Prediction of First-Year Corrosion Losses of Carbon Steel and Zinc in Continental Regions
Abstract
:1. Introduction
2. Results
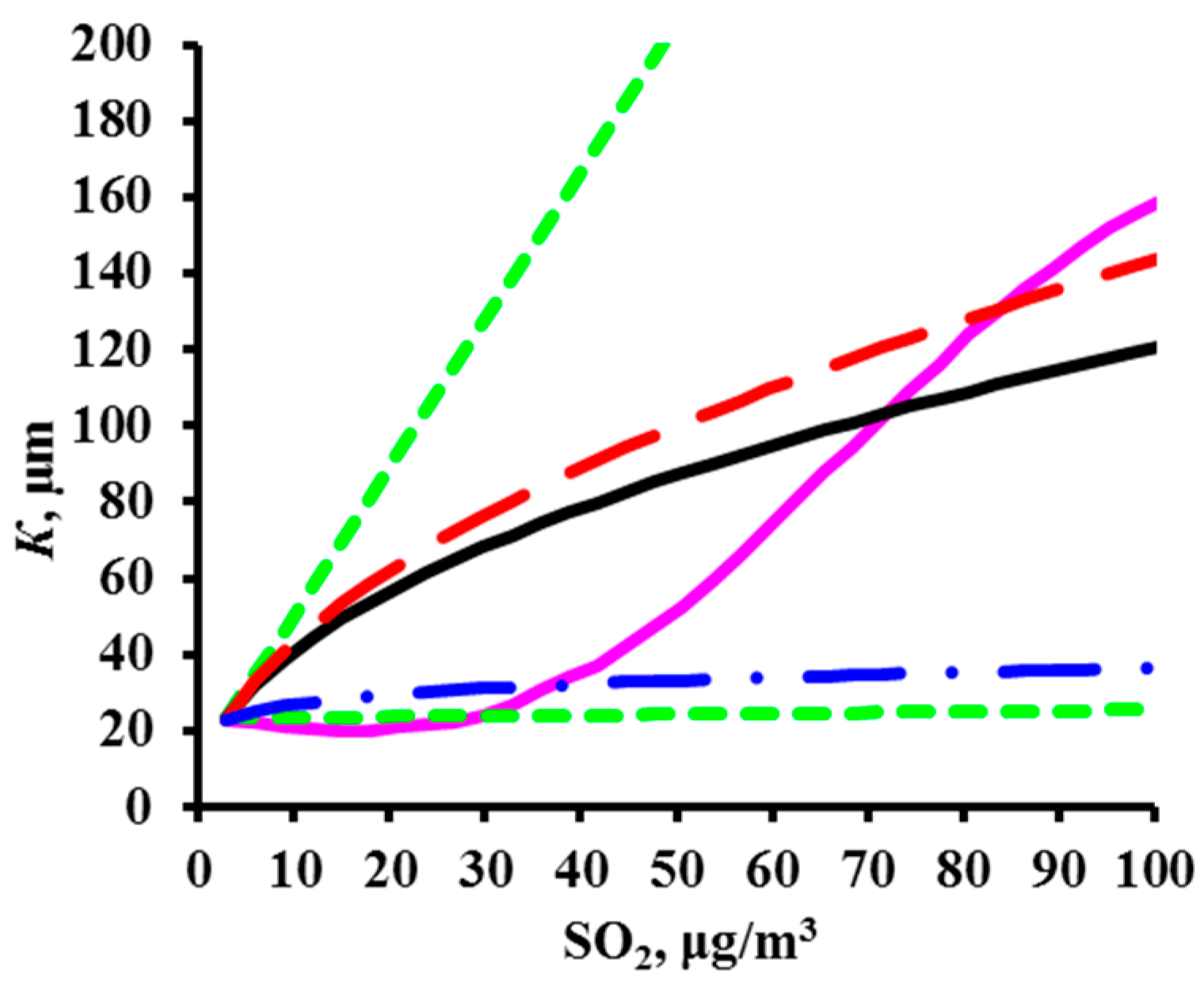
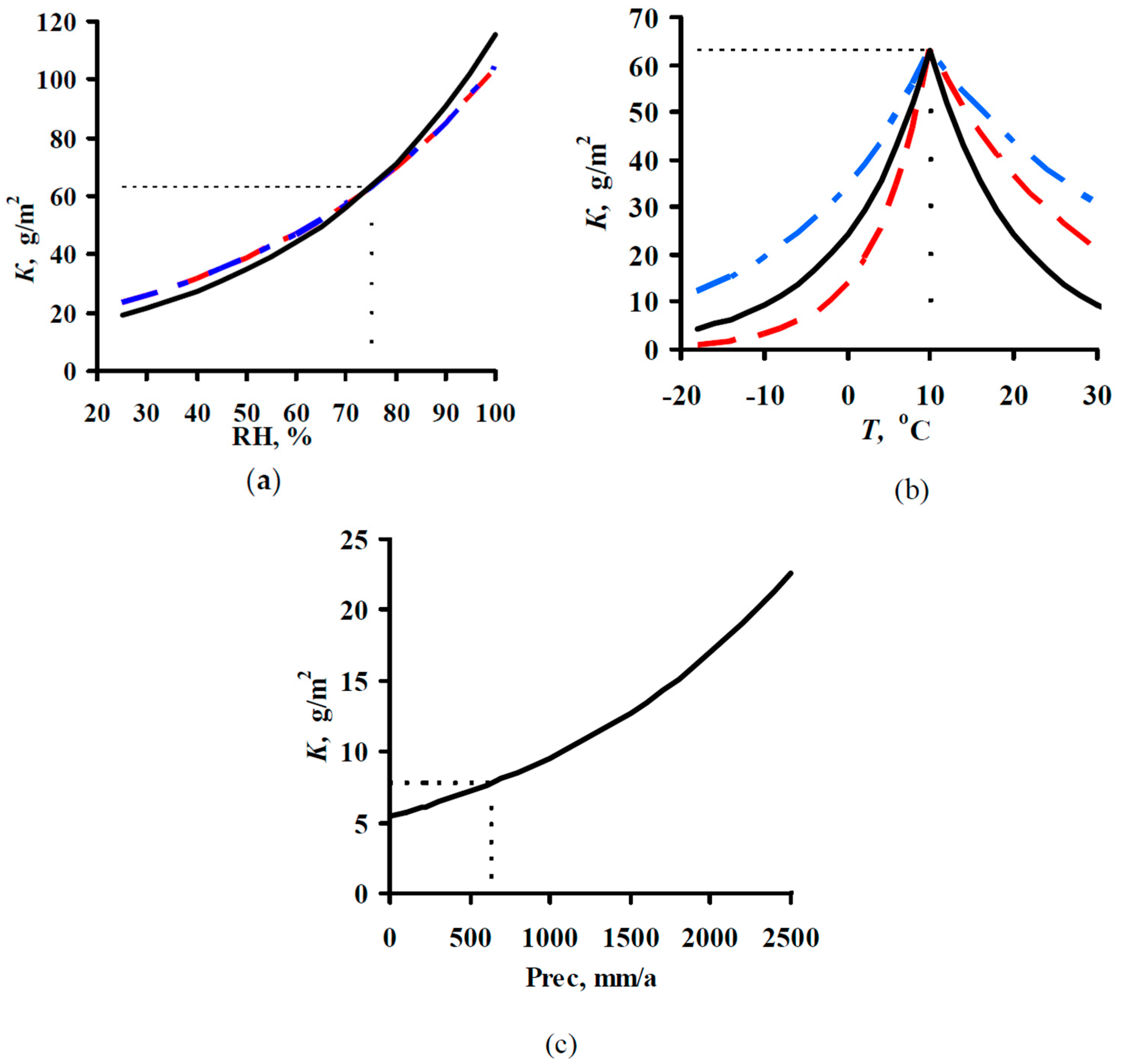
2.1. Development of DRFs for Continental Territories
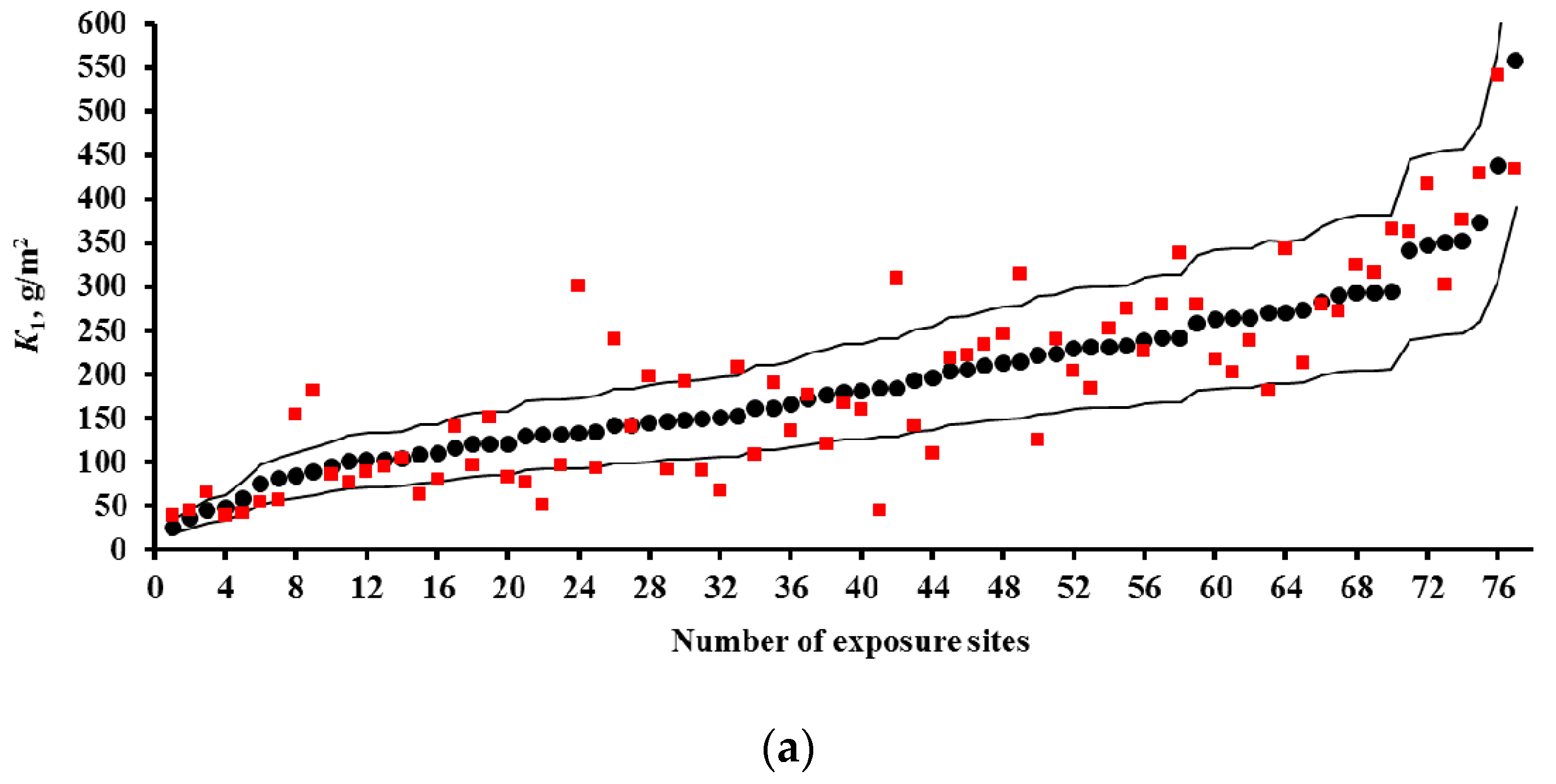
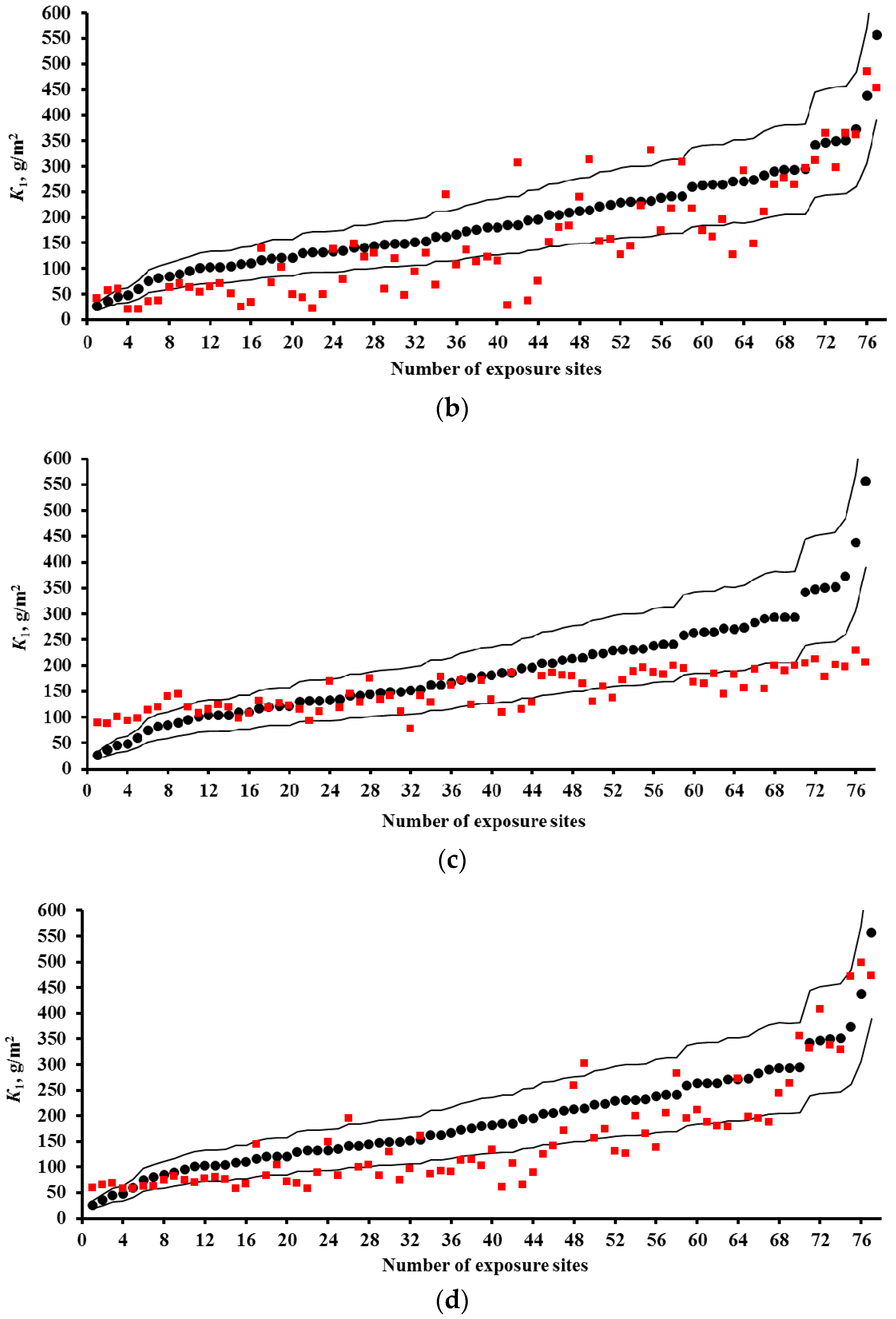
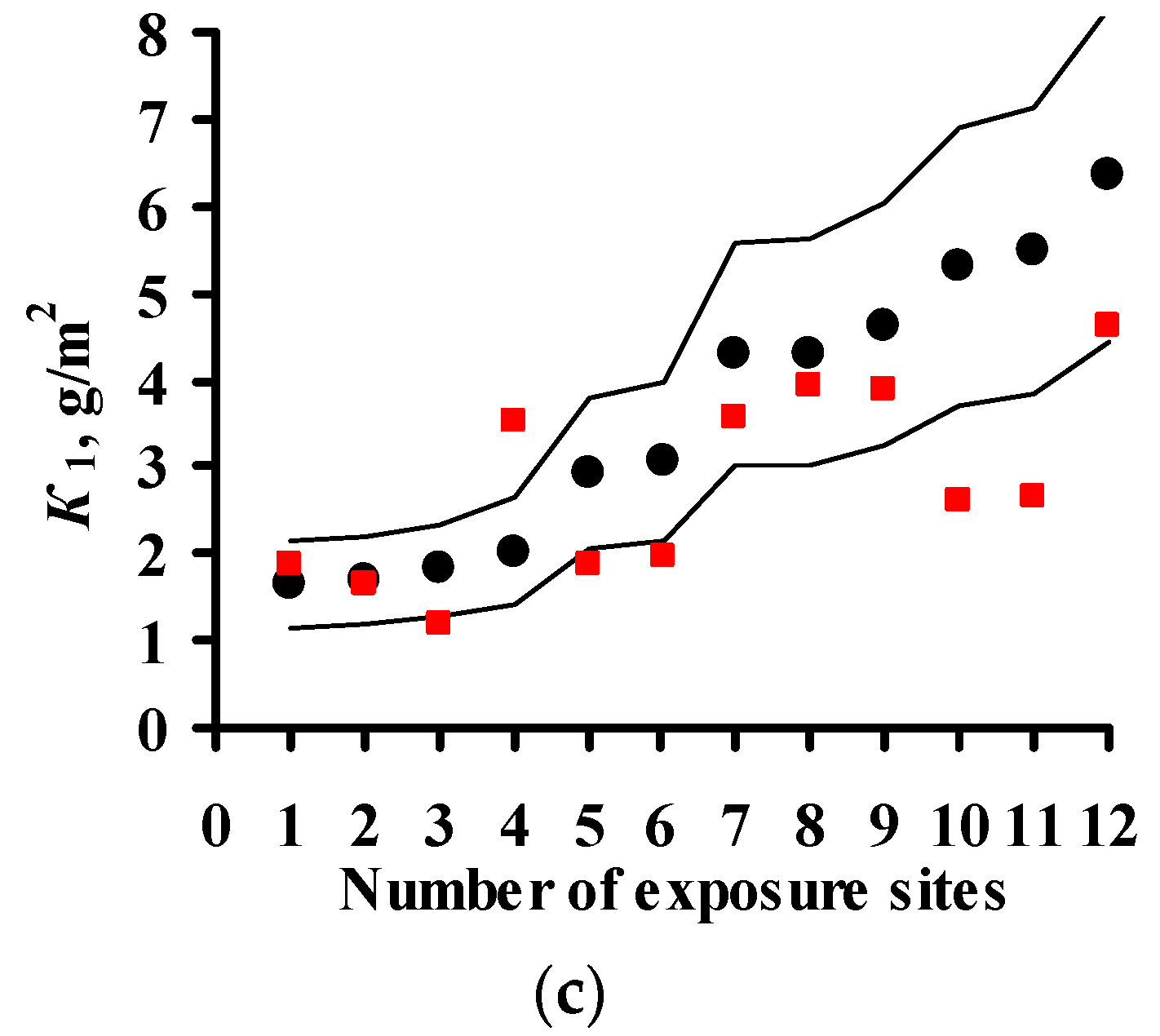
2.2. Predictions of First-Year Corrosion Losses
K = 3.54 × [SO2]0.13 × exp{0.020 × RH − 0.036 × (T-10)} × τ0.33 T > 10 °C.
K = 1.35 × [SO2]0.22 × exp{0.018 × RH − 0.021 × (T-10)} × τ0.85 + 0.029 × Rain[H+] × τ T > 10 °C.
3. Results
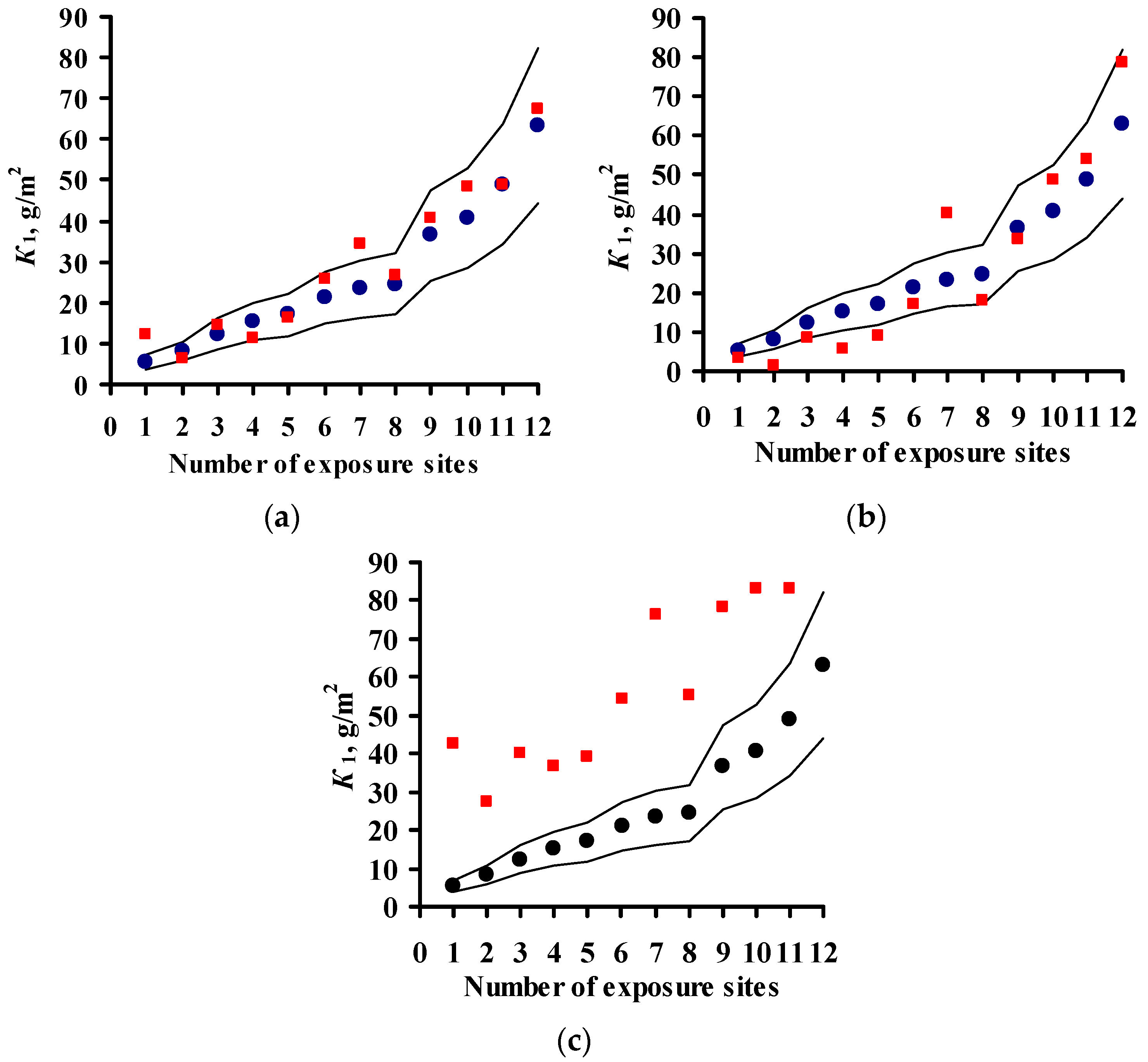
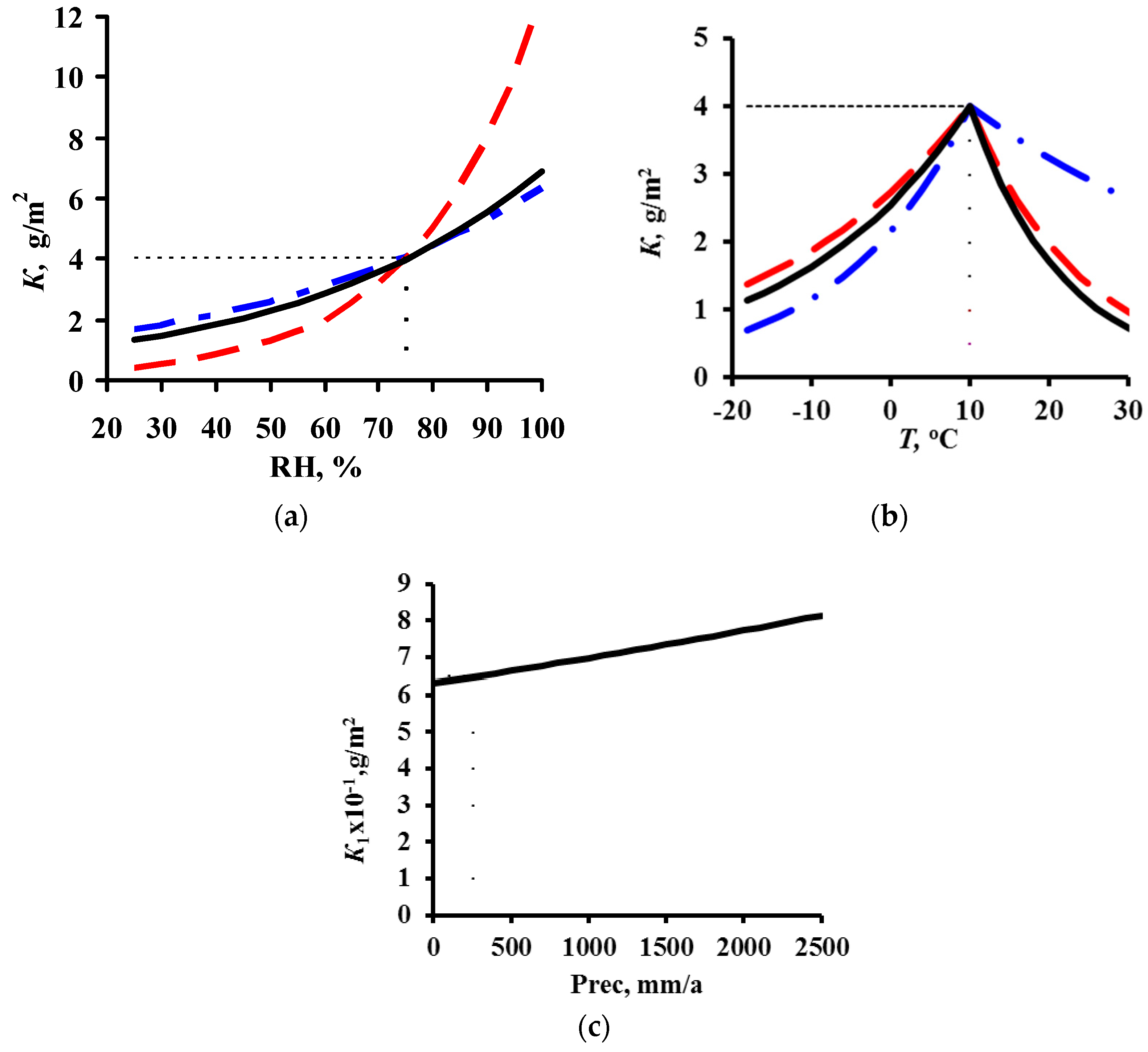
3.1. DRF Development
K1 = 7.7 × [SO2]0.47 × exp{0.024 × RH − 0.095 × (T-10) + 0.00056 × Prec} T > 10 °C,
K1 = 0.71 × [SO2]0.28 × exp{0.022 × RH − 0.085 × (T-10) + 0.0001 × Prec} T > 10 °C.
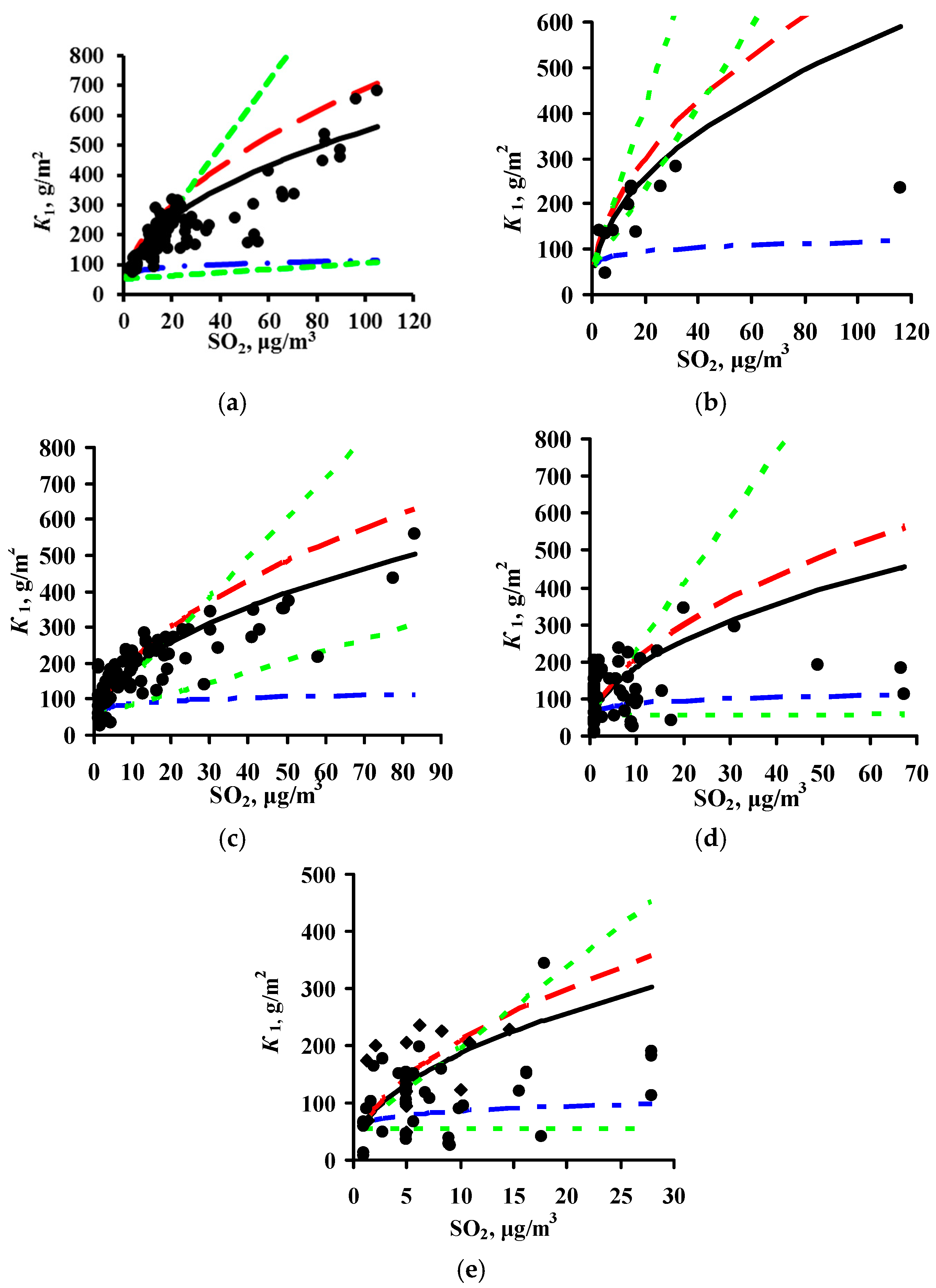
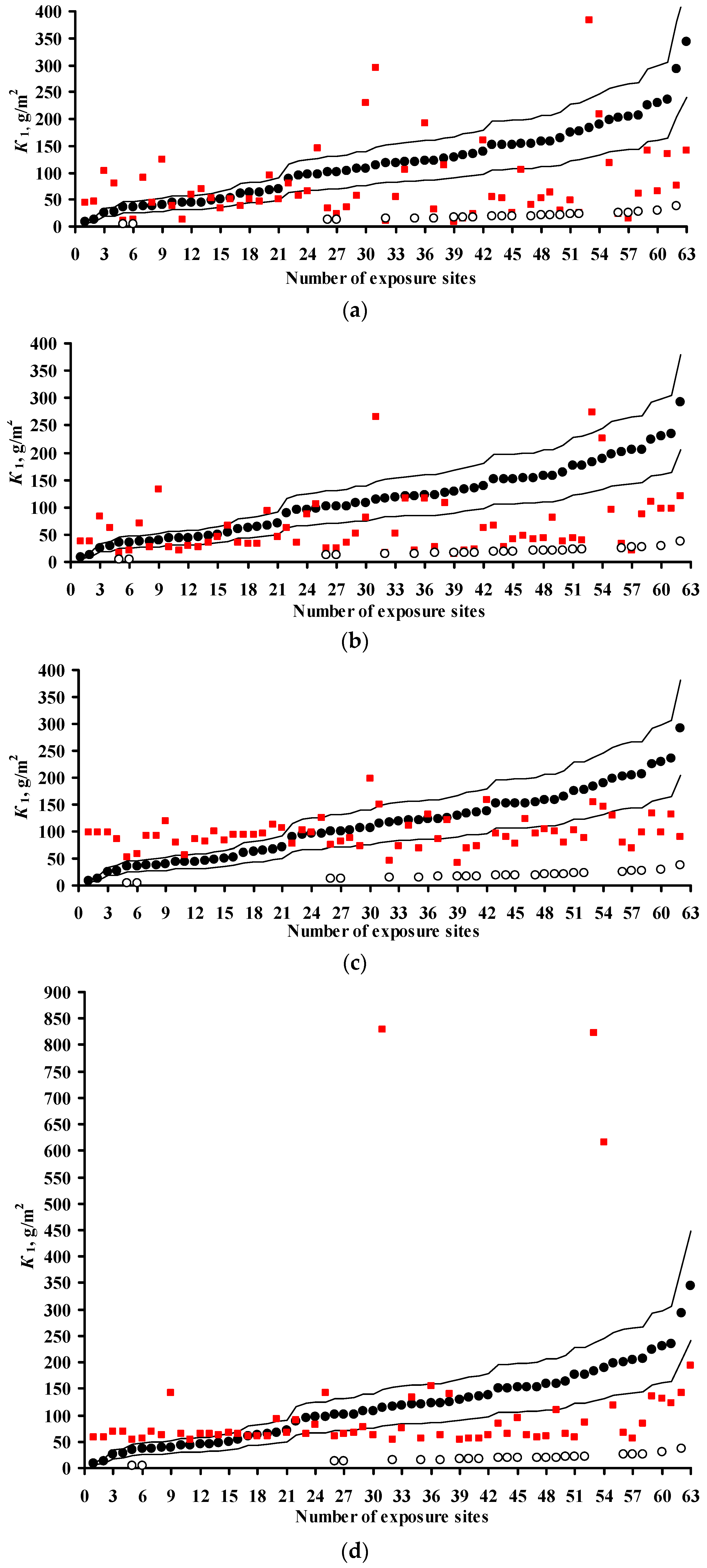
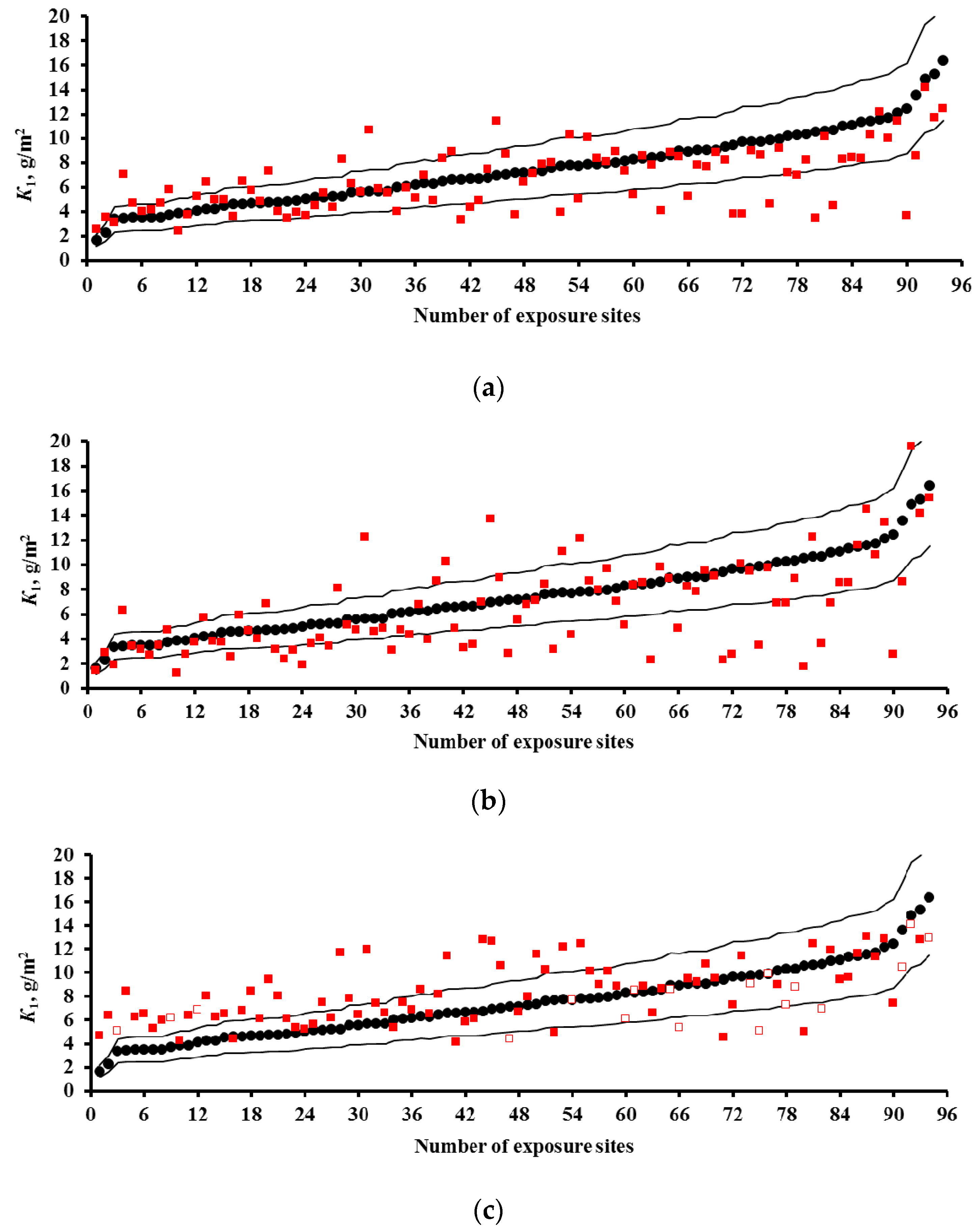
3.2. Predictions of K1 Using Various DRFs for Carbon Steel
3.3. Analysis of DRFs for Carbon Steel
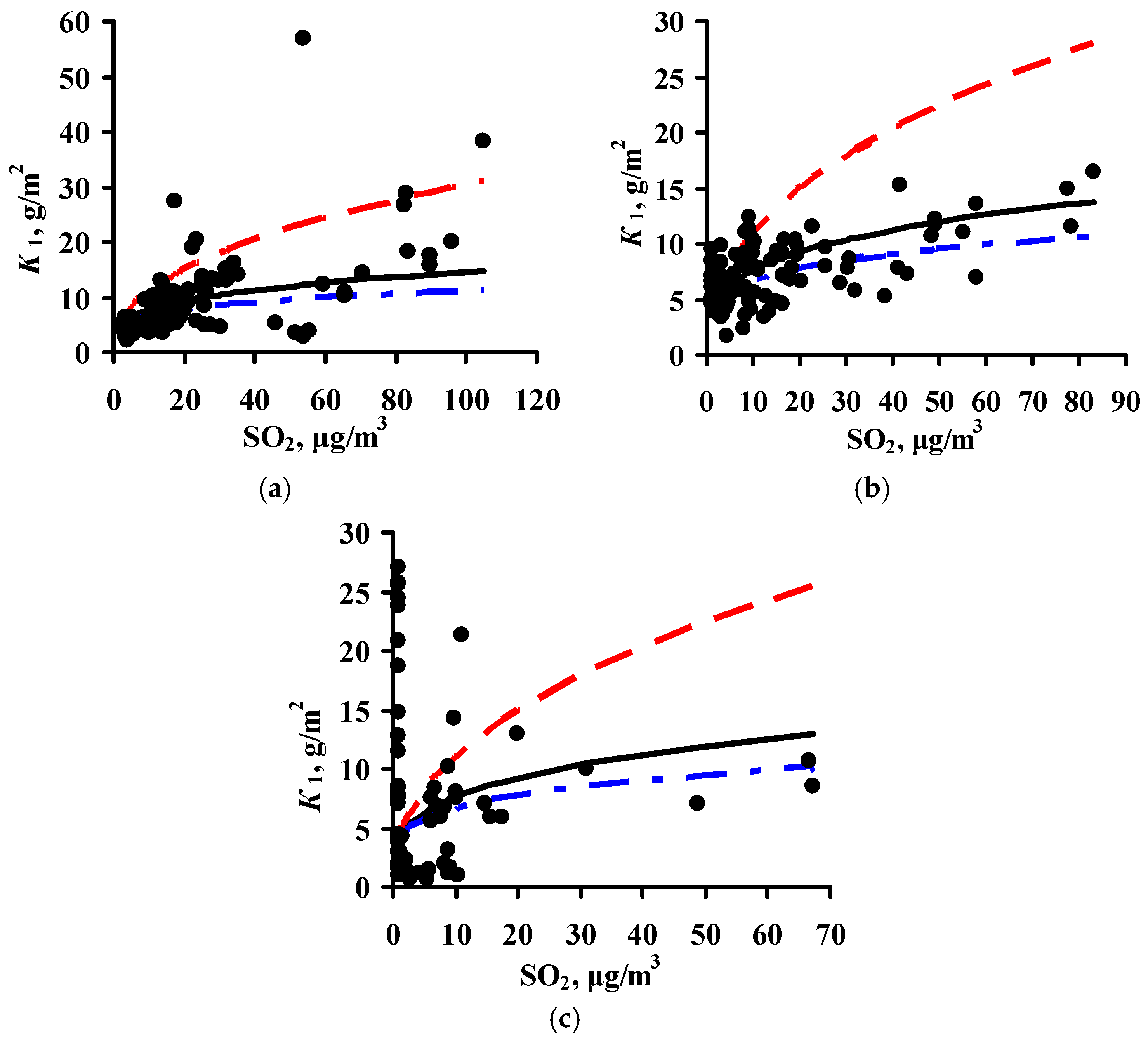
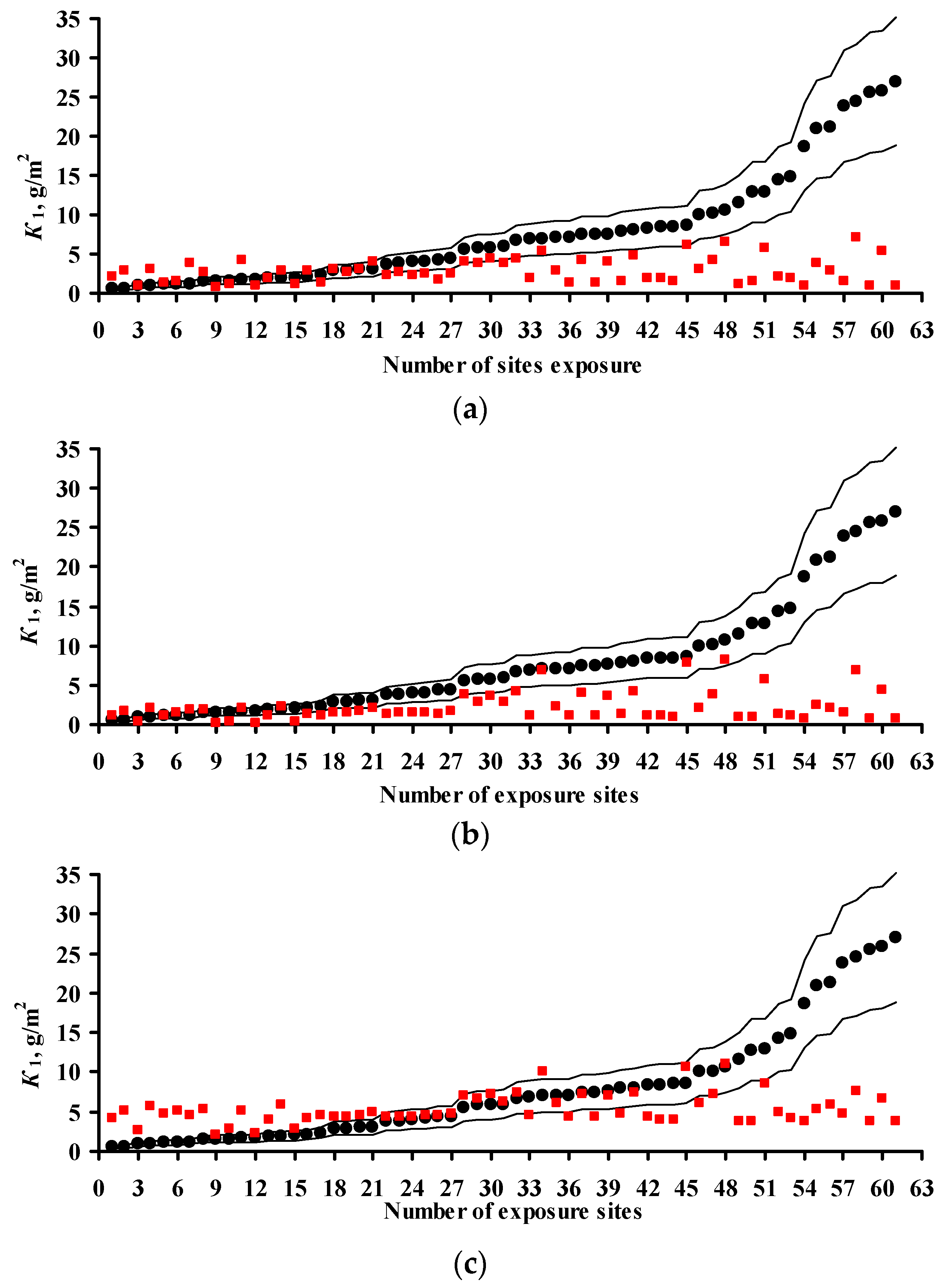
3.4. Predictions of K1 Using Various DRFs for Zinc
3.5. Analysis of DRFs for Zinc
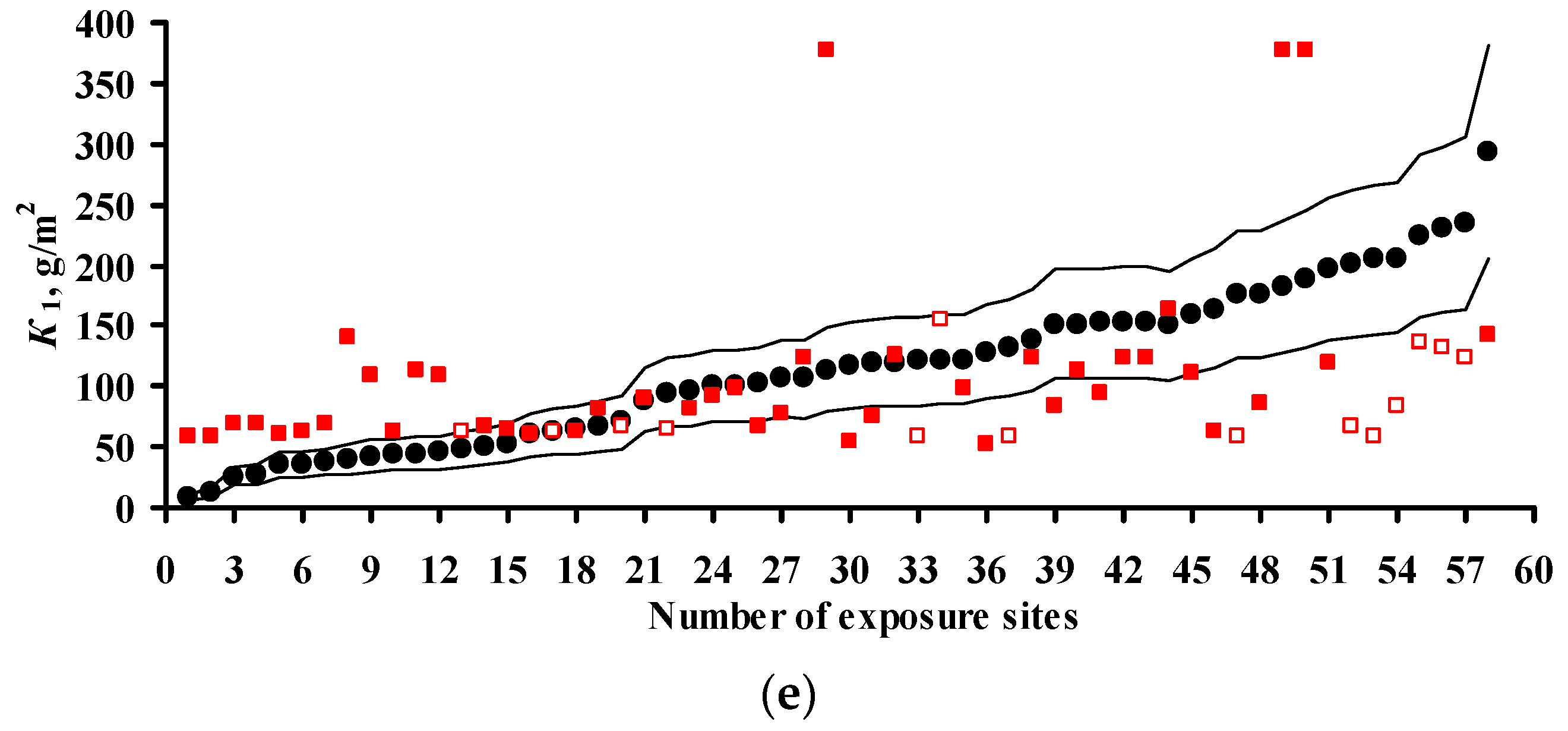
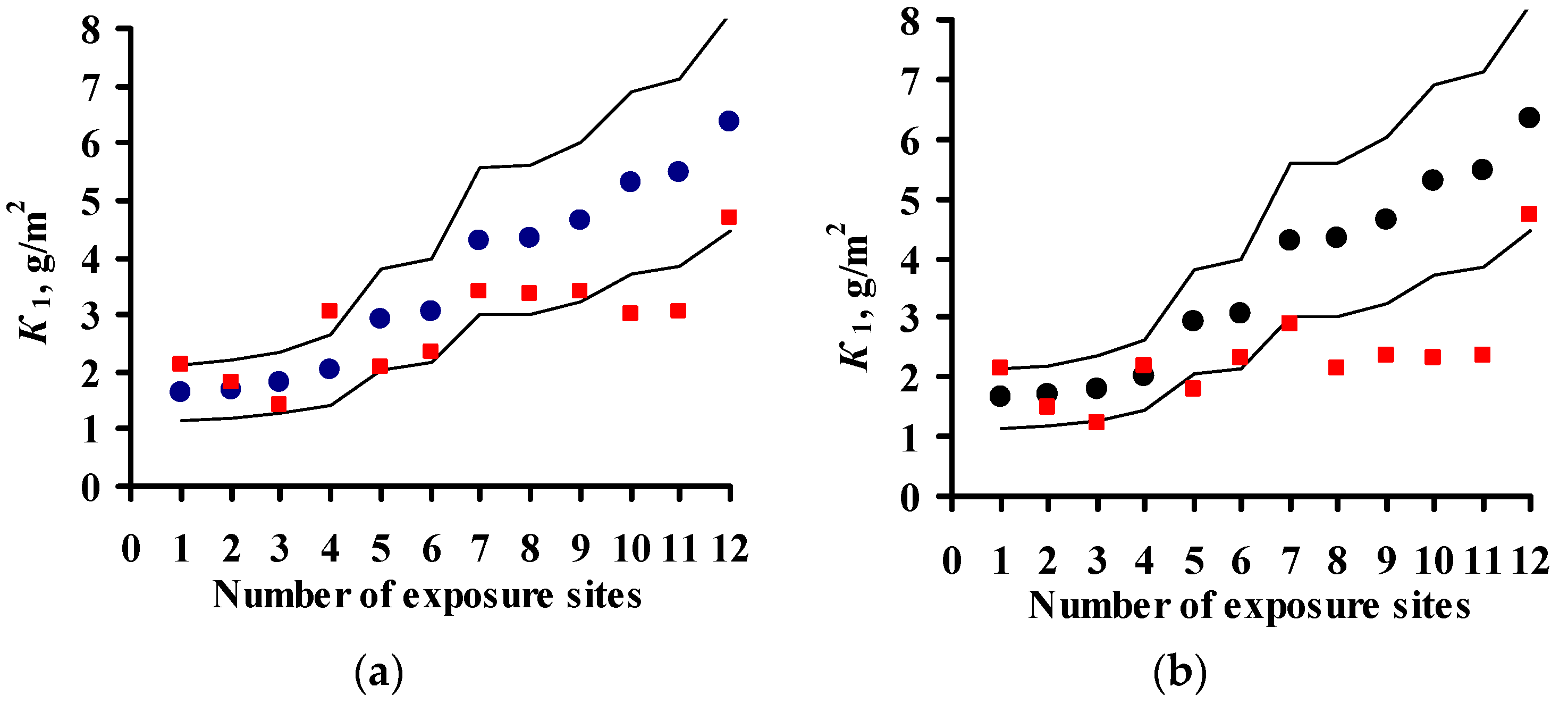
4. Estimation of Coefficients in DRFs for Carbon Steel and Zinc
5. Conclusions
- K = f(SO2) plots of corrosion losses of carbon steel and zinc vs. sulfur dioxide concentration were obtained to match, to a first approximation, the mean meteorological parameters of atmosphere corrosivity.
- Based on the K = f(SO2) relationships obtained, with consideration for the nonlinear effect of temperature on corrosion, New DRFs for carbon steel and zinc in continental territories were developed.
- Based on the corrosivity parameters at test locations under the UN /ECE and RF programs and the MICAT project, predictions of first-year corrosion losses of carbon steel and zinc were given using the New DRF, Standard DRF, and Unified DRF, as well as the linear model for carbon steel obtained in [20] with the aid of an artificial neural network. The predicted corrosion losses are compared with the experimental data for each DRF. It was shown that the predictions provided by the New DRFs for the first-year match the experimental data most accurately.
- An analysis of the values of the coefficients used in the DRFs for the prediction of corrosion losses of carbon steel and zinc is presented. It is shown that more accurate DRFs can be developed based on quantitative estimations of the effects of each atmosphere corrosivity parameter on corrosion.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCuen, R.H.; Albrecht, P.; Cheng, J.G. A New Approach to Power-Model Regression of Corrosion Penetration Data. In Corrosion Forms and Control for Infrastructure; Chaker, V., Ed.; American Society for Testing and Materials: Philadelphia, PA, USA, 1992. [Google Scholar]
- Syed, S. Atmospheric corrosion of materials. Emir. J. Eng. Res. 2006, 11, 1–24. [Google Scholar]
- De la Fuente, D.; Castano, J.G.; Morcillo, M. Long-term atmospheric corrosion of zinc. Corros. Sci. 2007, 49, 1420–1436. [Google Scholar] [CrossRef]
- Landolfo, R.; Cascini, L.; Portioli, F. Modeling of metal structure corrosion damage: A state of the art report. Sustainability 2010, 2, 2163–2175. [Google Scholar] [CrossRef]
- Morcillo, M.; de la Fuente, D.; Diaz, I.; Cano, H. Atmospheric corrosion of mild steel (article review). Rev. Metal. 2011, 47, 426–444. [Google Scholar] [CrossRef]
- De la Fuente, D.; Diaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Diaz, I.; Cano, H.; de la Fuente, D. Atmospheric corrosion data of weathering steels: A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef]
- Surnam, B.Y.R.; Chiu, C.W.; Xiao, H.P.; Liang, H. Long term atmospheric corrosion in Mauritius. CEST 2015, 50, 155–159. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I. Long-term prediction of metal corrosion losses in atmosphere using a power-linear function. Corros. Sci. 2016, 109, 217–229. [Google Scholar] [CrossRef]
- Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Guiding Values for the Corrosivity Categories; ISO 9224:2012(E); International Standards Organization: Geneva, Switzerlands, 2012.
- Rosales, B.M.; Almeida, M.E.M.; Morcillo, M.; Uruchurtu, J.; Marrocos, M. Corrosion y Proteccion de Metales en las Atmosferas de Iberoamerica; Programma CYTED: Madrid, Spain, 1998; pp. 629–660. [Google Scholar]
- Tidblad, J.; Kucera, V.; Mikhailov, A.A. Statistical Analysis of 8 Year Materials Exposure and Acceptable Deterioration and Pollution Levels; UN/ECE ICP on Effects on Materials; Swedish Corrosion Institute: Stockholm, Sweden, 1998; p. 49. [Google Scholar]
- Tidblad, J.; Mikhailov, A.A.; Kucera, V. Unified Dose-Response Functions after 8 Years of Exposure. In Quantification of Effects of Air Pollutants on Materials; UN ECE Workshop Proceedings; Umweltbundesamt: Berlin, Germany, 1999; pp. 77–86. [Google Scholar]
- Tidblad, J.; Kucera, V.; Mikhailov, A.A.; Henriksen, J.; Kreislova, K.; Yaites, T.; Stöckle, B.; Schreiner, M. UN ECE ICP Materials. Dose-response functions on dry and wet acid deposition effects after 8 years of exposure. Water Air Soil Pollut. 2001, 130, 1457–1462. [Google Scholar] [CrossRef]
- Tidblad, J.; Mikhailov, A.A.; Kucera, V. Acid Deposition Effects on Materials in Subtropical and Tropical Climates. Data Compilation and Temperate Climate Comparison; KI Report 2000:8E; Swedish Corrosion Institute: Stockholm, Sweden, 2000; pp. 1–34. [Google Scholar]
- Tidblad, J.; Mikhailov, A.A.; Kucera, V. Application of a Model for Prediction of Atmospheric Corrosion for Tropical Environments. In Marine Corrosion in Tropical Environments; Dean, S.W., Delgadillo, G.H., Bushman, J.B., Eds.; American Society for Testing and Materials: West Conshohocken, PA, USA, 2000; p. 18. [Google Scholar]
- Tidblad, J.; Kucera, V.; Mikhailov, A.A.; Knotkova, D. Improvement of the ISO Classification System Based on Dose-Response Functions Describing the Corrosivity of Outdoor Atmospheres. In Outdoor Atmospheric Corrosion, ASTM STP 1421; Townsend, H.E., Ed.; American Society for Testing and Materials: West Conshohocken, PA, USA, 2002; p. 73. [Google Scholar]
- Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation; ISO 9223:2012(E); International Standards Organization: Geneva, Switzerlands, 2012.
- Cai, J.; Cottis, R.A.; Lyon, S.B. Phenomenological modelling of atmospheric corrosion using an artificial neural network. Corros. Sci. 1999, 41, 2001–2030. [Google Scholar] [CrossRef]
- Pintos, S.; Queipo, N.V.; de Rincon, O.T.; Rincon, A.; Morcillo, M. Artificial neural network modeling of atmospheric corrosion in the MICAT project. Corros. Sci. 2000, 42, 35–52. [Google Scholar] [CrossRef]
- Diaz, V.; Lopez, C. Discovering key meteorological variables in atmospheric corrosion through an artificial neural network model. Corros. Sci. 2007, 49, 949–962. [Google Scholar] [CrossRef]
- Kenny, E.D.; Paredes, R.S.C.; de Lacerda, L.A.; Sica, Y.C.; de Souza, G.P.; Lazaris, J. Artificial neural network corrosion modeling for metals in an equatorial climate. Corros. Sci. 2009, 51, 2266–2278. [Google Scholar] [CrossRef]
- Reddy, N.S. Neural Networks Model for Predicting Corrosion Depth in Steels. Indian J. Adv. Chem. Sci. 2014, 2, 204–207. [Google Scholar]
- Knotkova, D.; Kreislova, K.; Dean, S.W. ISOCORRAG International Atmospheric Exposure Program: Summary of Results; ASTM Series 71; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Morcillo, M. Atmospheric corrosion in Ibero-America. The MICAT project. In Atmospheric Corrosion; Kirk, W.W., Lawson, H.H., Eds.; ASTM STP 1239; American Society for Testing and Materials: Philadelphia, PA, USA, 1995; pp. 257–275. [Google Scholar]
- Panchenko, Y.M.; Shuvakhina, L.N.; Mikhailovsky, Y.N. Atmospheric corrosion of metals in Far Eastern regions. Zashchita Metallov 1982, 18, 575–582. (In Russian) [Google Scholar]
- Knotkova, D.; Vlckova, J.; Honzak, J. Atmospheric Corrosion of Weathering Steels. In Atmospheric Corrosion of Metals; Dean, S.W., Jr., Rhea, E.C., Eds.; ASTM STP 767; American Society for Testing and Materials: Philadelphia, PA, USA, 1982; pp. 7–44. [Google Scholar]
- Tidblad, J.; Mikhailov, A.A.; Kucera, V. Model for the prediction of the time of wetness from average annual data on relative air humidity and air temperature. Prot. Met. 2000, 36, 533–540. [Google Scholar] [CrossRef]
- Feliu, S.; Morcillo, M.; Feliu, J.S. The prediction of atmospheric corrosion from meteorological and pollution parameters. I. Annual corrosion. Corros. Sci. 1993, 34, 403–422. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Nikolaeva, L.A.; Kovtanyuk, V.V.; Igonin, T.N.; Andryushchenko, T.A. Comparative estimation of long-term predictions of corrosion losses for carbon steel and zinc using various models for the Russian territory. CEST 2017, 52, 149–157. [Google Scholar] [CrossRef]
| MICAT Project | UN/ECE Program | ||||
|---|---|---|---|---|---|
| Country | Test Location | Designation | Country | Test Location | Designation |
| Argentina | Villa Martelli | A2 | Czech Republic | Prague | CS1 |
| Argentina | Iguazu | A3 | Czech Republic | Kasperske Hory | CS2 |
| Argentina | San Juan | A4 | Czech Republic | Kopisty | CS3 |
| Argentina | La Plata | A6 | Finland | Espoo | FIN4 |
| Brasil | Caratinga | B1 | Finland | Ähtäri | FIN5 |
| Brasil | Sao Paulo | B6 | Finland | Helsinki Vallila | FIN6 |
| Brasil | Belem | B8 | Germany | Waldhof Langenbrügge | GER7 |
| Brasil | Brasilia | B10 | Germany | Aschaffenburg | GER8 |
| Brasil | Paulo Afonso | B11 | Germany | Langenfeld Reusrath | GER9 |
| Brasil | Porto | B12 | Germany | Bottrop | GER10 |
| Colombia | San Pedro | CO2 | Germany | Essen Leithe | GER11 |
| Colombia | Cotove | CO3 | Germany | Garmisch Partenkirchen | GER12 |
| Ecuador | Guayaquil | EC1 | Netherlands | Eibergen | NL18 |
| Ecuador | Riobamba | EC2 | Netherlands | Vredepeel | NL19 |
| Spain | Leon | E1 | Netherlands | Wijnandsrade | NL20 |
| Spain | Tortosa | E4 | Norway | Oslo | NOR21 |
| Spain | Granada | E5 | Norway | Birkenes | NOR23 |
| Spain | Arties | E8 | Sweden | Stockholm South | SWE24 |
| Mexico | Mexico (a) | M1 | Sweden | Stockholm Centre | SWE25 |
| Mexico | Mexico (b) | M2 | Sweden | Aspvreten | SWE26 |
| Mexico | Cuernavaca | M3 | Spain | Madrid | SPA31 |
| Mexico | San Luis Potosi | PE4 | Spain | Toledo | SPA33 |
| Peru | Arequipa | PE5 | Russian Federation | Moscow | RUS34 |
| Peru | Arequipa | PE6 | Estonia | Lahemaa | EST35 |
| Peru | Pucallpa | U1 | Canada | Dorset | CAN37 |
| Uruguay | Trinidad | U3 | USA | Research Triangle Park | US38 |
| - | - | - | USA | Steubenville | US39 |
| Designation | T, °C | RH, % | TOW, Hours/a | Prec, mm/a | [SO2], μg/m3 | [H+], mg/L | Steel | Zinc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| g/m2 | No. | g/m2 | No. | |||||||
| CS1 | 9.5 | 79 | 2830 | 639.3 | 77.5 | - | 438.0 | 76 | 14.89 | 92 |
| CS1 | 10.3 | 74 | 2555 | 380.8 | 58.1 | 0.0221 | - | - | 6.98 | 45 |
| CS1 | 9.1 | 73 | 2627 | 684.3 | 41.2 | 0.0714 | 270.7 | 64 | 7.78 | 53 |
| CS1 | 9.8 | 77 | 3529 | 581.1 | 32.1 | 0.0342 | 241.0 | 58 | 5.69 | 31 |
| CS2 | 7.0 | 77 | 3011 | 850.2 | 19.7 | - | 224.0 | 51 | 8.95 | 65 |
| CS2 | 7.4 | 76 | 3405 | 703.4 | 25.6 | 0.045 | - | - | 7.99 | 58 |
| CS2 | 6.6 | 73 | 2981 | 921 | 17.9 | 0.1921 | 152.9 | 33 | 6.77 | 44 |
| CS2 | 7.2 | 74 | 3063 | 941.2 | 12.2 | 0.0366 | 148.2 | 30 | 3.46 | 4 |
| CS3 | 9.6 | 73 | 2480 | 426.4 | 83.3 | - | 557.0 | 77 | 16.41 | 94 |
| CS3 | 9.9 | 72 | 2056 | 416.6 | 78.4 | 0.0242 | - | - | 11.59 | 87 |
| CS3 | 8.9 | 71 | 2866 | 431.6 | 49 | 0.058 | 350.2 | 73 | 11.74 | 88 |
| CS3 | 9.7 | 75 | 2759 | 512.7 | 49.2 | 0.0567 | 351.8 | 74 | 12.17 | 89 |
| FIN4 | 5.9 | 76 | 3322 | 625.9 | 18.6 | - | 271.0 | 63 | - | - |
| FIN4 | 6.4 | 80 | 4127 | 657 | 13.9 | 0.0392 | - | - | 8.42 | 62 |
| FIN4 | 5.6 | 79 | 3446 | 754.6 | 2.3 | 0.0231 | 130.3 | 21 | 5.18 | 25 |
| FIN4 | 6.0 | 80 | 3607 | 698.1 | 2.6 | 0.0334 | 120.9 | 20 | 4.68 | 19 |
| FIN5 | 3.1 | 78 | 2810 | 801.3 | 6.3 | - | 132.0 | 23 | 8.92 | 66 |
| FIN5 | 3.9 | 80 | 3342 | 670.7 | 1.8 | 0.0271 | - | - | 7.70 | 52 |
| FIN5 | 3.4 | 81 | 2994 | 609.7 | 0.9 | 0.0201 | 48.4 | 4 | 6.62 | 41 |
| FIN5 | 3.9 | 83 | 3324 | 675.4 | 0.8 | 0.0247 | 59.3 | 5 | 4.61 | 16 |
| FIN6 | 6.3 | 78 | 3453 | 673.1 | 20.7 | - | 273.0 | 65 | - | - |
| FIN6 | 6.8 | 80 | 4017 | 665.6 | 15.3 | 0.0554 | - | - | 9.29 | 70 |
| FIN6 | 6.2 | 78 | 3360 | 702.4 | 4.8 | 0.0221 | 162.2 | 34 | 5.69 | 33 |
| FIN6 | 6.6 | 76 | 3288 | 649.2 | 5.5 | 0.0139 | 195.8 | 44 | 5.62 | 30 |
| GER7 | 9.3 | 80 | 4561 | 630.6 | 13.7 | - | 264.0 | 62 | - | - |
| GER7 | 10.2 | 80 | 4390 | 499.7 | 11 | 0.0358 | - | - | 7.85 | 56 |
| GER7 | 8.9 | 81 | 4382 | 624.4 | 8.2 | 0.0342 | 230.9 | 53 | 9.07 | 68 |
| GER7 | 9.5 | 81 | 4676 | 595.6 | 3.9 | 0.0265 | 166.1 | 36 | 4.25 | 13 |
| GER8 | 12.3 | 77 | 4282 | 626.9 | 23.7 | - | 213.0 | 48 | - | - |
| GER8 | 12.2 | 67 | 2541 | 655.4 | 14.2 | 0.0411 | - | - | 4.68 | 18 |
| GER8 | 11.4 | 64 | 3563 | 561.2 | 12.6 | 0.0183 | 116.2 | 17 | 5.18 | 26 |
| GER8 | 11.6 | 65 | 2359 | 779 | 9.6 | - | 141.2 | 27 | 4.10 | 12 |
| GER9 | 10.8 | 77 | 4220 | 782.9 | 24.5 | - | 293.0 | 69 | - | - |
| GER9 | 11.7 | 80 | 4940 | 697.6 | 20.3 | 0.0366 | - | - | 6.62 | 40 |
| GER9 | 10.7 | 79 | 4437 | 619.1 | 16.3 | 0.0291 | 230.9 | 54 | 9.07 | 69 |
| GER9 | 11.4 | 81 | 5210 | 841 | 11.1 | 0.0278 | 209.8 | 47 | 7.63 | - |
| GER10 | 11.2 | 75 | 4077 | 873.8 | 50.6 | - | 373.0 | 75 | - | - |
| GER10 | 12 | 76 | 4107 | 696.6 | 48.5 | 0.0253 | - | - | 10.66 | 81 |
| GER10 | 10.3 | 78 | 4201 | 707.3 | 41.6 | 0.0211 | 347.1 | 72 | 15.34 | 93 |
| GER10 | 11.8 | 80 | 4930 | 912.9 | 30.2 | 0.0334 | 294.1 | 70 | 7.85 | 55 |
| GER11 | 10.5 | 79 | 4537 | 713.1 | 30.3 | - | 342.0 | 71 | - | - |
| GER11 | 11.5 | 77 | 4040 | 644.5 | 25.6 | 0.042 | - | - | 9.72 | 73 |
| GER11 | 10.1 | 79 | 4120 | 683.6 | 22.9 | 0.0253 | 293.3 | 68 | 11.45 | 86 |
| GER11 | 10.9 | 78 | 4632 | 889.3 | 16.2 | 0.0247 | 241.0 | 57 | 7.06 | 46 |
| GER12 | 8.0 | 82 | 4989 | 1491.5 | 9.4 | - | 133.0 | 24 | 8.35 | 61 |
| GER12 | 7.3 | 82 | 4201 | 1183.1 | 6.1 | 0.0171 | - | - | 7.27 | 49 |
| GER12 | 7.1 | 84 | 4545 | 1552.4 | 3.2 | 0.0018 | 89.7 | 9 | 7.20 | 48 |
| GER12 | 7.4 | 83 | 4375 | 1503 | 2.4 | - | 85.0 | 8 | 3.74 | 9 |
| NL18 | 9.9 | 83 | 5459 | 904.2 | 10.1 | - | 232.0 | 55 | 9.93 | 76 |
| NL18 | 10.9 | 79 | 4482 | 705.9 | 8.5 | 0.0046 | - | - | 8.14 | 59 |
| NL18 | 9.5 | 82 | 4808 | 872.8 | 7.4 | 0.004 | 204.4 | 45 | 7.92 | 57 |
| NL18 | 10.3 | 83 | 5358 | 987.1 | 4.7 | 0.0366 | 144.3 | 28 | 4.75 | 20 |
| NL19 | 10.3 | 81 | 5354 | 845 | 13 | - | 283.0 | 66 | - | - |
| NL19 | 11 | 81 | 4969 | 569.1 | 9.9 | 0.0049 | - | - | 9.07 | 67 |
| NL19 | 10 | 82 | 5084 | 749.2 | 8.3 | 0.0021 | 238.7 | 56 | 11.09 | 84 |
| NL19 | 10.9 | 83 | 5454 | 828.9 | 4.5 | - | 180.2 | 39 | - | - |
| NL20 | 10.3 | 81 | 5125 | 801.3 | 13.7 | - | 259.0 | 59 | - | - |
| NL20 | 11.1 | 77 | 4424 | 608.8 | 10.3 | 0.0106 | - | - | 10.22 | 77 |
| NL20 | 10.1 | 81 | 4688 | 679.6 | 9.3 | 0.0113 | 205.1 | 46 | 11.38 | 85 |
| NL20 | 11.1 | 82 | 5141 | 789.9 | 5.8 | 0.0038 | 172.4 | 37 | 6.34 | 37 |
| NOR21 | 7.6 | 70 | 2673 | 1023.8 | 14.4 | - | 229.0 | 52 | - | - |
| NOR21 | 8.8 | 70 | 2864 | 526.6 | 7.9 | 0.0326 | - | - | 5.69 | 32 |
| NOR21 | 7.7 | 68 | 2471 | 440.1 | 6 | 0.0156 | 134.9 | 25 | 6.70 | 43 |
| NOR21 | 7.5 | 69 | 2827 | 680 | 2.9 | 0.0136 | 100.6 | 11 | 3.53 | 7 |
| NOR23 | 6.5 | 80 | 4831 | 2144.3 | 1.3 | - | 194.0 | 43 | - | - |
| NOR23 | 7.4 | 77 | 4193 | 1762.2 | 0.9 | 0.042 | - | - | 8.50 | 63 |
| NOR23 | 5.9 | 75 | 3341 | 1188.6 | 0.7 | 0.0374 | 131.8 | 22 | 10.58 | 80 |
| NOR23 | 6.4 | 76 | 3779 | 1419.7 | 0.7 | 0.0326 | 109.2 | 15 | 5.04 | 24 |
| SWE24 | 7.6 | 78 | 3959 | 531 | 16.8 | - | 264.0 | 61 | 10.36 | 79 |
| SWE24 | 8.7 | 70 | 3074 | 473.2 | 8.4 | 0.0366 | - | - | 6.12 | 35 |
| SWE24 | 7 | 70 | 2580 | 577 | 5.7 | 0.043 | 120.1 | 18 | 4.54 | 15 |
| SWE24 | 7.5 | 73 | 3160 | 580.6 | 4.2 | 0.0231 | 103.0 | 13 | 4.25 | 14 |
| SWE25 | 7.6 | 78 | 3959 | 531 | 19.6 | - | 263.0 | 60 | 9.76 | 74 |
| SWE25 | 8.7 | 70 | 3074 | 473.2 | 10.3 | 0.0366 | - | - | 5.62 | 29 |
| SWE25 | 7 | 70 | 2580 | 577 | 4.7 | 0.043 | 103.0 | 12 | 3.53 | 5 |
| SWE25 | 7.5 | 73 | 3160 | 580.6 | 3.4 | 0.0231 | 95.2 | 10 | 3.53 | 8 |
| SWE26 | 6.0 | 83 | 4534 | 542.7 | 3.3 | - | 147.0 | 29 | 8.31 | 60 |
| SWE26 | 7.6 | 77 | 3469 | 342.3 | 2 | 0.043 | - | - | 6.70 | 42 |
| SWE26 | 6 | 81 | 3592 | 467.8 | 1.3 | 0.043 | 74.9 | 6 | 4.90 | 23 |
| SWE26 | 6.8 | 82 | 4118 | 525.2 | 1.1 | 0.0278 | 81.1 | 7 | 6.05 | 34 |
| SPA31 | 14.1 | 66 | 2762 | 398 | 18.4 | - | 222.0 | 50 | 7.74 | 54 |
| SPA31 | 15.2 | 56 | 1160 | 331.5 | 15.3 | 0.0073 | - | - | 4.82 | 22 |
| SPA31 | 14.3 | 67 | 2319 | 360.1 | 8.2 | 0.0003 | 162.2 | 35 | 3.53 | 6 |
| SPA31 | 15.7 | 68 | 2766 | 223.9 | 7.8 | 0.0002 | 151.3 | 32 | 2.30 | 2 |
| SPA33 | 14.0 | 64 | 2275 | 785 | 3.3 | - | 45.0 | 3 | 3.37 | 3 |
| SPA33 | 15.5 | 61 | 2147 | 610.4 | 13.5 | 0.0006 | - | - | 3.89 | 11 |
| SPA33 | 13.4 | 61 | 1888 | 432.5 | 1.7 | 0.0012 | 25.7 | 1 | 3.89 | 10 |
| SPA33 | 14.8 | 57 | 1465 | 327.4 | 4.2 | 0.0006 | 35.9 | 2 | 1.66 | 1 |
| RUS34 | 5.5 | 73 | 2084 | 575.4 | 19.2 | - | 181.0 | 40 | 10.32 | 78 |
| RUS34 | 5.7 | 76 | 2894 | 860.2 | 30.8 | 0.0006 | - | - | 8.64 | 64 |
| RUS34 | 5.7 | 74 | 2444 | 880.6 | 28.7 | 0.0009 | 141.2 | 26 | 6.48 | 39 |
| RUS34 | 5.6 | 71 | 1514 | 666.7 | 16.4 | 0.0008 | 120.9 | 19 | 4.61 | 17 |
| EST35 | 5.5 | 83 | 4092 | 447.8 | 0.9 | - | 185.0 | 41 | 7.18 | 47 |
| EST35 | 6.7 | 81 | 4332 | 532.7 | 0.6 | 0.0226 | - | - | 9.43 | 71 |
| CAN37 | 5.5 | 75 | 3252 | 961.1 | 3.3 | - | 149.0 | 31 | 9.88 | 75 |
| CAN37 | 5 | 79 | 3431 | 1103 | 3 | 0.042 | - | - | 6.26 | 38 |
| CAN37 | 4.3 | 80 | 3302 | 1080 | 2.1 | 0.0482 | 110.0 | 16 | 5.26 | 27 |
| CAN37 | 5.2 | 80 | 3386 | 1022.8 | 3.3 | 0.0461 | 103.7 | 14 | 6.19 | 36 |
| US38 | 14.6 | 69 | 3178 | 846.7 | 9.6 | - | 176.0 | 38 | 10.72 | 82 |
| US38 | 16.3 | 66 | 3026 | 1106.7 | 9.2 | 0.0358 | - | - | 12.46 | 90 |
| US38 | 15.5 | 64 | 2644 | 982.3 | 10.1 | 0.0349 | 184.9 | 42 | 9.72 | 72 |
| US38 | 15.8 | 68 | - | 1037.6 | 9.3 | 0.0482 | - | - | 4.75 | 21 |
| US39 | 12.3 | 67 | 2111 | 733.1 | 58.1 | - | 214.0 | 49 | 13.61 | 91 |
| US39 | 11.2 | 61 | 1391 | 967.4 | 55.2 | 0.0838 | - | - | 11.02 | 83 |
| US39 | 11.8 | 65 | 1532 | 729.4 | 43.1 | 0.0941 | 290.2 | 67 | 7.34 | 50 |
| US39 | 11.8 | 69 | - | 756.8 | 38.3 | 0.0765 | - | - | 5.26 | 28 |
| Designation | T, °C | RH, % | Rain, mm/a | [SO2], μg/m3 | Cl−, mg/(m2·Day) | TOW, h/a | Steel | Zinc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| g/m2 | No. | g/m2 | No. | |||||||
| A2 * | 16.7 | 75 | 1729 | 10 | Ins | 5063 | 122.5 | 36 (34) | 8.06 | 41 |
| A2 | 17.1 | 72 | 983 | 10 | Ins | 4222 | 125.6 | 38 | 7.56 | 39 |
| A2 | 17.0 | 74 | 1420 | 9 | Ins | 4862 | 96.7 | 25 | 10.15 | 47 |
| A3 | 20.6 | 76 | 2158 | Ins (5) ** | Ins (1.5) | 5825 | 44.5 | 12 (11) | 14.76 | 53 |
| A3 | 20.9 | 74 | 2624 | Ins (5) | Ins (1.5) | 5528 | 45.2 | 13 (12) | 8.42 | 43 |
| A3 | 22.1 | 75 | 1720 | Ins (5) | Ins (1.5) | 5545 | 43.7 | 10 (9) | 8.50 | 44 |
| A4 | 18.0 | 51 | 35 | Ins (5) | Ins (1.5) | 999 | 35.9 | 6 (6) | 2.02 | 15 |
| A4 | 20.0 | 49 | 111 | Ins (5) | Ins (1.5) | 850 | 35.1 | 5 (5) | 0.94 | 3 |
| A4 | 18.3 | 51 | 93 | Ins (5) | Ins (1.5) | 867 | 43.7 | 11 (10) | 1.58 | 10 |
| A6 | 17.0 | 78 | 1178 | 6.22 | Ins | 5195 | 197.3 | 55 (51) | 5.54 | 28 |
| A6 * | 16.7 | 77 | 1263 | 8.21 | Ins | 4949 | 224.6 | 59 (55) | 6.70 | 32 |
| A6 * | 16.6 | 78 | 1361 | 6.2 | Ins | 5528 | 234.8 | 61 (57) | 7.49 | 37 |
| B1 | 21.2 | 75 | 996 | 1.67 | 1.57 | 4222 | 102.2 | 28(26) | 4.32 | 26 |
| B6 | 19.7 | 75 | 1409 | 67.2 (28) | Ins (1.5) | 5676 | 113.9 | 31 (29) | 8.57 | 45 |
| B6 | 19.5 | 76 | 1810 (1910) | 66.8 (28) | Ins (1.5) | 5676 | 182.5 | 53 (49) | 10.66 | 48 |
| B6 | 19.6 | 75 | 1034 | 48.8 (28) | Ins (1.5) | 5676 | 188.8 | 54 (50) | 6.98 | 34 |
| B8 | 26.1 | 88 | 2395 | Ins (5) | Ins (1.5) | 5974 | 151.3 | 44 (40) | 7.92 | 40 |
| B10 | 20.4 | 69 (72) | 1440 | Ins (5) | Ins (1.5) | 3872 | 100.6 | 26 (24) | 12.82 | 50 |
| B11 | 25.9 | 77 | 1392 | Ins | Ins | 1507 | 134.9 | 41 | 11.52 | 49 |
| B12 | 26.6 | 90 | 2096 | Ins | Ins | 4222 | 38.2 | 8 | 23.83 | 57 |
| CO2 | 9.6 (14.1) | 98 (81) | 1800 | 0.56 (5) | Ins (1.5) | 8760 (7008) | 106.9 | 30 (28) | 24.48 | 58 |
| CO2 | 11.4 | 90 | 1800 | 0.56 (5) | Ins (1.5) | 8760 (7808) | 138.1 | 42 (38) | 25.78 | 60 |
| CO2 | 13.5 (14.2) | 81 (73) | 1800 | 0.56 (5) | Ins (1.5) | 8760 (7808) | 152.9 | 46 (42) | 20.88 | 55 |
| CO3 * | 27.0 | 76 | 900 | 0.33 | Ins | 2891 | 120.9 | 35 (33) | 18.65 | 54 |
| CO3 * | 27.0 | 76 | 900 | 0.33 | Ins | 2891 | 204.4 | 57 (53) | 27.00 | 61 |
| CO3 * | 27.0 | 76 | 900 | 0.33 | Ins | 2891 | 132.6 | 40 (37) | 25.56 | 59 |
| EC1 | 26.1 | 71 | 936 | 4.20 | 1.5 | 4853 | 152.1 | 45 (41) | 1.08 | 5 |
| EC1 | 26.9 | 82 | 635 | 2.72 | 1.31 | 5790 | 176.3 | 52 (48) | 1.15 | 6 |
| EC1 * | 24.8 | 75 | 564 | 2.1 | 1.66 | 3101 | 201.2 | 56 (52) | 2.38 | 17 |
| EC2 | 12.9 | 66 | 554 | 1.0 | 0.4 | 3583 | 60.8 | 17 (16) | - | - |
| EC2 * | 13.2 | 71 | 598 | 1.35 | 1.14 | 4932 | 70.2 | 21 (20) | - | - |
| E1 | 12.0 | 69 | 652 | 1.18 (16.2) | 1.5 | 3364 | 158.3 (150.5) | 48 (44) | 3.02 | 20 |
| E1 * | 10.6 | 65 | 495 | 1.18 | 1.5 | 2374 | 175.5 | 51 (47) | 2.88 | 18 |
| E1 | 11.1 | 63 | 334 | 1.18 (16.2) | 1.5 | 2111 | 153.7 | 47 (43) | 2.09 | 16 |
| E4 | 18.1 | 65 | 554 | 8.3 | 1.5 | 3416 | 158.3 | 49 (45) | 1.94 | 14 |
| E4 | 17.0 | 63 | 521 | 5.7 | 1.5 | 2646 | 151.3 | 43 (39) | 1.51 | 8 |
| E4 | 17.2 | 62 | 374 | 1.9 | 1.5 | 2768 | 163.8 | 50 (46) | 1.94 | 13 |
| E5 | 16.3 | 59 | 416 | 10.3 | 1.5 | 1323 | 95.9 | 24 (23) | 1.01 | 4 |
| E5 | 15.0 (15.8) | 59 (58) | 258 (239) | 5.4 | 1.5 | 1104 | 53.0 | 16 (15) | 0.65 | 2 |
| E5 | 15.6 | 58 | 266 | 2.8 | 1.5 | 2400 | 49.9 | 15 (14) | 0.65 | 1 |
| E8 | 8.8 | 52 (72) | 738 | 9.1 | 1.8 | 876 | 25.7 | 3 (3) | 1.66 | 11 |
| E8 | 6.9 | 52 (72) | 624 | 8.9 | 1.6 | 876 | 28.1 | 4 (4) | 1.22 | 7 |
| E8 | 7.8 | 52 (72) | 681 | 9.0 | 1.7 | 876 | 37.4 | 7 (7) | 3.10 | 21 |
| M1 | 16.0 | 62 | 743 | 15.6 | 1.5 | 2523 (2321) | 120.1 | 34 (32) | 5.83 | 29 |
| M1 | 14.8 (15.2) | 66 (65) | 747 | 7.7 (5.6) | 1.5 | 2523 | 67.1 | 20 (19) | 5.98 | 31 |
| M1 | 15.4 | 64 (63) | 747 | 17.5 | 1.5 | 2523 (2427) | 39.8 | 9 (8) | 5.83 | 30 |
| M2 | 21.0 | 56 | 1352 | 6.7 | 1.5 | 1664 | 118.6 | 33 (31) | 8.35 | 42 |
| M2 | 21.0 | 56 | 1724 | 9.9 | Ins (1.5) | 1857 | 88.9 | 22 (21) | 14.33 | 52 |
| M2 | 21.0 | 56 | 1372 | 7.1 | Ins (1.5) | 1752 | 106.9 | 29 (27) | 6.84 | 33 |
| M3 | 18.0 | 51 | 374 | 31.1 | Ins | 1410 | 292.5 | 62 (58) | 10.01 | 46 |
| M3 * | 18.0 | 62 | 374 | 10.9 | Ins | 1410 | 205.9 | 58 (54) | 21.24 | 56 |
| M3 * | 18.0 | 60 | 374 | 14.6 | Ins | 2646 | 229.3 | 60 (56) | 7.06 | 35 |
| PE4 | 16.4 | 37 | 17 | Ins (5) | Ins (1.5) | 26 | 117.0 | 32 (30) | 1.66 | 12 |
| PE4 | 17.2 | 33 | 34 (89) | Ins (5) | Ins (1.5) | 175 (26) | 128.7 | 39 (36) | 1.58 | 9 |
| PE5 | 12.2 | 67 | 632 | Ins (0) | Ins (0) | 2847 | 7.8 | 1 (1) | 3.89 | 23 |
| PE5 | 12.2 | 67 | 672 (792) | Ins (0) | Ins (0) | 2689 (2847) | 13.3 | 2 (2) | 2.88 | 19 |
| PE6 | 25.4 | 84 | 1523 | Ins (5) | Ins (1.5) | 5037 (4580) | 122.5 | 37 (35) | 7.06 | 36 |
| PE6 | 25.8 | 83 | 1158 (1656) | Ins (5) | Ins (1.5) | 5790 (4380) | 100.6 | 27 (25) | 7.49 | 38 |
| U1 | 16.8 | 74 | 1182 | 0.6 (1) | 1.8 (2.2) | 5133 | 64.0 | 19 (18) | 4.03 | 24 |
| U1 * | 16.6 | 73 | 1324 | 0.8 | 1.2 | 4976 | 62.4 | 18 (17) | 3.74 | 22 |
| U1 * | 16.7 | 76 | 1306 | Ins | Ins | 4792 | 47.6 | 14 (13) | 4.10 | 25 |
| U3 * | 17.7 | 79 | 1490 | Ins | Ins | 5764 | 94.4 | 23 (22) | 4.39 | 27 |
| CH1 | 14.2 | 71 | 355 | 20 | 2.18 | 3469 | 221.5 | 63 | 12.89 | 51 |
| Test Location | T, °C | RH, % | Prec, mm/a | [SO2], μg/m3 | Steel | Zinc | ||
|---|---|---|---|---|---|---|---|---|
| g/m2 | No. | g/m2 | No. | |||||
| Bilibino | −12.2 | 80 | 218 | 3 | 5.4 | 1 | 1.64 | 1 |
| Oimyakon | −16.6 | 71 | 175 | 3 | 8.1 | 2 | 1.81 | 3 |
| Ust-Omchug | −11 | 70 | 317 | 5 | 12.4 | 3 | 2.91 | 5 |
| Atka | −12 | 72 | 376 | 3 | 15.2 | 4 | 1.69 | 2 |
| Susuman | −13.2 | 71 | 283 | 10 | 17.0 | 5 | 3.07 | 6 |
| Tynda | −6.5 | 72 | 525 | 5 | 21.2 | 6 | 5.30 | 10 |
| Klyuchi | 1.4 | 69 | 253 | 3 | 23.4 | 7 | 2.03 | 4 |
| Aldan | −6.2 | 72 | 546 | 5 | 24.6 | 8 | 5.47 | 11 |
| Pobedino | −0.9 | 77 | 604 | 3 | 36.5 | 9 | 4.30 | 7 |
| Yakovlevka | 2.5 | 70 | 626 | 3 | 40.6 | 10 | 4.64 | 9 |
| Pogranichnyi | 3.6 | 67 | 595 | 3 | 49.0 | 11 | 4.32 | 8 |
| Komsomolsk-on-Amur | −0.7 | 76 | 499 | 10 | 63.2 | 12 | 6.35 | 12 |
| [SO2], μg/m3 | Cl−, mg/(m2·Day) | K1, g/m2 |
|---|---|---|
| 3 | 2 | 137.7 |
| 5 | 0,3 | 46.1 |
| 5 | 0,7 | 130.7 |
| 8 | 1 | 137.7 |
| 8 | 0 | 140.0 |
| 14 | 2 | 193.8 |
| 15 | 2 | 228.4 |
| 15 | 1 | 236.1 |
| 17 | 0,16 | 136.1 |
| 26 | 1 | 236.1 |
| 32 | 2 | 276.1 |
| 116 | 0,62 | 232.2 |
| Locations with Uncertain Data | Locations with Trusted Data | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Designation | No. | T, °C | RH, % | TOW, 1/a | Prec, mm/a | [SO2], μg/m3 | K1exp | Designation | No. | T, °C | RH, % | TOW, 1/a | Prec, mm/a | [SO2], μg/m3 | K1exp | ||
| µm | g/m2 | µm | g/m2 | ||||||||||||||
| PE4 | 32 | 16.4 | 37 | 0.003 | 17 | 1 | 15.0 | 117.0 | E8 | 3 | 8.8 | 52 | 0.100 | 738 | 9.1 | 3.3 | 25.7 |
| PE4 | 39 | 17.2 | 33 | 0.020 | 34 | 1 | 16.5 | 128.7 | E8 | 4 | 6.9 | 52 | 0.100 | 624 | 8.9 | 3.6 | 28.1 |
| A4 | 5 | 20.0 | 49 | 0.097 | 111 | 1 | 4.5 | 35.1 | E8 | 7 | 7.8 | 52 | 0.100 | 681 | 9 | 4.8 | 37.4 |
| A4 | 6 | 18.0 | 51 | 0.114 | 35 | 1 | 4.6 | 35.9 | M2 | 29 | 21.0 | 56 | 0.200 | 1372 | 7.1 | 13.7 | 106.9 |
| M3 | 58 | 18.0 | 62 | 0.161 | 374 | 10.9 | 26.4 | 205.9 | M2 | 33 | 21.0 | 56 | 0.190 | 1352 | 6.7 | 15.2 | 118.6 |
| M3 | 62 | 18.0 | 51 | 0.161 | 374 | 31.1 | 37.5 | 292.5 | M2 | 22 | 21.0 | 56 | 0.212 | 1724 | 9.9 | 11.4 | 88.9 |
| M3 | 60 | 18.0 | 60 | 0.302 | 374 | 14.6 | 29.4 | 229.3 | E5 | 15 | 15.6 | 58 | 0.161 | 266 | 2.8 | 6.4 | 49.9 |
| E1 | 47 | 11.1 | 63 | 0.241 | 334 | 1.18 | 19.7 | 153.7 | E5 | 16 | 15.0 | 59 | 0.126 | 258 | 5.4 | 6.8 | 53.0 |
| E1 | 48 | 12.0 | 69 | 0.384 | 652 | 1.18 | 20.3 | 158.3 | M1 | 34 | 16.0 | 62 | 0.288 | 743 | 15.6 | 15.4 | 120.1 |
| E1 | 51 | 10.6 | 65 | 0.271 | 495 | 1.18 | 22.5 | 175.5 | M1 | 9 | 15.4 | 64 | 0.288 | 743 | 17.5 | 5.1 | 39.8 |
| E4 | 43 | 17.0 | 63 | 0.302 | 521 | 5.7 | 19.4 | 151.3 | M1 | 20 | 14.8 | 66 | 0.288 | 743 | 7.7 | 8.6 | 67.1 |
| E4 | 49 | 18.1 | 65 | 0.390 | 554 | 8.3 | 20.3 | 158.3 | A2 | 38 | 17.1 | 72 | 0.482 | 983 | 10.0 | 16.1 | 125.6 |
| E4 | 50 | 17.2 | 62 | 0.316 | 374 | 1.9 | 21.0 | 163.8 | A2 | 36 | 16.7 | 75 | 0.578 | 1729 | 10.0 | 15.7 | 122.5 |
| B10 | 26 | 20.4 | 69 | 0.442 | 1440 | 1 | 12.9 | 100.6 | A2 | 25 | 17.0 | 74 | 0.555 | 1420 | 9 | 12.4 | 96.7 |
| B1 | 28 | 21.2 | 75 | 0.484 | 996 | 1.67 | 13.1 | 102.2 | A3 | 12 | 20.6 | 76 | 0.665 | 2158 | 1 | 5.7 | 44.5 |
| CO3 | 40 | 27.0 | 76 | 0.330 | 900 | 1 | 17.0 | 132.6 | A3 | 13 | 20.9 | 74 | 0.631 | 2624 | 1 | 5.8 | 45.2 |
| CO3 | 57 | 27.0 | 76 | 0.330 | 900 | 1 | 26.2 | 204.4 | A3 | 10 | 22.1 | 75 | 0.633 | 1720 | 1 | 5.6 | 43.7 |
| B11 | 41 | 25.9 | 77 | 0.172 | 1392 | 1 | 17.3 | 134.9 | - | - | - | - | - | - | - | - | - |
| EC1 | 56 | 24.8 | 75 | 0.354 | 564 | 2.1 | 25.8 | 201.2 | - | - | - | - | - | - | - | - | - |
| EC1 | 52 | 26.9 | 82 | 0.661 | 635 | 2.72 | 22.6 | 176.3 | - | - | - | - | - | - | - | - | - |
| DRF | A | α | k1 | k2 | k3 | ||
|---|---|---|---|---|---|---|---|
| µm | g/m2 | T ≤ 10 | T > 10 | ||||
| New | 0.99 | 7.7 | 0.47 | 0.024 | 0.095 | −0.095 | 0.00056 |
| Standard | 1.77 | 13.8 | 0.52 | 0.020 | 0.150 | −0.054 | - |
| Unified | 3.54 | 27.6 | 0.13 | 0.020 | 0.059 | −0.036 | - |
| DRF | A | α | k1 | k2 | k3 | B | |||
|---|---|---|---|---|---|---|---|---|---|
| µm | g/m2 | T ≤ 10 | T > 10 | µg | g/m2 | ||||
| New | 0.0986 | 0.71 | 0.28 | 0.022 | 0.045 | −0.085 | 0.0001 | - | - |
| Standard | 0.0129 | 0.0929 | 0.44 | 0.046 | 0.038 | −0.071 | - | - | - |
| Unified | 0.188 | 1.35 | 0.22 | 0.018 | 0.062 | −0.021 | - | 0.00403 | 0.029 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchenko, Y.M.; Marshakov, A.I. Prediction of First-Year Corrosion Losses of Carbon Steel and Zinc in Continental Regions. Materials 2017, 10, 422. https://doi.org/10.3390/ma10040422
Panchenko YM, Marshakov AI. Prediction of First-Year Corrosion Losses of Carbon Steel and Zinc in Continental Regions. Materials. 2017; 10(4):422. https://doi.org/10.3390/ma10040422
Chicago/Turabian StylePanchenko, Yulia M., and Andrey I. Marshakov. 2017. "Prediction of First-Year Corrosion Losses of Carbon Steel and Zinc in Continental Regions" Materials 10, no. 4: 422. https://doi.org/10.3390/ma10040422





