Strong Photoluminescence Enhancement of Silicon Oxycarbide through Defect Engineering
Abstract
:1. Introduction
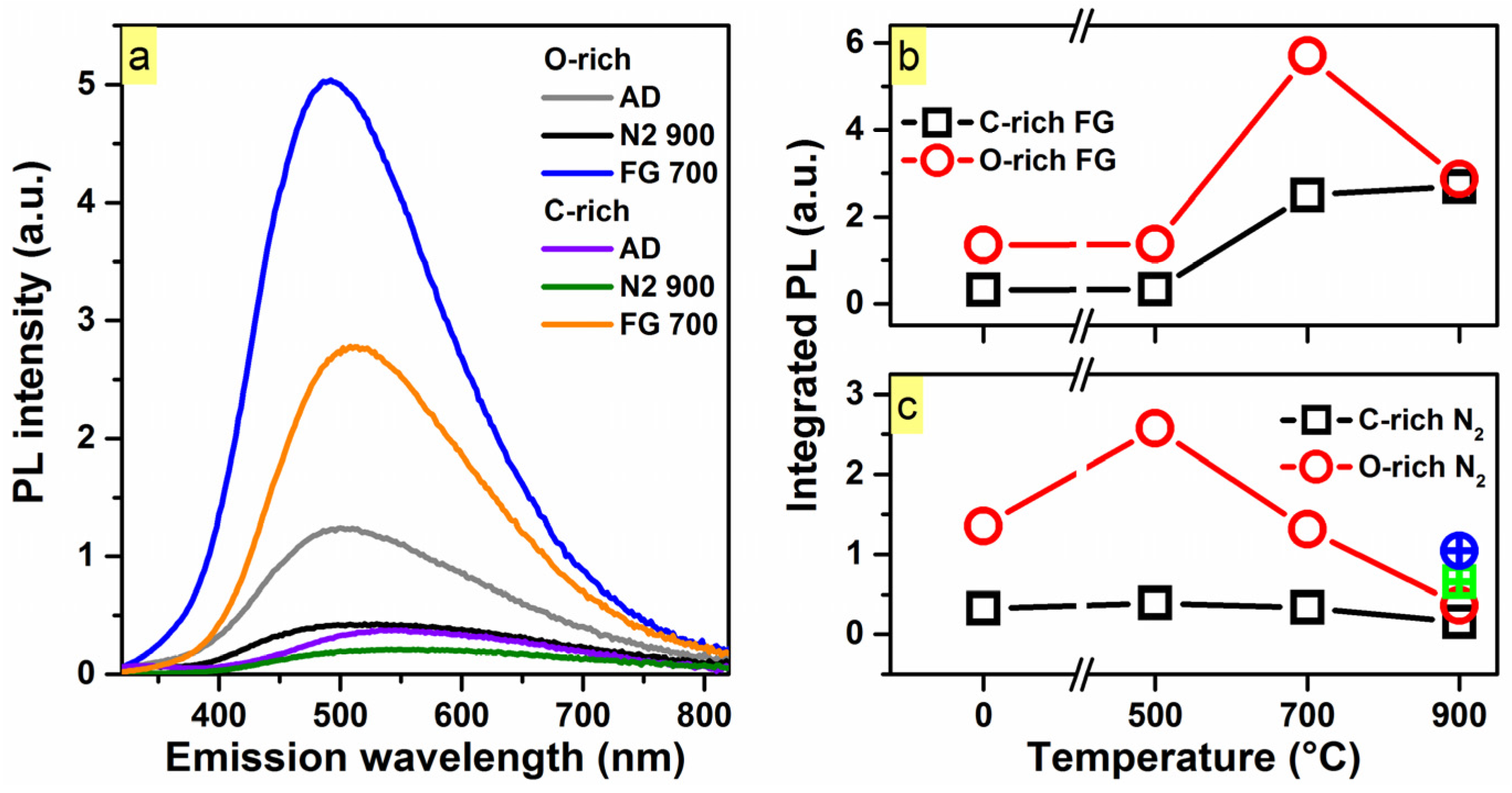
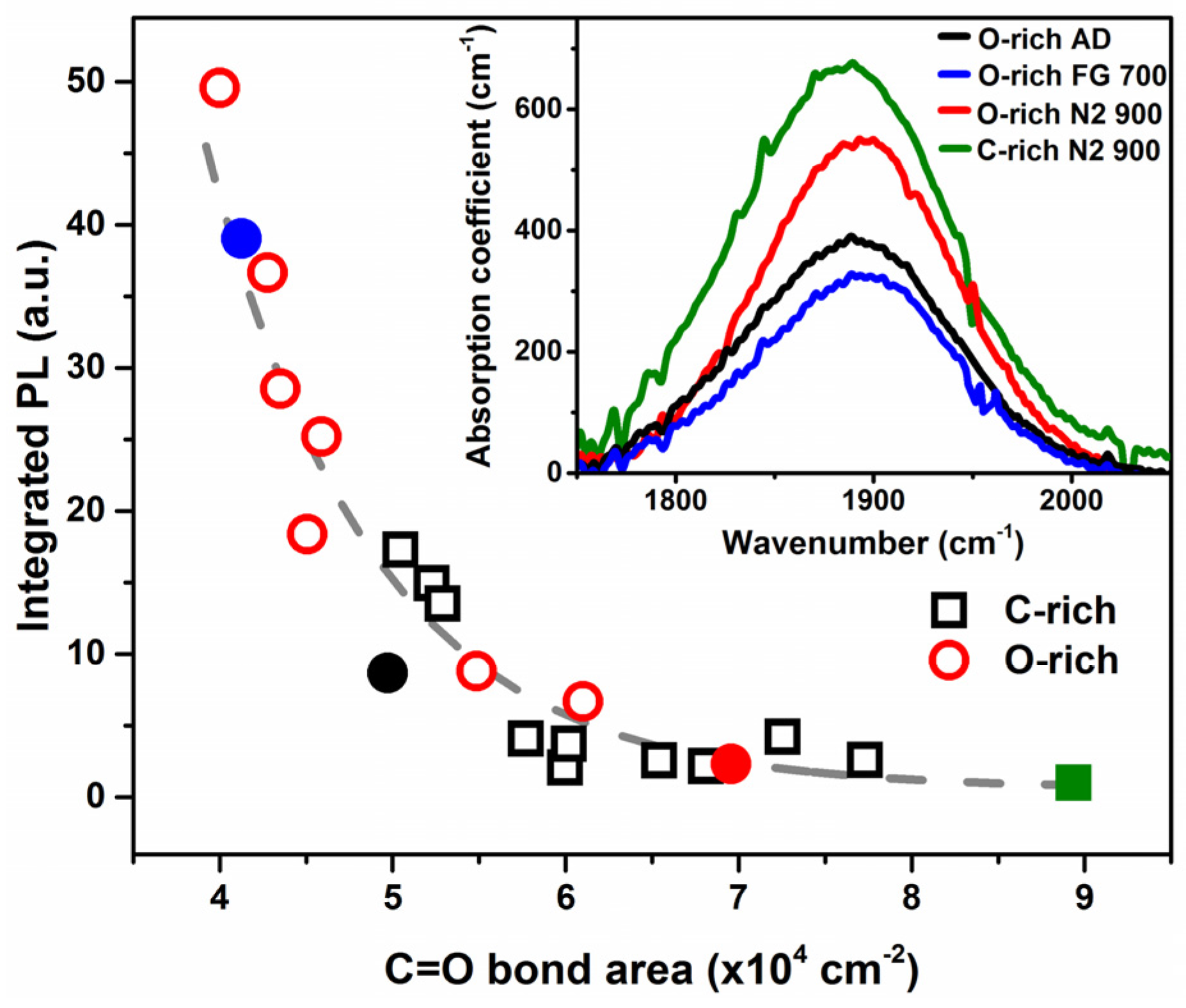
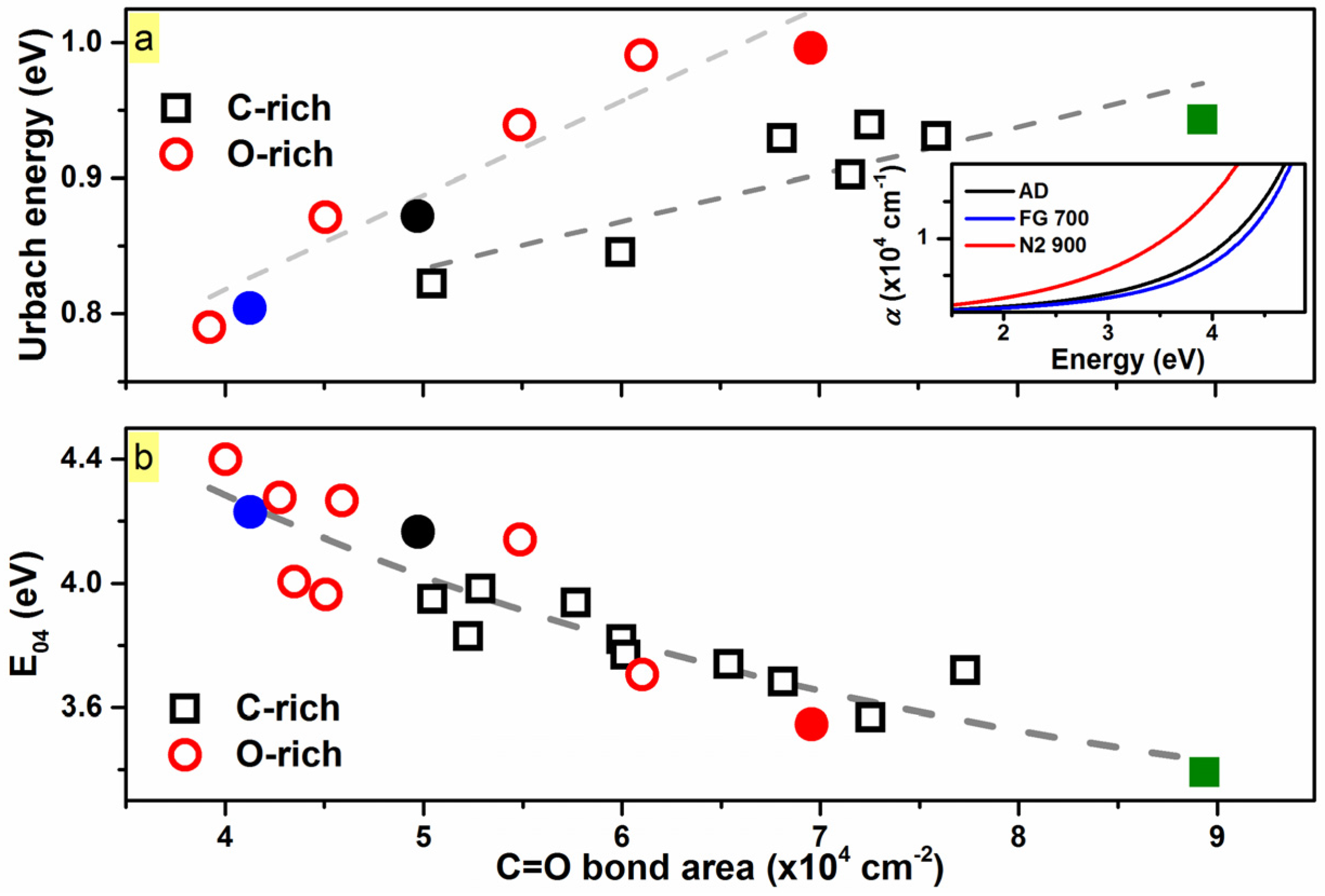
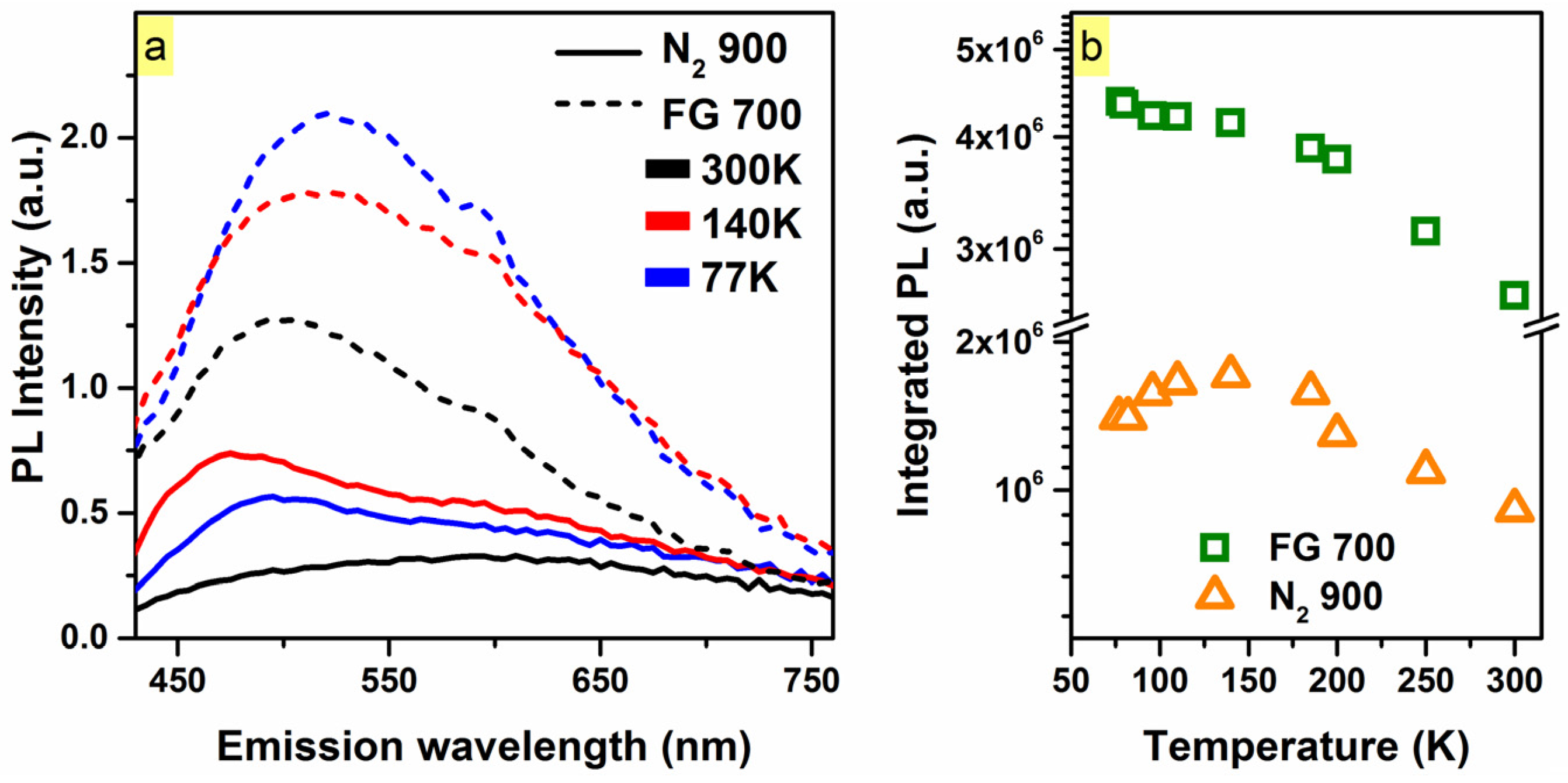
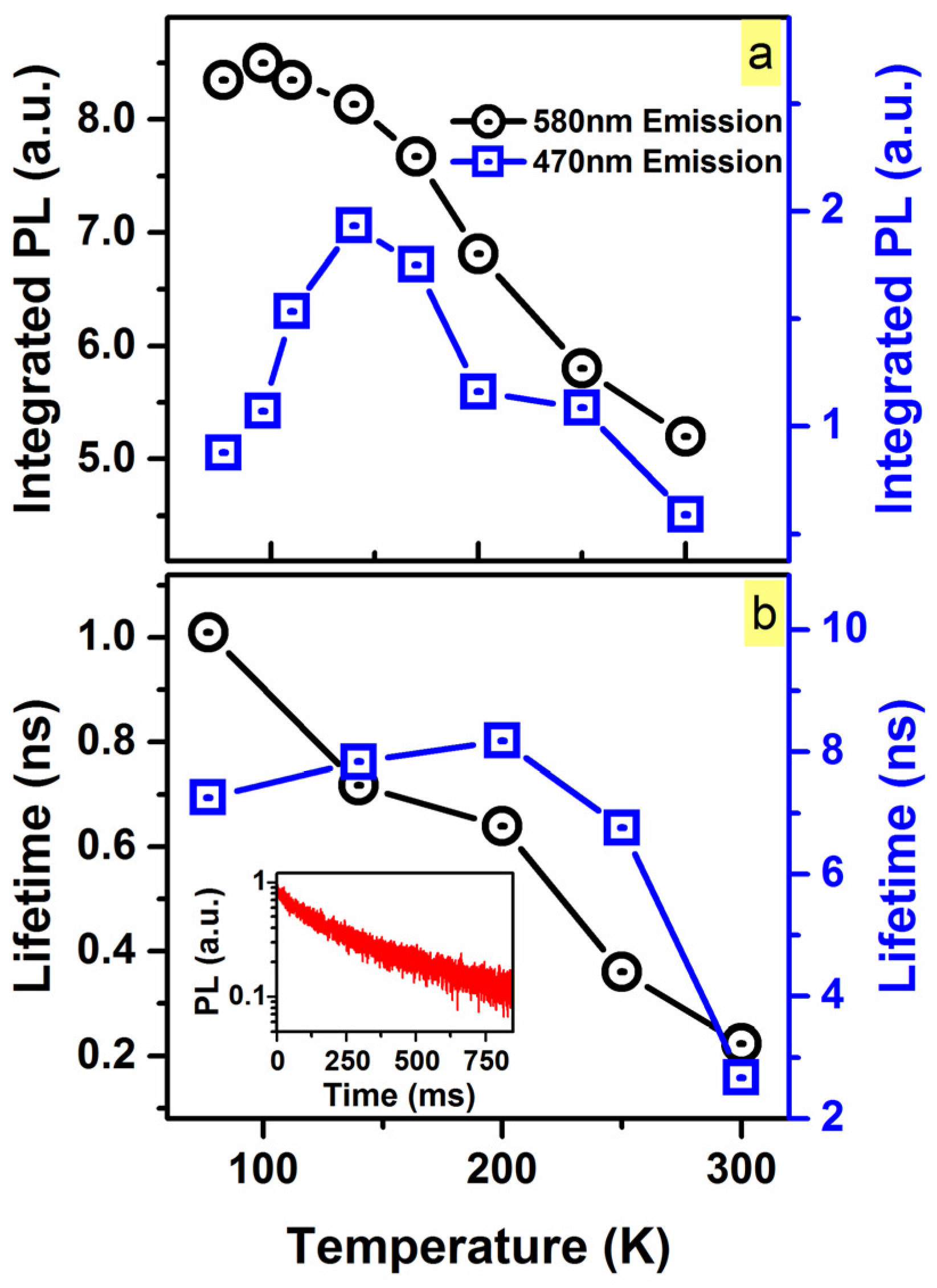
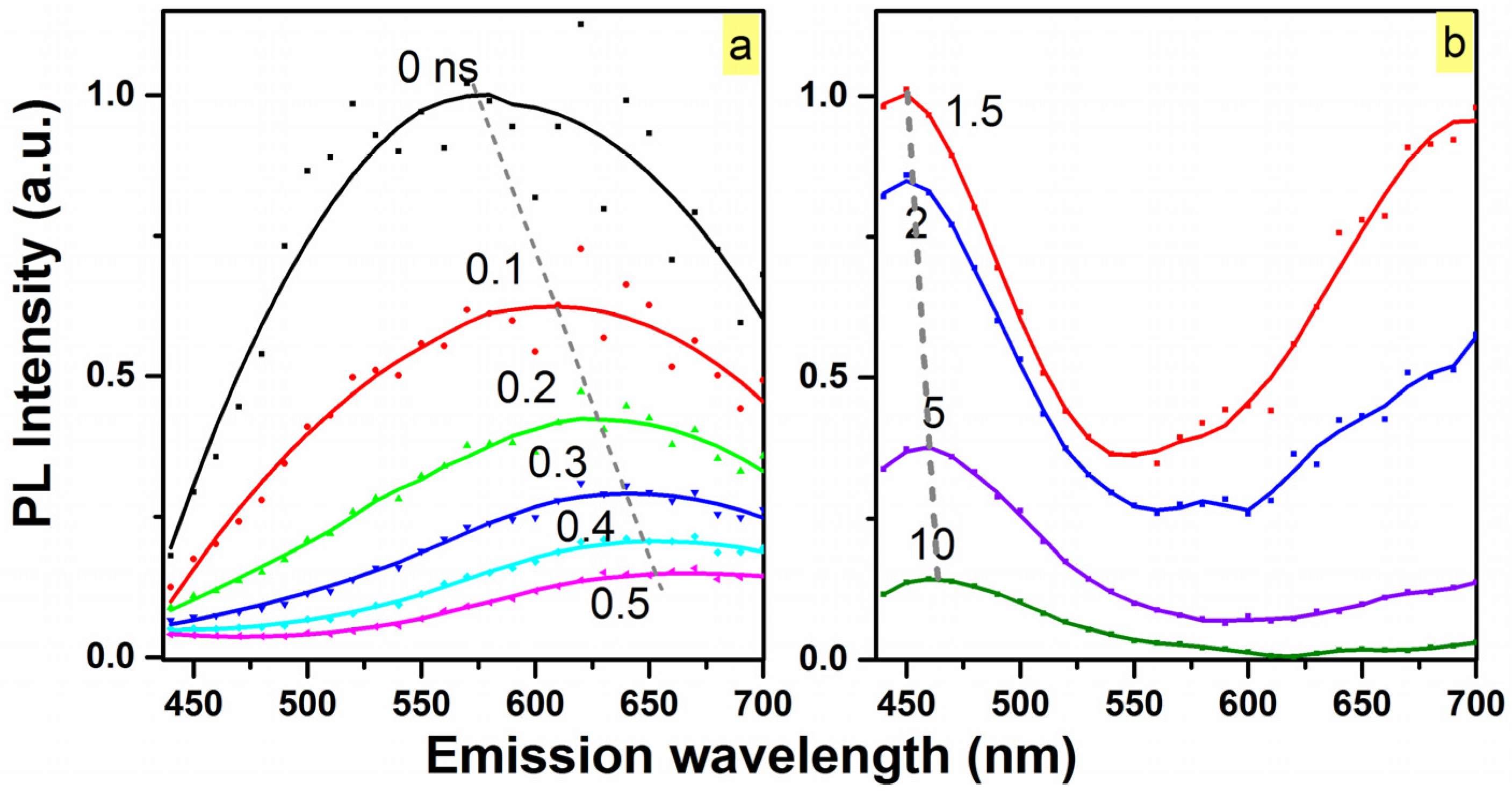
2. Results and Discussion
3. Materials and Methods
3.1. Thin Film Synthesis
3.2. Thermal Passivation of the SiCxOy Thin Films
3.3. Characterization of the SiCxOy Thin Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon nanostructures for photonics and photovoltaics. Nat. Nanotechnol. 2014, 9, 19–32. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fan, C.; Lee, S.T. Silicon nanostructures for bioapplications. Nano Today 2010, 5, 282–295. [Google Scholar] [CrossRef]
- Grill, A.; Gates, S.M.; Ryan, T.E.; Nguyen, S.V.; Priyadarshini, D. Progress in the development and understanding of advanced low k and ultralow k dielectrics for very large-scale integrated interconnects—State of the art. Appl. Phys. Rev. 2014, 1, 11306. [Google Scholar] [CrossRef]
- King, S.W. Dielectric Barrier, Etch Stop, and Metal Capping Materials for State of the Art and beyond Metal Interconnects. ECS J. Solid State Sci. Technol. 2014, 4, N3029–N3047. [Google Scholar] [CrossRef]
- Vasin, A.V. Structural and Luminescent Properties of Carbonized Silicon Oxide Thin Layers. In Functional Nanomaterials and Devices for Electronics, Sensors and Energy Harvesting, 1st ed.; Nazarov, A., Balestra, F., Kilchytska, V., Flandre, D., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 297–321. [Google Scholar]
- Gallis, S.; Nikas, V.; Suhag, H.; Huang, M.; Kaloyeros, A.E. White light emission from amorphous silicon oxycarbide (a-SiCxOy) thin films: Role of composition and postdeposition annealing. Appl. Phys. Lett. 2010, 97, 81905. [Google Scholar] [CrossRef]
- Nghiem, Q.D.; Cho, S.J.; Kim, D.-P. Synthesis of heat-resistant mesoporous SiOC ceramic and its hydrogen adsorption. J. Mater. Chem. 2006, 16, 558–562. [Google Scholar] [CrossRef]
- Karakuscu, A.; Ponzoni, A.; Aravind, P.R.; Sberveglieri, G.; Soraru, G.D. Gas Sensing Behavior of Mesoporous SiOC Glasses. J. Am. Ceram. Soc. 2013, 96, 2366–2369. [Google Scholar] [CrossRef]
- Zhuo, R.; Colombo, P.; Pantano, C.; Vogler, E.A. Silicon oxycarbide glasses for blood-contact applications. Acta Biomater. 2005, 1, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Nikas, V.; Tabassum, N.; Ford, B.; Smith, L.; Kaloyeros, A.E.; Gallis, S. Strong visible light emission from silicon-oxycarbide nanowire arrays prepared by electron beam lithography and reactive ion etching. J. Mater. Res. 2015, 30, 1–8. [Google Scholar] [CrossRef]
- Tabassum, N.; Nikas, V.; Ford, B.; Huang, M.; Kaloyeros, A.E.; Gallis, S. Time-resolved analysis of the white photoluminescence from chemically synthesized SiCxOy thin films and nanowires. Appl. Phys. Lett. 2016, 109, 43104. [Google Scholar] [CrossRef]
- Bellocchi, G.; Franzò, G.; Boninelli, S.; Miritello, M.; Cesca, T.; Iacona, F.; Priolo, F. Structural and luminescence properties of undoped and Eu-doped SiOC thin films. IOP Conf. Ser. Mater. Sci. Eng. 2014, 56, 12009. [Google Scholar] [CrossRef]
- Gallis, S.; Huang, M.; Kaloyeros, A.E. Efficient energy transfer from silicon oxycarbide matrix to Er ions via indirect excitation mechanisms. Appl. Phys. Lett. 2007, 90, 10–13. [Google Scholar] [CrossRef]
- Kilina, S.V.; Tamukong, P.K.; Kilin, D.S. Surface Chemistry of Semiconducting Quantum Dots: Theoretical Perspectives. Acc. Chem. Res. 2016, 49, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Wells, S.A.; Jariwala, D.; Chen, K.-S.; Cho, E.; Sangwan, V.K.; Liu, X.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Effective Passivation of Exfoliated Black Phosphorus Transistors against Ambient Degradation. Nano Lett. 2014, 14, 6964–6970. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; LeCroy, G.E.; Wang, P.; Liang, W.; Chen, J.; Fernando, K.A.S.; Bunker, C.E.; Qian, H.; Sun, Y.-P. Functionalization of Carbon Nanoparticles and Defunctionalization—Toward Structural and Mechanistic Elucidation of Carbon “Quantum” Dots. J. Phys. Chem. C 2016, 120, 25604–25611. [Google Scholar] [CrossRef]
- Nikas, V.; Gallis, S.; Huang, M.; Kaloyeros, A.E.; Nguyen, A.P.D.; Stesmans, A.; Afanas’ev, V.V. The origin of white luminescence from silicon oxycarbide thin films. Appl. Phys. Lett. 2014, 104, 61906. [Google Scholar] [CrossRef]
- Rui, Y.; Chen, D.; Xu, J.; Zhang, Y.; Yang, L.; Mei, J.; Ma, Z.; Cen, Z.; Li, W.; Xu, L.; et al. Hydrogen-induced recovery of photoluminescence from annealed a-Si:H/a-SiO2 multilayers. J. Appl. Phys. 2005, 98, 33532. [Google Scholar] [CrossRef]
- Gallis, S.; Nikas, V.; Huang, M.; Eisenbraun, E.; Kaloyeros, A.E. Comparative study of the effects of thermal treatment on the optical properties of hydrogenated amorphous silicon-oxycarbide. J. Appl. Phys. 2007, 102. [Google Scholar] [CrossRef]
- Papadimitrakopoulos, F.; Konstadinidis, K.; Miller, T.M.; Opila, R.; Chandross, E.A.; Galvin, M.E. The Role of Carbonyl Groups in the Photoluminescence of Poly(p-phenylenevinylene). Chem. Mater. 1994, 6, 1563–1568. [Google Scholar] [CrossRef]
- Yan, M.; Rothberg, L.J.; Papadimitrakopoulos, F.; Galvin, M.E.; Miller, T.M. Defect Quenching of Conjugated Polymer Luminescence. Phys. Rev. Lett. 1994, 73, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.R.; Lim, J.; Oh, J.; Kim, J.-W.; Shin, H.S.; Kim, J.H.; Lee, S.; Jun, S.C. Epoxy to Carbonyl Group Conversion in Graphene Oxide Thin Films: Effect on Structural and Luminescent Characteristics. J. Phys. Chem. C 2012, 116, 19010–19017. [Google Scholar] [CrossRef]
- Tolstoy, V.P.; Chernyshova, I.V.; Skryshevsky, V.A. Handbook of Infrared Spectroscopy of Ultrathin Films, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 416–475. [Google Scholar]
- Zanatta, A.R.; Chambouleyron, I. Absorption edge, band tails, and disorder of amorphous semiconductors. Phys. Rev. B 1996, 53, 3833–3836. [Google Scholar] [CrossRef]
- Knief, S.; von Niessen, W. Disorder, defects, and optical absorption in a-Si and a-Si:H. Phys. Rev. B 1999, 59, 12940–12946. [Google Scholar] [CrossRef]
- Robertson, J. Recombination and photoluminescence mechanism in hydrogenated amorphous carbon. Phys. Rev. B 1996, 53, 16302–16305. [Google Scholar] [CrossRef]
- Street, R.A.; Zesch, J.; Thompson, M.J. Effects of doping on transport and deep trapping in hydrogenated amorphous silicon. Appl. Phys. Lett. 1983, 43, 672–674. [Google Scholar] [CrossRef]
- Monroe, D. Hopping in Exponential Band Tails. Phys. Rev. Lett. 1985, 54, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H. Negative Thermal Quenching Curves in Photoluminescence of Solids. Jpn. J. Appl. Phys. 1998, 37, 550–553. [Google Scholar] [CrossRef]
- Kuzmany, H. Solid-State Spectroscopy: An Introduction, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 144–146. [Google Scholar]
- Klopffer, W. Introduction to Polymer Spectroscopy, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 48–49. [Google Scholar]
- Estes, M.J.; Moddel, G. Luminescence from amorphous silicon nanostructures. Phys. Rev. B 1996, 54, 14633–14642. [Google Scholar] [CrossRef]
- Skuja, L. Optically active oxygen-deficiency-related centers in amorphous silicon dioxide. J. Non Cryst. Solids 1998, 239, 16–48. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ford, B.; Tabassum, N.; Nikas, V.; Gallis, S. Strong Photoluminescence Enhancement of Silicon Oxycarbide through Defect Engineering. Materials 2017, 10, 446. https://doi.org/10.3390/ma10040446
Ford B, Tabassum N, Nikas V, Gallis S. Strong Photoluminescence Enhancement of Silicon Oxycarbide through Defect Engineering. Materials. 2017; 10(4):446. https://doi.org/10.3390/ma10040446
Chicago/Turabian StyleFord, Brian, Natasha Tabassum, Vasileios Nikas, and Spyros Gallis. 2017. "Strong Photoluminescence Enhancement of Silicon Oxycarbide through Defect Engineering" Materials 10, no. 4: 446. https://doi.org/10.3390/ma10040446




