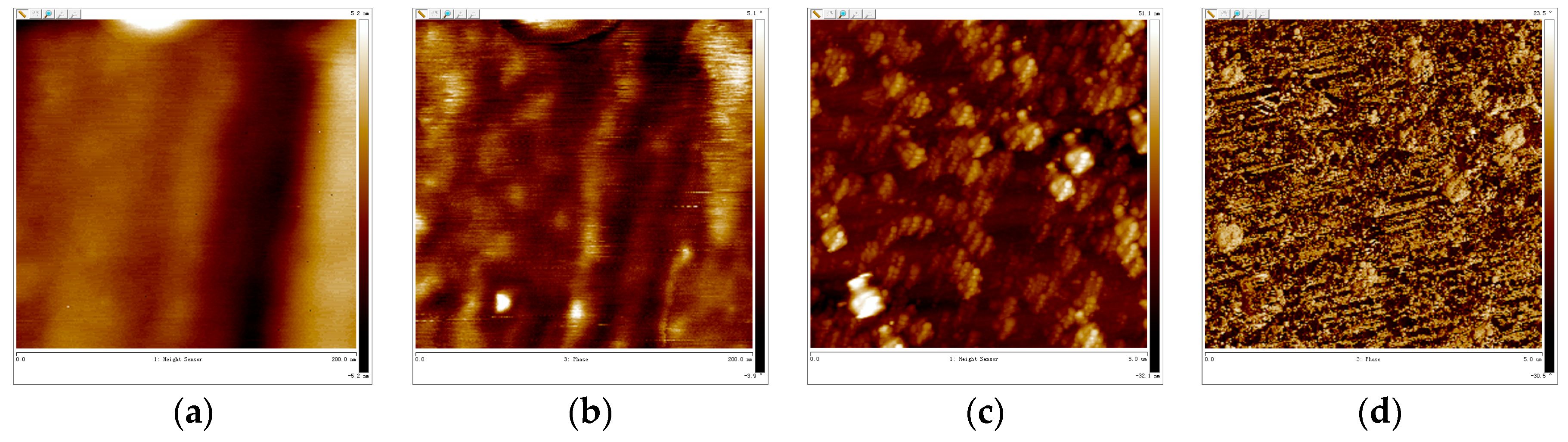
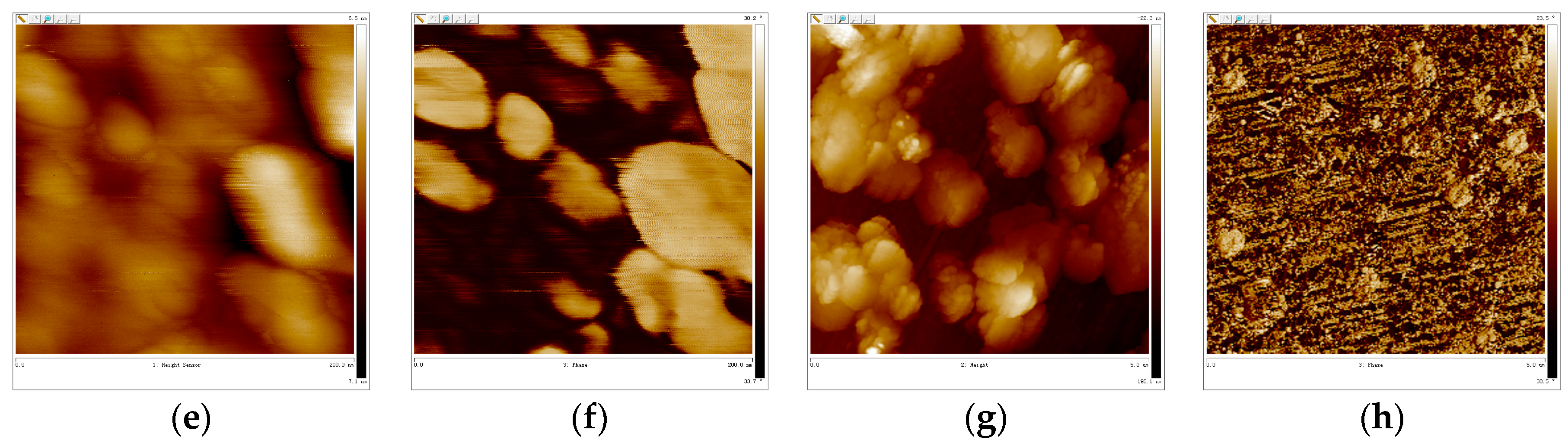
3.5. AFM-Cross Section Image Analysis
4. Discussion
4.1. The Effect of the Cr Element
4.2. The Relationship Between Passive Film Structure and Electrochemical Property
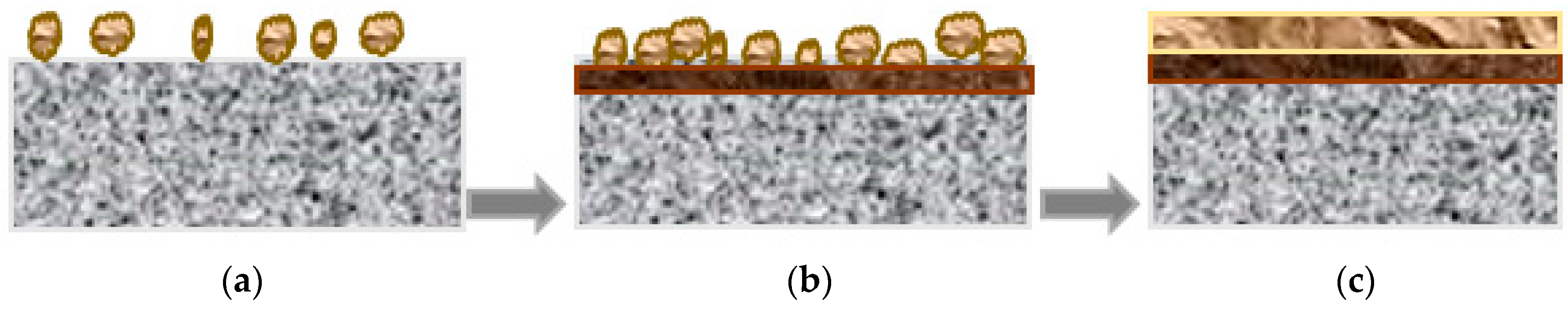
4.3. Growth Mechanism of the Passive Film
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Xu, J.; Yao, W.; Jiang, Z. Non-ureolytic bacterial carbonate precipitation as a surface treatment strategy on cementitious materials. J. Mater. Civ. Eng. 2013, 26, 983–991. [Google Scholar] [CrossRef]
- Venkatesan, P.; Palaniswamy, N.; Rajagopal, K. Corrosion performance of coated reinforcing bars embedded in concrete and exposed to natural marine environment. Prog. Org. Coat. 2006, 56, 8–12. [Google Scholar] [CrossRef]
- Monticelli, C.; Criado, M.; Fajardo, S.; Bastidas, J.M.; Abbottoni, M.; Balbo, A. Corrosion behaviour of a Low Ni austenitic stainless steel in carbonated chloride-polluted alkali-activated fly ash mortar. Cem. Concr. Res. 2014, 55, 49–58. [Google Scholar] [CrossRef]
- Duarte, R.G.; Castela, A.S.; Neves, R.; Freire, L.; Montemor, M.F. Corrosion behavior of stainless steel rebars embedded in concrete: An electrochemical impedance spectroscopy study. Electrochim. Acta 2014, 124, 218–224. [Google Scholar] [CrossRef]
- Pokorný, P.; Tej, P.; Kouřil, M. Evaluation of the impact of corrosion of hot‑dip galvanized reinforcement on bond strength with concrete–A review. Constr. Build. Mater. 2017, 132, 271–289. [Google Scholar] [CrossRef]
- Sun, F.; Li, X.; Zhang, F.; Cheng, X.; Zhou, C.; Wu, N.; Yin, Y.; Zhao, J. Corrosion mechanism of corrosion‑resistant steel developed for bottom plate of cargo oil tanks. Acta Metall. Sinica (Eng. Lett.). 2013, 26, 257–264. [Google Scholar] [CrossRef]
- Medina, E.; Medina, J.M.; Cobo, A.; Bastidas, D.M. Evaluation of mechanical and structural behavior of austenitic and duplex stainless steel reinforcements. Constr. Build. Mater. 2015, 78, 1–7. [Google Scholar] [CrossRef]
- Sedriks, A.J. Corrosion of Stainless Steel, 2nd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Hartt, W.H.; Powers, R.G.; Leroux, V.; Lysogorski, D.K. A Critical Literature Review of High-Performance Corrosion Reinforcements in Concrete Bridge Applications; Final Report for Office of Infrastructure Research and Development Federal Highway Administration: Geoorgetown Pike, VA, USA, July 2004.
- Nurnbeger, U. Stainless steel in concrete structures. In Corrosion in Reinforced Concrete Structures, 1st ed.; Böhni, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2005; pp. 135–162. [Google Scholar]
- Presuel-Moreno, F.; Scully, J.R.; Sharp, S.R. Literature review of commercially available alloys that have potential as low-cost, corrosion-resistant concrete reinforcement. Corrosion 2010, 66. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Olsson, C.O.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Machet, A.; Galtayries, A.; Zanna, S.; Klein, L.; Maurice, V.; Jolivet, P.; Foucault, M.; Combrade, P.; Scott, P.; Marcus, P.X. XPS and STM study of the growth and structure of passive films in high temperature water on a nickel‑base alloy. Electrochim. Acta 2004, 49, 3957–3964. [Google Scholar] [CrossRef]
- Dutta, R.S.; Dey, G.K.; Lobo, A.; Purandare, R.; Kulkarni, S.K. Corrosion behavior of austenitic alloy 690 under anodic and cathodic potentials. Metall. Mater. Trans. A 2002, 33, 1437–1447. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Jones, M.E.; Combs, K.E. Solubility and phase behavior of Cr (III) oxides in alkaline media at elevated temperatures. J. Solut. Chem. 1998, 27, 33–66. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Li, Y.; Wang, S.; Wang, F. Passive film growth mechanism of nanocrystalline 304 stainless steel prepared by magnetron sputtering and deep rolling techniques. Electrochim. Acta 2011, 56, 7740–7748. [Google Scholar] [CrossRef]
- Poursaee, A. Corrosion of steel bars in saturated Ca(OH)2 and concrete pore solution. Concr. Res. Lett. 2010, 1, 90–97. [Google Scholar]
- Sikora, J.; Sikora, E.; Macdonald, D.D. The electronic structure of the passive film on tungsten. Electrochim. Acta 2000, 45, 1875–1883. [Google Scholar] [CrossRef]
- Park, K.; Ahn, S.; Kwon, H. Effects of solution temperature on the kinetic nature of passive film on Ni. Electrochim. Acta 2011, 56, 1662–1669. [Google Scholar] [CrossRef]
- Hamada, E.; Yamada, K.; Nagoshi, M.; Makiishi, N.; Sato, K.; Ishii, T.; Fukuda, K.; Ishikawa, S.; Ujiro, T. Direct imaging of native passive film on stainless steel by aberration corrected STEM. Corros. Sci. 2010, 52, 3851–3854. [Google Scholar] [CrossRef]
- Gunay, H.B.; Gunay, P.; Isgor, O.B.; Carpenter, G.J.; Wu, X. Characterization of atomic structure of oxide films on carbon steel in simulated concrete pore solutions using EELS. Appl. Surf. Sci 2013, 274, 195–202. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Abreu, C.M.; Cristóbal, M.J.; Losada, R.; Nóvoa, X.R.; Pena, G.; Pérez, M.C. Long-term behaviour of AISI 304L passive layer in chloride containing medium. Electrochim. Acta 2006, 51, 1881–1890. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.; Montemor, M.F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Hakiki, N.E.; Montemor, M.F.; Ferreira, M.G.; da Cunha Belo, M. Semiconducting properties of thermally grown oxide films on AISI 304 stainless steel. Corros. Sci. 2000, 42, 687–702. [Google Scholar] [CrossRef]
- Carmezim, M.J.; Simoes, A.M.; Montemor, M.F.; Belo, M.D. Capacitance behaviour of passive films on ferritic and austenitic stainless steel. Corros. Sci. 2005, 47, 581–591. [Google Scholar] [CrossRef]
- Freire, L.; Nóvoa, X.R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Hussain, R.R. Passive layer development and corrosion of steel in concrete at the nano-scale. J. Civ. Environ. Eng. 2014, 4, 1. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Cheng, X.Q.; Xiao, K.; Li, X.G. Electrochemical Behavior of 2205 Duplex Stainless Steel in NaCl Solution with Different Chromate Contents. J. Mater. Eng. Perform. 2012, 21, 1283–1291. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Impedance investigation of thermally formed oxide films on AISI 304L stainless steel. Corros. Sci. 2010, 52, 859–864. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Han, E.H. Electrochemical properties and growth mechanism of passive films on Alloy 690 in high‑temperature alkaline environments. Corros. Sci. 2010, 52, 3444–3452. [Google Scholar] [CrossRef]
- Oh, K.; Ahn, S.; Eom, K.; Jung, K.; Kwon, H. Observation of passive films on Fe–20Cr–xNi (x = 0, 10, 20 wt.%) alloys using TEM and Cs-corrected STEM–EELS. Corros. Sci. 2014, 79, 34–40. [Google Scholar] [CrossRef]
- Robertson, J. The mechanism of high temperature aqueous corrosion of steel. Corros. Sci. 1989, 29, 1275–1291. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Högström, J.; Fredriksson, W.; Edstrom, K.; Björefors, F.; Nyholm, L.; Olsson, C.O. Cation profiling of passive films on stainless steel formed in sulphuric and acetic acid by deconvolution of angle-resolved X‑ray photoelectron spectra. Appl. Surf. Sci. 2013, 284, 700–714. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Zhang, B.; Li, Y.; Yan, F.; Tao, N.; Wang, F. Passive behavior of a bulk nanostructured 316L austenitic stainless steel consisting of nanometer-sized grains with embedded nano-twin bundles. Corros. Sci. 2014, 85, 331–342. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Pan, Y.; Li, J. Effect of Cr on the passive film formation mechanism of steel rebar in saturated calcium hydroxide solution. Appl. Surf. Sci. 2016, 389, 1182–1191. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Blasco-Tamarit, E.; García-García, D.M.; García-Antón, J. Effect of alloying elements on the electronic properties of thin passive films formed on carbon steel, ferritic and austenitic stainless steels in a highly concentrated LiBr solution. Thin Solid Films 2014, 558, 252–258. [Google Scholar] [CrossRef]


| Steel | C | Si | Mn | Cr | Cu | Ni | Al | Mo |
|---|---|---|---|---|---|---|---|---|
| LC | 0.22 | 0.53 | 1.44 | - | - | - | - | - |
| CR | 0.01 | 0.487 | 1.49 | 10.36 | - | - | - | 1.162 |
| SS | 0.02 | 0.48 | 1.09 | 23.31 | - | 4.35 | - | 0.42 |
| Parameters | CR | Fitting Error | SS | Fitting Error |
|---|---|---|---|---|
| Rct (Ω·cm2) | 848,000 | 0.00% | 903,000 | 0.00% |
| Rf (Ω·cm2) | 1,210,000 | 0.00% | 1,930,000 | 0.00% |
| Cdl × 10−5 (μF·cm−2) | 2.25 | 12.60% | 2.77 | 26.10% |
| n | 0.9756 | 34.95% | 0.9411 | 66.13% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.-y.; Wang, D.; Chu, H.-y.; Ma, H.; Liu, Y.; Gao, Y.; Shi, J.; Sun, W. The Passive Film Growth Mechanism of New Corrosion-Resistant Steel Rebar in Simulated Concrete Pore Solution: Nanometer Structure and Electrochemical Study. Materials 2017, 10, 412. https://doi.org/10.3390/ma10040412
Jiang J-y, Wang D, Chu H-y, Ma H, Liu Y, Gao Y, Shi J, Sun W. The Passive Film Growth Mechanism of New Corrosion-Resistant Steel Rebar in Simulated Concrete Pore Solution: Nanometer Structure and Electrochemical Study. Materials. 2017; 10(4):412. https://doi.org/10.3390/ma10040412
Chicago/Turabian StyleJiang, Jin-yang, Danqian Wang, Hong-yan Chu, Han Ma, Yao Liu, Yun Gao, Jinjie Shi, and Wei Sun. 2017. "The Passive Film Growth Mechanism of New Corrosion-Resistant Steel Rebar in Simulated Concrete Pore Solution: Nanometer Structure and Electrochemical Study" Materials 10, no. 4: 412. https://doi.org/10.3390/ma10040412






