A High Performance Lithium-Ion Capacitor with Both Electrodes Prepared from Sri Lanka Graphite Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the GPC
2.2. Characterization
2.3. Electrochemical Measurements
2.4. Pre-Lithiation of PVG
3. Results and Discussion
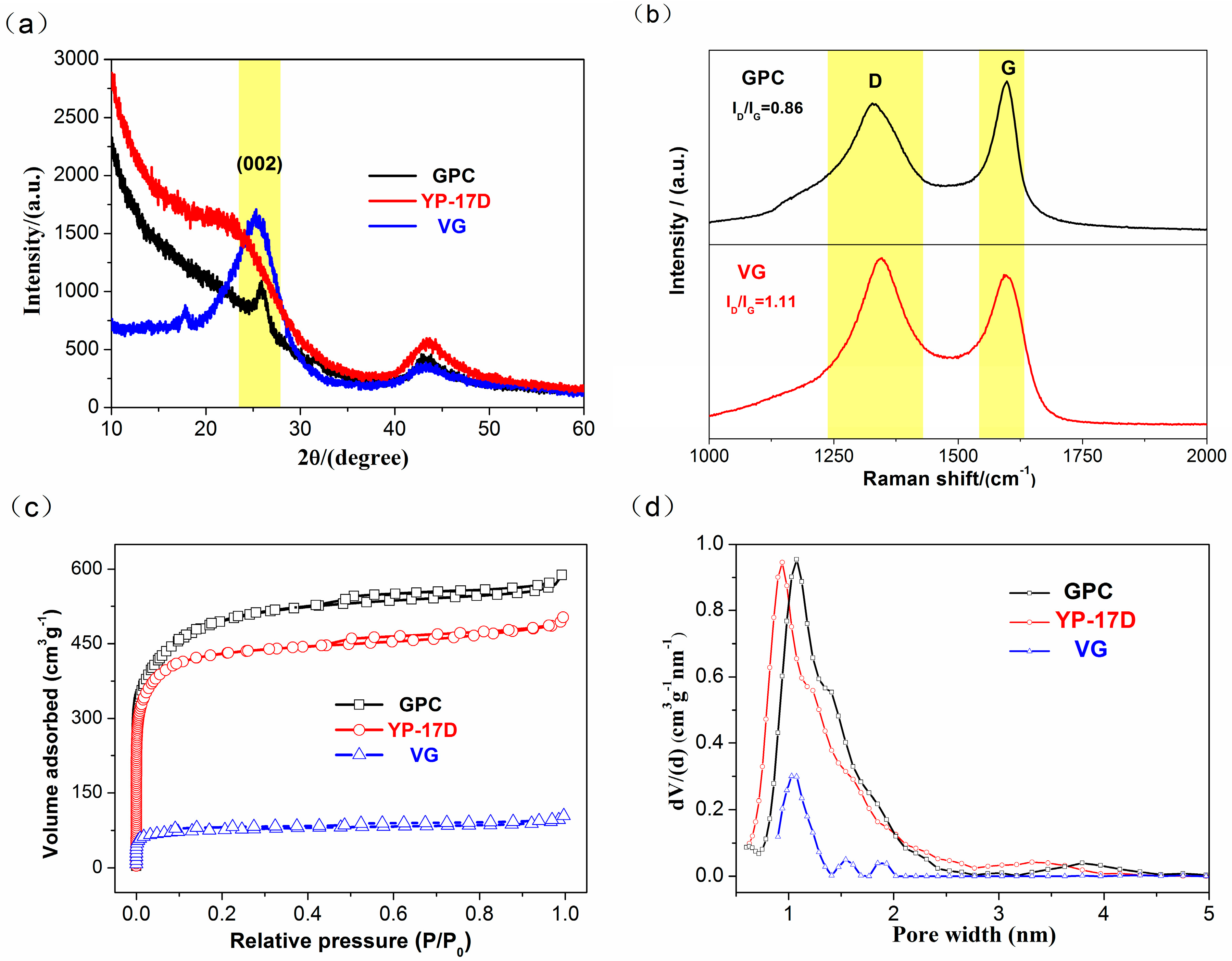
3.1. Structure and Morphology
3.2. Electrochemical Performance of PVG in LIB
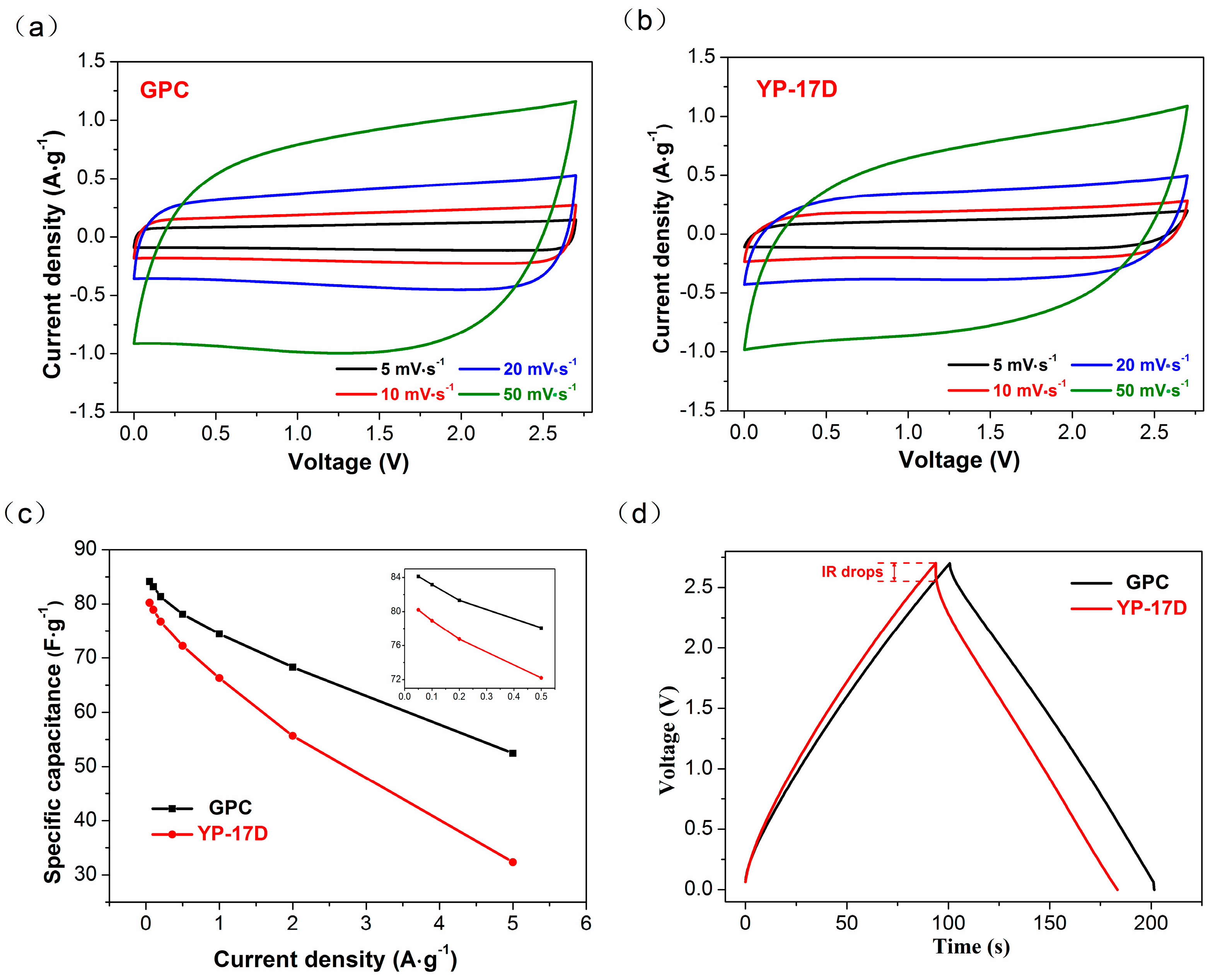
3.3. Electrochemical Performance of GPC in SCs
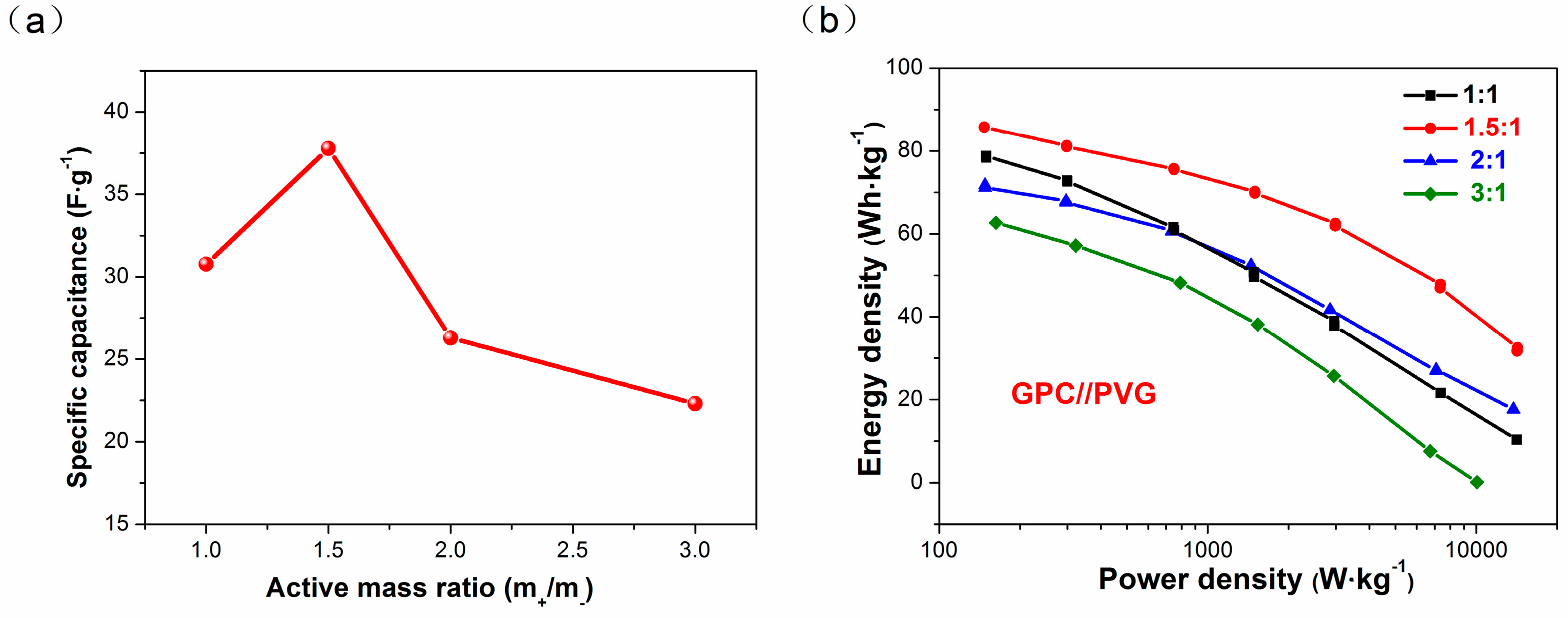
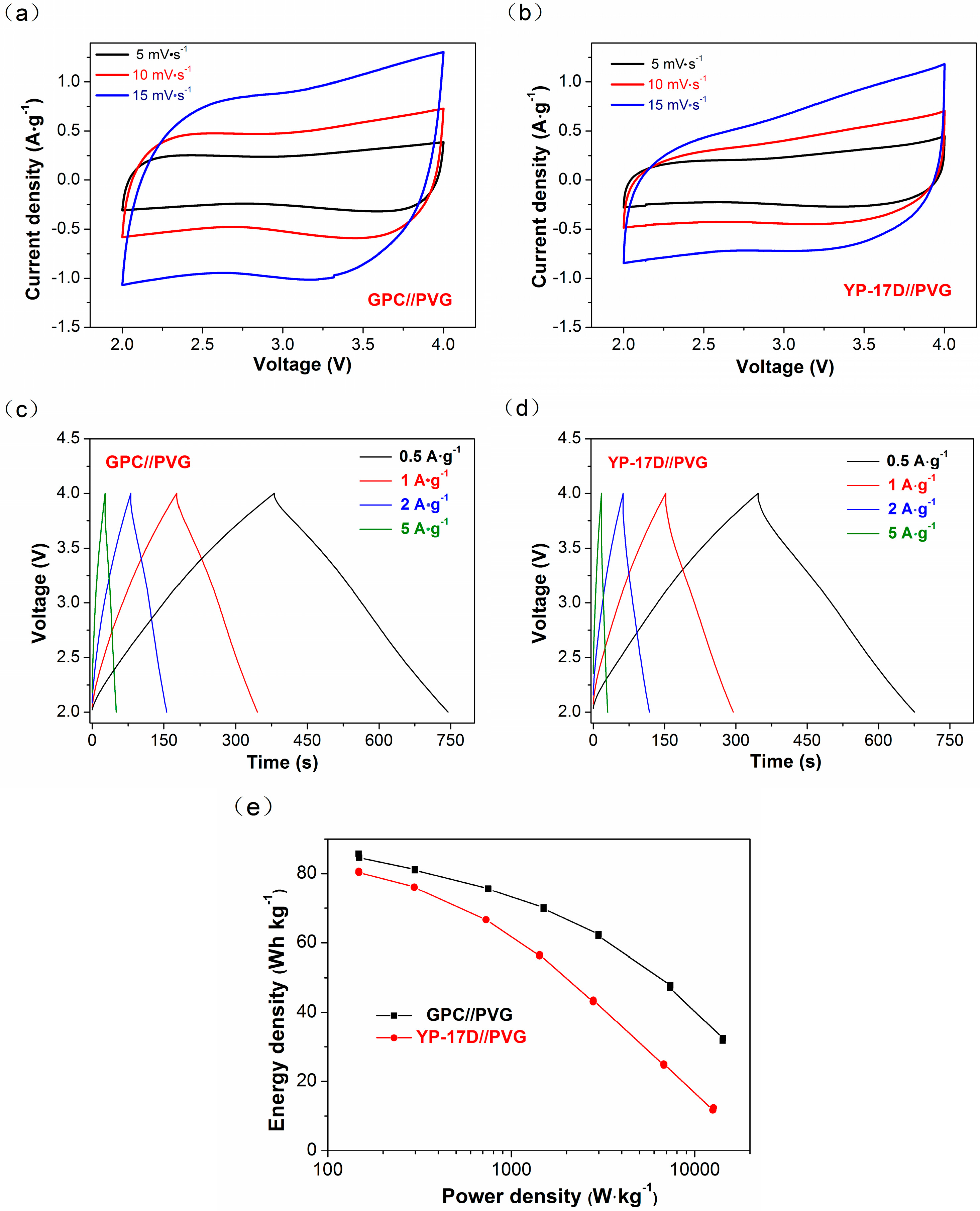
3.4. Electrochemical Performance of GPC in LICs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Ren, G.; Ma, G.; Cong, N. Review of electrical energy storage system for vehicular applications. Renew. Sustain. Energy Rev. 2015, 41, 225–236. [Google Scholar] [CrossRef]
- Choi, N.S.; Chen, Z.H.; Freunberger, S.A.; Ji, X.L.; Sun, Y.K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar]
- Gu, H.C.; Zhu, Y.E.; Yang, J.Q.; Wei, J.P.; Zhou, Z. Nanomaterials and technologies for lithium-ion hybrid supercapacitors. Chemnanomat 2016, 2, 578–587. [Google Scholar] [CrossRef]
- Wen, L.; Liu, C.M.; Song, R.S.; Luo, H.Z.; Shi, Y.; Li, F.; Cheng, H.M. Lithium storage characteristics and possible applications of graphene materials. Acta Chim. Sin. 2014, 72, 333–344. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, H.; Zhang, M.; Chen, Y. Graphene-based materials for lithium-ion hybrid supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Kim, Y.R.; Jeong, M.G.; Yuk, Y.J.; Kim, H.J.; Park, S.G. Synthesis and electrochemical characteristics of spherical Li4Ti5O12/CNT composite materials for hybrid capacitors. J. Electrochem. Sci. Technol. 2015, 6, 59–64. [Google Scholar] [CrossRef]
- Han, X.; Han, P.; Yao, J.; Zhang, S.; Cao, X.; Xiong, J.; Zhang, J.; Cui, G. Nitrogen-doped carbonized polyimide microsphere as a novel anode material for high performance lithium ion capacitors. Electrochim. Acta 2016, 196, 603–610. [Google Scholar] [CrossRef]
- Yu, X.; Zhan, C.; Lv, R.; Bai, Y.; Lin, Y.; Huang, Z.-H.; Shen, W.; Qiu, X.; Kang, F. Ultrahigh-rate and high-density lithium-ion capacitors through hybriding nitrogen-enriched hierarchical porous carbon cathode with prelithiated microcrystalline graphite anode. Nano Energy 2015, 15, 43–53. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, Z.H.; Yang, Y.; Shen, W.C.; Zheng, Y.P.; Sun, H.Y.; Kang, F.Y. Porous mesocarbon microbeads with graphitic shells: Constructing a high-rate, high-capacity cathode for hybrid supercapacitor. Sci. Rep. UK 2013, 3, 2477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.F.; Wang, J.; Shi, J.L.; Shi, Z.Q. Different types of pre-lithiated hard carbon as negative electrode material for lithium-ion capacitors. Electrochim. Acta 2016, 187, 134–142. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Piñero, E.; Béguin, F. High-energy density graphite/ac capacitor in organic electrolyte. J. Power Sources 2008, 177, 643–651. [Google Scholar] [CrossRef]
- Wang, H.; Yoshio, M. Performance of ac/graphite capacitors at high weight ratios of AC/graphite. J. Power Sources 2008, 177, 681–684. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Wang, J.; Shi, J. Composite of mesocarbon microbeads/hard carbon as anode material for lithium ion capacitor with high electrochemical performance. J. Electroanal. Chem. 2015, 747, 20–28. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Wang, C. Effect of pre-lithiation degrees of mesocarbon microbeads anode on the electrochemical performance of lithium-ion capacitors. Electrochim. Acta 2014, 125, 22–28. [Google Scholar] [CrossRef]
- Wang, H.W.; Guan, C.; Wang, X.F.; Fan, H.J. A high energy and power Li-ion capacitor based on a TiO2 nanobelt array anode and a graphene hydrogel cathode. Small 2015, 11, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Yan, J.; Wei, T.; Zhi, L.J.; Ning, G.Q.; Li, T.Y.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- Ma, S.B.; Nam, K.W.; Yoon, W.S.; Yang, X.Q.; Ahn, K.Y.; Oh, K.H.; Kim, K.B. A novel concept of hybrid capacitor based on manganese oxide materials. Electrochem. Commun. 2007, 9, 2807–2811. [Google Scholar] [CrossRef]
- Luo, L.; Tan, X.; Tian, J. Research progress of graphite purification. Non-Met. Min. 2007, 30, 2110–2116. [Google Scholar]
- Puthusseri, D.; Aravindan, V.; Madhavi, S.; Ogale, S. Improving the energy density of li-ion capacitors using polymer-derived porous carbons as cathode. Electrochim. Acta 2014, 130, 766–770. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Mahanty, S. Reduced graphene oxide anchored Cu(OH)2 as a high performance electrochemical supercapacitor. Dalton Trans. 2015, 44, 14604–14612. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Q.; Zhang, J.; Wang, J.; Shi, J.L.; Wang, C.Y. Effect of the capacity design of activated carbon cathode on the electrochemical performance of lithium-ion capacitors. Electrochim. Acta 2015, 153, 476–483. [Google Scholar] [CrossRef]
- Ye, L.; Liang, Q.; Lei, Y.; Yu, X.; Han, C.; Shen, W.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. A high performance li-ion capacitor constructed with Li4Ti5O12/c hybrid and porous graphene macroform. J. Power Sources 2015, 282, 174–178. [Google Scholar] [CrossRef]
- Tu, F.Y.; Liu, S.Q.; Wu, T.H.; Jin, G.H.; Pan, C.Y. Porous graphene as cathode material for lithium ion capacitor with high electrochemical performance. Powder Technol. 2014, 253, 580–583. [Google Scholar] [CrossRef]
- Lu, L.; Sahajwalla, V.; Kong, C.; Harris, D. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Juan-Juan, J.; Cazorla-Amoros, D.; Linares-Solano, A. About reactions occurring during chemical activation with hydroxides. Carbon 2004, 42, 1371–1375. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite nanofibers as advanced materials for Li-ion, Li-O2 and Li-S batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef]
- Ji, L.W.; Lin, Z.; Alcoutlabi, M.; Zhang, X.W. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Zou, L.; Kang, F.; Li, X.; Zheng, Y.-P.; Shen, W.; Zhang, J. Investigations on the modified natural graphite as anode materials in lithium ion battery. J. Phys. Chem. Solids 2008, 69, 1265–1271. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Zhan, C.; Yu, X.; Liang, Q.; Lv, R.; Gai, G.; Shen, W.; Kang, F.; Huang, Z.-H. A High Performance Lithium-Ion Capacitor with Both Electrodes Prepared from Sri Lanka Graphite Ore. Materials 2017, 10, 414. https://doi.org/10.3390/ma10040414
Gao X, Zhan C, Yu X, Liang Q, Lv R, Gai G, Shen W, Kang F, Huang Z-H. A High Performance Lithium-Ion Capacitor with Both Electrodes Prepared from Sri Lanka Graphite Ore. Materials. 2017; 10(4):414. https://doi.org/10.3390/ma10040414
Chicago/Turabian StyleGao, Xiaoyu, Changzhen Zhan, Xiaoliang Yu, Qinghua Liang, Ruitao Lv, Guosheng Gai, Wanci Shen, Feiyu Kang, and Zheng-Hong Huang. 2017. "A High Performance Lithium-Ion Capacitor with Both Electrodes Prepared from Sri Lanka Graphite Ore" Materials 10, no. 4: 414. https://doi.org/10.3390/ma10040414




