Effect of Graphite Nanoplate Morphology on the Dispersion and Physical Properties of Polycarbonate Based Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing
2.3. Characterization Methods
2.3.1. Particle Size Distribution of Pristine Carbon Fillers
2.3.2. Investigation of the Pristine Morphology of Carbon Fillers
2.3.3. Bulk Density Measurement of Pristine Carbon Fillers
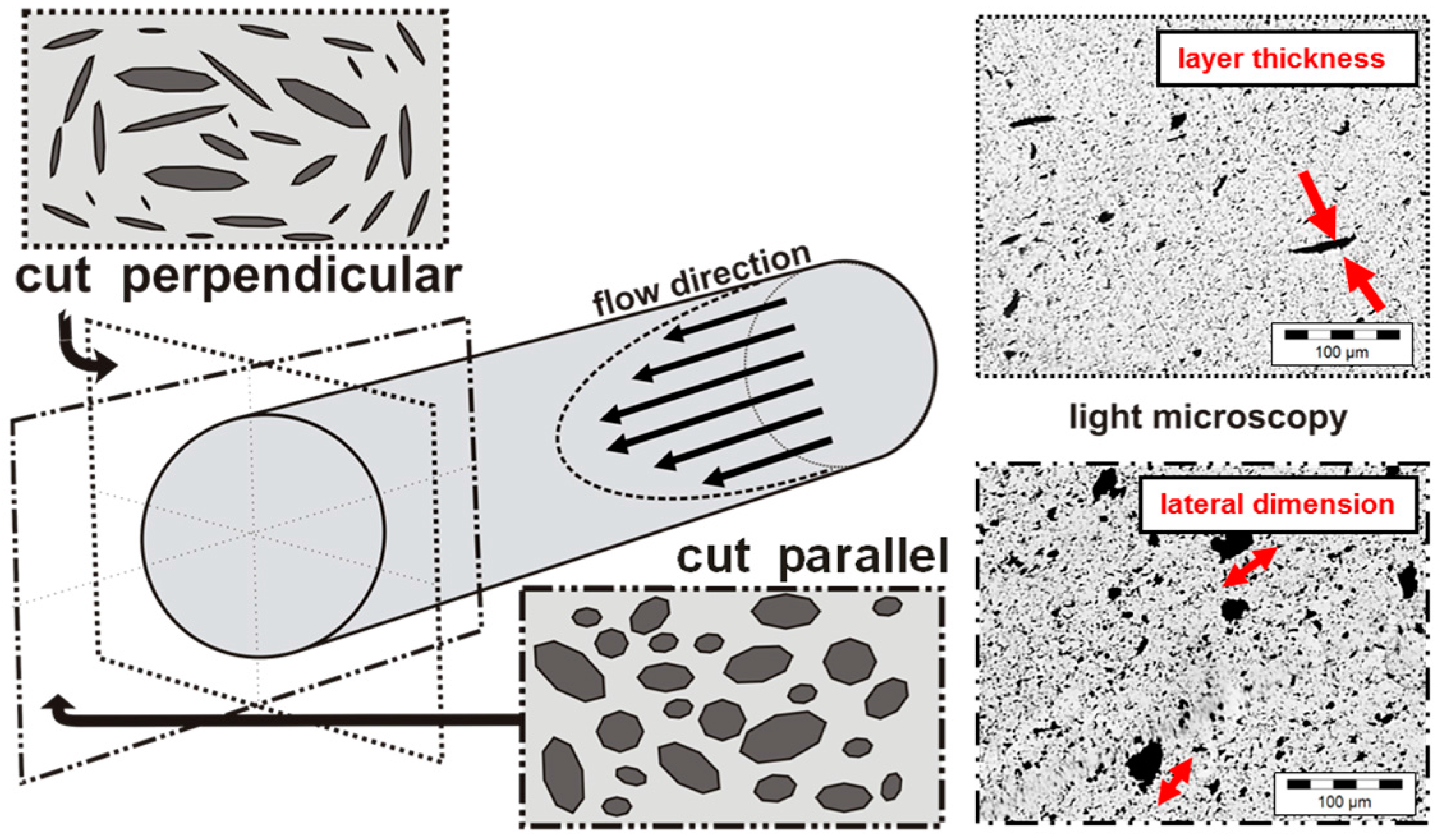
2.3.4. Carbon Filler Dispersion
2.3.5. Electrical Properties
2.3.6. Mechanical Properties
2.3.7. Thermal Behavior (DSC)
2.3.8. Thermal Conductivity
2.3.9. Gas Permeability
2.3.10. Mixing Energy
3. Results and Discussion
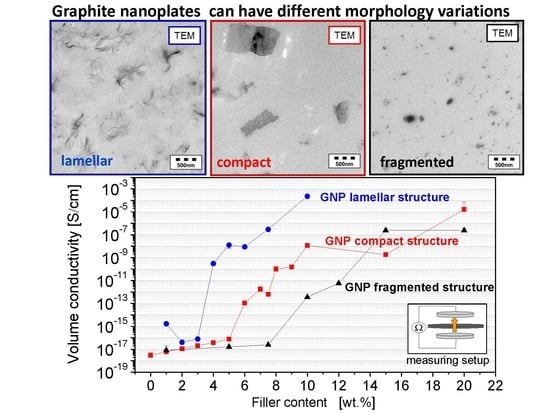
3.1. Morphology of the Filler Materials
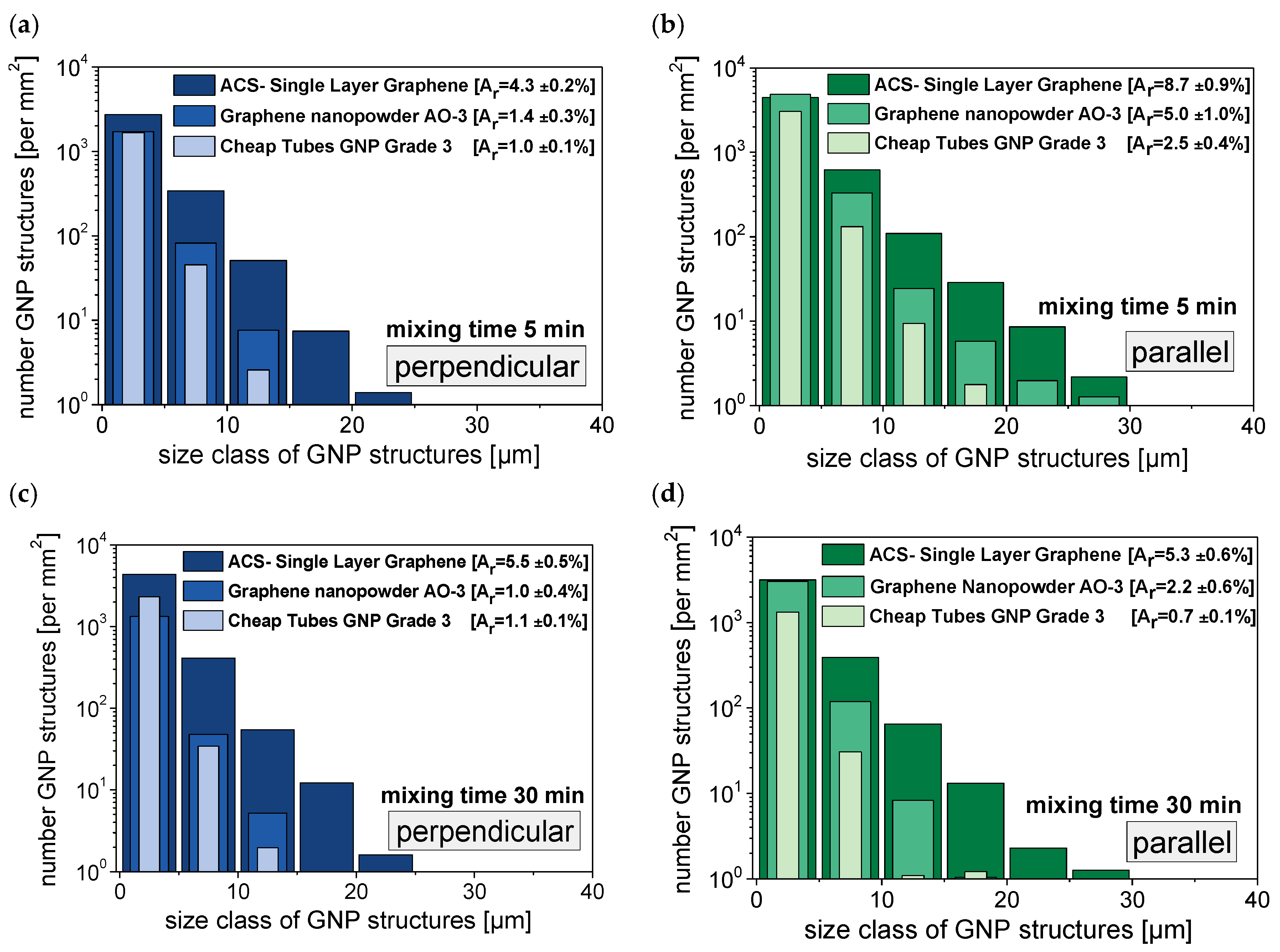
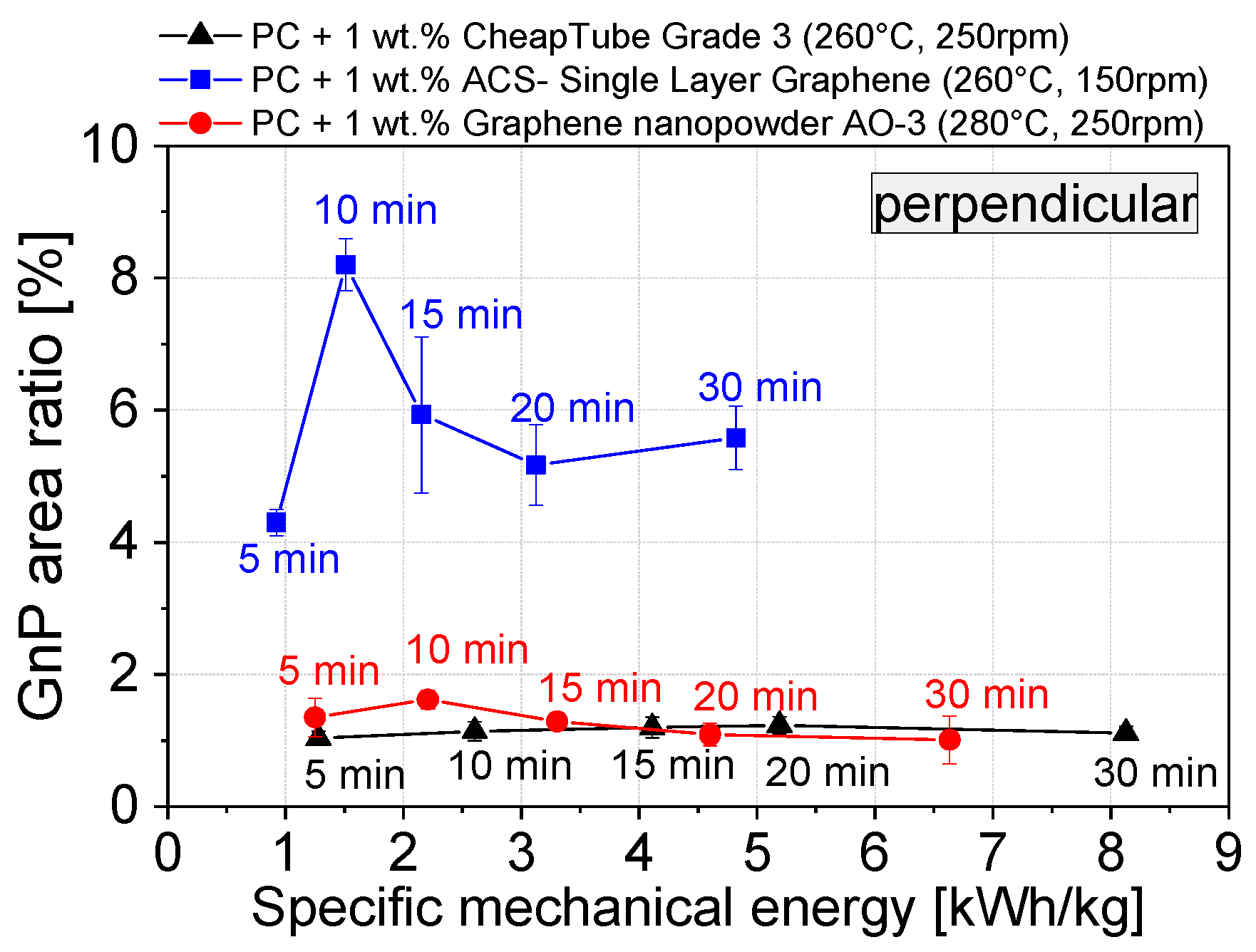
3.2. Dispersion Behavior of the GNP Fillers
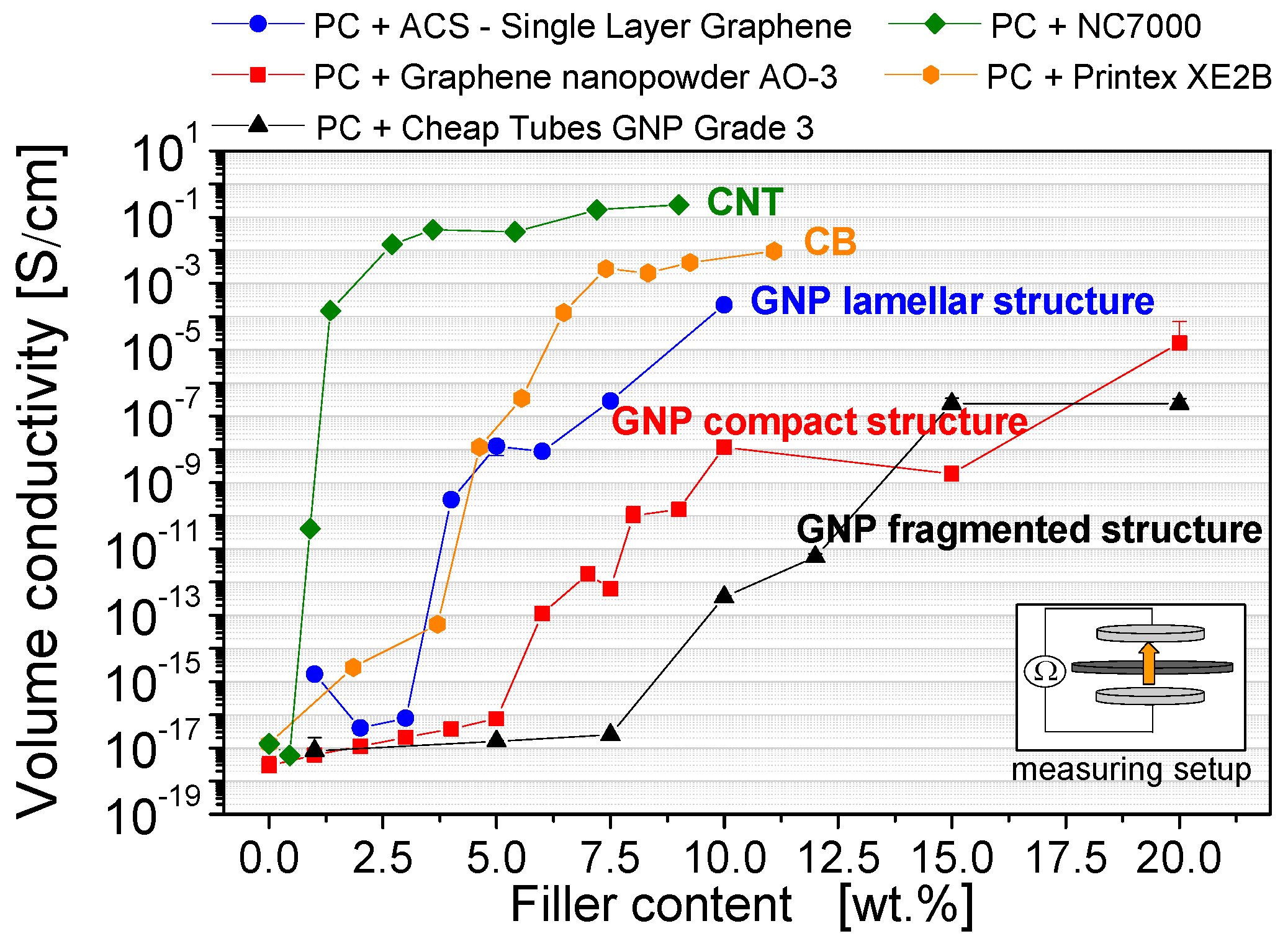
3.3. Electrical Properties
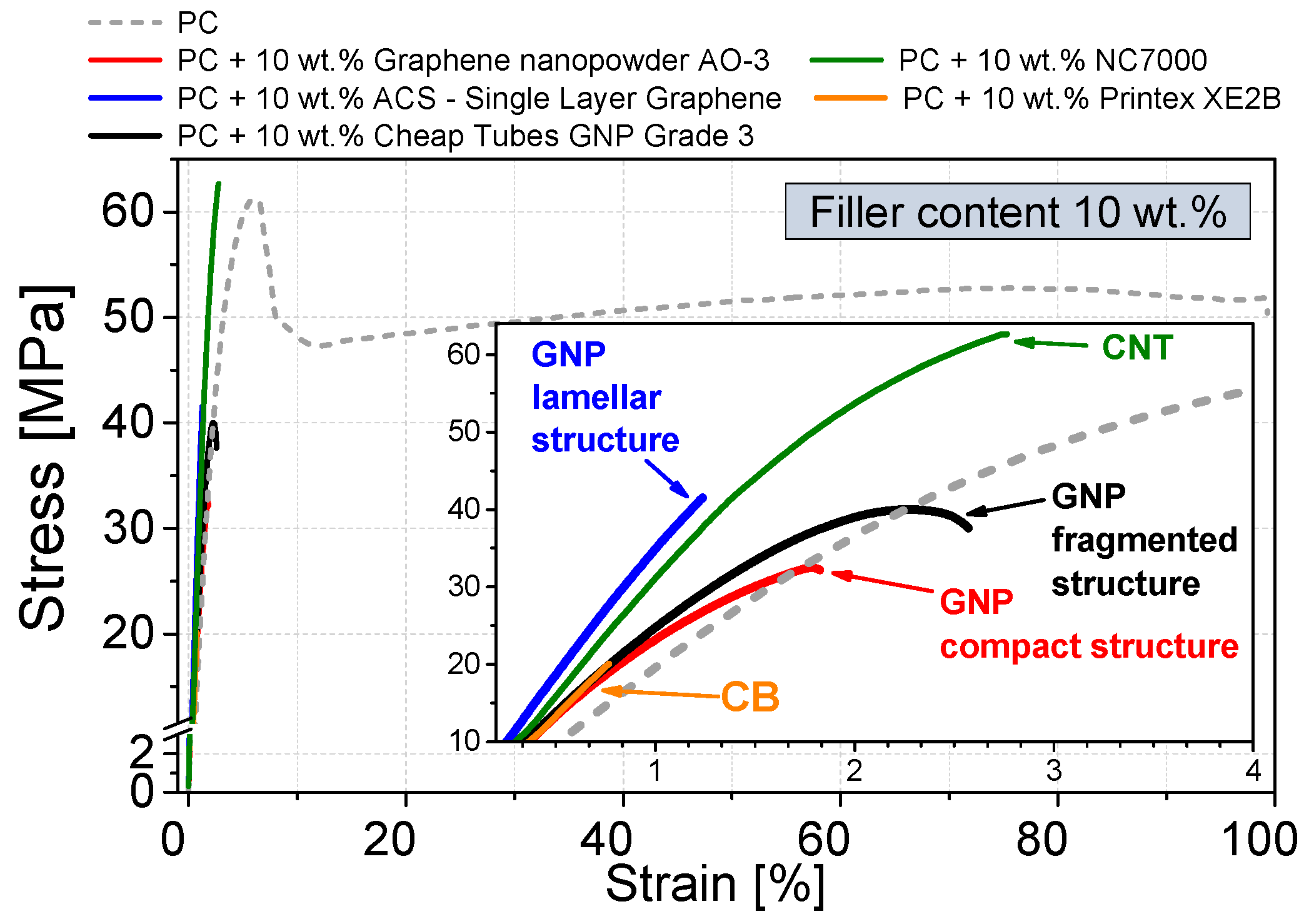
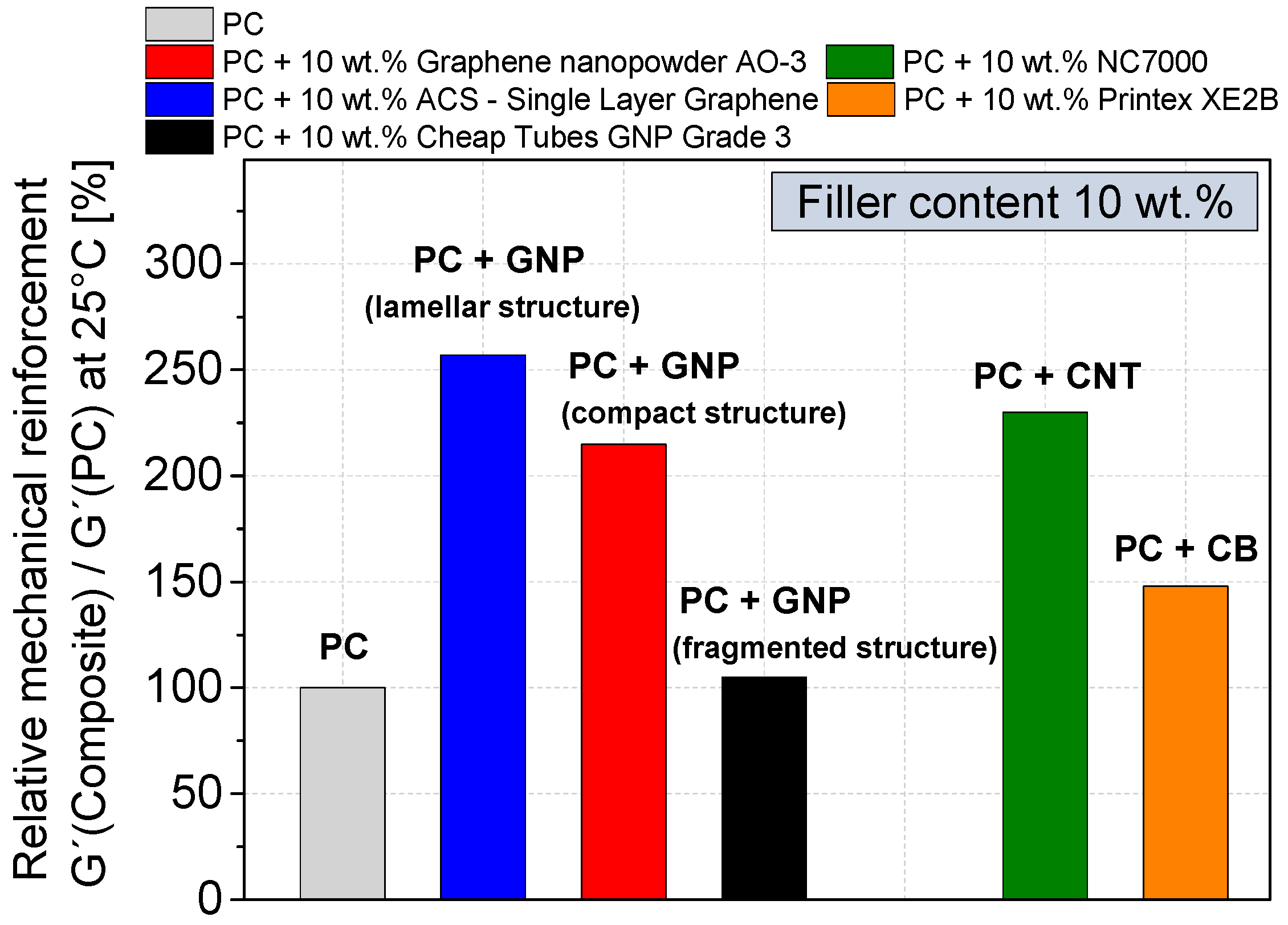
3.4. Mechanical Properties
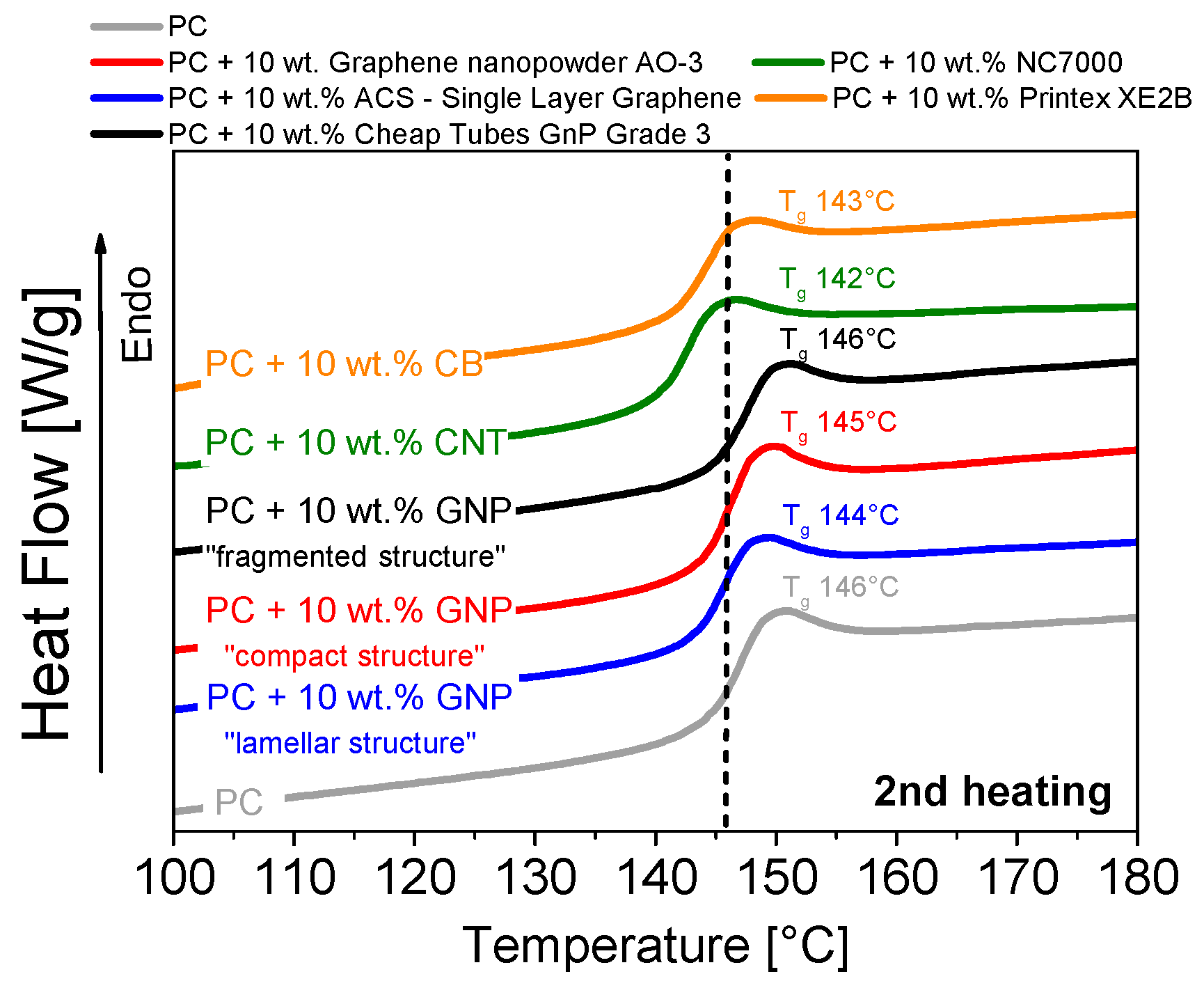
3.5. Thermal Properties
3.6. Gas Barrier Properties
4. Conclusions
- (1)
- Among the GNP types, the lamellar GNP has the lowest electrical percolation threshold of 2 wt %, which is only slightly higher than that of CNTs with 1 wt %. However, the highest achieved electrical conductivity level (≈10−4 S∙cm−1 at 10 wt % loading) is significantly lower than for CNT or carbon black (≈10−1–10−2 S∙cm−1).
- (2)
- The highest relative mechanical reinforcement was obtained when using GNP with lamellar structure showing an increase of the relative G′ value by 160% (loading 10 wt %) which is even higher than that when using CNTs (130%).
- (3)
- The composite filled with compact GNP (10 wt %) exhibited the highest thermal conductivity of 0.71 W∙m−1∙K−1, which corresponds to an increase by 196% compared to virgin PC with only 0.24 W∙m−1∙K−1. With all other carbon based fillers a significantly lower thermal conductivity improvement was achieved.
- (4)
- The oxygen gas permeability can be decreased by 41% by using only 3 wt % of lamellar GNP material. Due to the plate-like shape combined with a high aspect ratio, composites with this GNP type showed better oxygen barrier properties than CNT or CB based composites.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, P.X. Fabrication and characterization of few-layer graphene. Carbon 2010, 48, 359–364. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Alexander, M.; Roumen, V.; Koen, S.; Alexander, V.; Liang, Z.; Van Tendeloo, G.; Annick, V.; Van Haesendonck, C. Synthesis of few-layer graphene via microwave plasma-enhanced chemical vapour deposition. Nanotechnology 2008, 19, 305604. [Google Scholar] [CrossRef]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as potential material for electronics. Chem. Rev. 2007, 107, 718–747. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Cao, J.; Qi, G.-Q.; Ke, K.; Luo, Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Effect of temperature and time on the exfoliation and de-oxygenation of graphite oxide by thermal reduction. J. Mater. Sci. 2012, 47, 5097–5105. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, L.; Wang, X.; Diankov, G.; Dai, H. Narrow graphene nanoribbons from carbon nanotubes. Nature 2009, 458, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Özyilmaz, B.; Zhang, Y.; Kim, P. Energy band-gap engineering of graphene nanoribbons. Phys. Rev. Lett. 2007, 98, 206805. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, W.; Li, X.; Zhu, H.; Wei, J.; Li, Z.; Shu, Q.; Wang, C.; Wang, K.; Shen, W.; et al. Graphene sheets from worm-like exfoliated graphite. J. Mater. Chem. 2009, 19, 3367–3369. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Mechanical properties and morphological characterization of exfoliated graphite–polypropylene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1675–1682. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Shin, Y.-R.; Sohn, G.-J.; Choi, H.-J.; Bae, S.-Y.; Mahmood, J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Wook Chang, D.; et al. Edge-carboxylated graphene nanosheets via ball milling. Proc. Natl. Acad. Sci. USA 2012, 109, 5588–5593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Wong, S.-C.; Sutherland, E.M.; Uhl, F.M. Materials processes of graphite nanostructured composites using ball milling. Mater. Manuf. Proc. 2006, 21, 159–166. [Google Scholar] [CrossRef]
- Vilaverde, C.; Santos, R.M.; Paiva, M.C.; Covas, J.A. Dispersion and re-agglomeration of graphite nanoplates in polypropylene melts under controlled flow conditions. Compos. Part A Appl. Sci. Manuf. 2015, 78, 143–151. [Google Scholar] [CrossRef]
- Santos, R.M.; Vilaverde, C.; Cunha, E.; Paiva, M.C.; Covas, J.A. Probing dispersion and re-agglomeration phenomena upon melt-mixing of polymer-functionalized graphite nanoplates. Soft Matter 2016, 12, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.J.; Joh, H.-I.; Yu, J.; Hwang, S.H.; Lee, S.; Lee, C.H.; Kim, S.Y.; Youn, J.R. Ultra-high dispersion of graphene in polymer composite via solvent free fabrication and functionalization. Sci. Rep. 2015, 5, 9141. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, M.; Blais, M.-O.; Pötschke, P.; Heinrich, G. A morphological study on the dispersion and selective localization behavior of graphene nanoplatelets in immiscible polymer blends of PC and SAN. Polymer 2013, 54, 5875–5882. [Google Scholar] [CrossRef]
- Pinto, A.M.; Cabral, J.; Tanaka, D.A.P.; Mendes, A.M.; Magalhães, F.D. Effect of incorporation of graphene oxide and graphene nanoplatelets on mechanical and gas permeability properties of poly(lactic acid) films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Cunha, E.; Paiva, M.C.; Hilliou, L.; Covas, J.A. Tracking the progression of dispersion of graphite nanoplates in a polypropylene matrix by melt mixing. Polym. Compos. 2015, 38, 947–954. [Google Scholar] [CrossRef]
- Pionteck, J.; Melchor Valdez, E.M.; Piana, F.; Omastová, M.; Luyt, A.S.; Voit, B. Reduced percolation concentration in polypropylene/expanded graphite composites: Effect of viscosity and polypyrrole. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Ramanathan, T.; Stankovich, S.; Dikin, D.A.; Liu, H.; Shen, H.; Nguyen, S.T.; Brinson, L.C. Graphitic nanofillers in PMMA nanocomposites—An investigation of particle size and dispersion and their influence on nanocomposite properties. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2097–2112. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Shirvanimoghaddam, K.; Naebe, M. PVDF/graphene composite nanofibers with enhanced piezoelectric performance for development of robust nanogenerators. Compos. Sci. Technol. 2017, 138, 49–56. [Google Scholar] [CrossRef]
- Oyarzabal, A.; Cristiano-Tassi, A.; Laredo, E.; Newman, D.; Bello, A.; Etxeberría, A.; Eguiazabal, J.I.; Zubitur, M.; Mugica, A.; Müller, A.J. Dielectric, mechanical and transport properties of bisphenol A polycarbonate/graphene nanocomposites prepared by melt blending. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Honaker, K.; Vautard, F.; Drzal, L.T. Investigating the mechanical and barrier properties to oxygen and fuel of high density polyethylene-graphene nanoplatelet composites. Mater. Sci. Eng. B 2017, 216, 23–30. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Joseph, G.; Shinu, K.P.; Jose, S.; Salim, N.V.; Hameed, N. Development of hybrid composites for automotive applications: Effect of addition of SEBS on the morphology, mechanical, viscoelastic, crystallization and thermal degradation properties of PP/PS-xGnP composites. RSC Adv. 2015, 5, 25634–25641. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, G. Graphene/polymer composites for energy applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 231–253. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Xie, X.-L.; Mai, Y.-W.; Zhou, X.-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Gruber, T.C.; Herd, C.R. Anisometry Measurements in Carbon Black Aggregate Populations. Rubber Chem. Technol. 1997, 70, 727–746. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Datasheet—12 nm Graphene Nanopowder AO-3; Graphene-Supermarket: Calverton, NY, USA, 2010.
- Datasheet—xGNP Grade M; XG-Sciences: Lansing, MI, USA, 2012.
- Datasheet—ACS Material Graphene Nanoplatelets; ACS-Material: Pasadena, CA, USA, 2010.
- Datasheet—Multilayer Graphene EXG 98 300; Graphit Kropfmühl GmbH: Hauzenberg, Germany, 2013.
- Product Data Sheet Graphene Nano Platelets Grade 3; Cheap-Tubes: Grafton, VT, USA, 2009.
- Krause, B.; Mende, M.; Pötschke, P.; Petzold, G. Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon 2010, 48, 2746–2754. [Google Scholar] [CrossRef]
- NC7000 Series—Product Datasheet—Thin Multi-Wall Carbon Nanotubes; Nanocyl S.A.: Sambreville, Belgium, 2010.
- Datasheet—Printex XE2B; Orion Engineered Carbons GmbH: Frankfurt am Main, Germany, 2010.
- Choudhury, S.; Agrawal, M.; Formanek, P.; Jehnichen, D.; Fischer, D.; Krause, B.; Albrecht, V.; Stamm, M.; Ionov, L. Nanoporous Cathodes for High-Energy Li-S Batteries from Gyroid Block Copolymer Templates. ACS Nano 2015, 9, 6147–6157. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.; Boldt, R.; Häußler, L.; Pötschke, P. Ultralow percolation threshold in polyamide 6.6/MWCNT composites. Compos. Sci. Technol. 2015, 114, 119–125. [Google Scholar] [CrossRef]
- Li, J.; Kim, J.-K. Percolation threshold of conducting polymer composites containing 3D randomly distributed graphite nanoplatelets. Compos. Sci. Technol. 2007, 67, 2114–2120. [Google Scholar] [CrossRef]
- King, J.A.; Via, M.D.; Morrison, F.A.; Wiese, K.R.; Beach, E.A.; Cieslinski, M.J.; Bogucki, G.R. Characterization of exfoliated graphite nanoplatelets/polycarbonate composites: Electrical and thermal conductivity, and tensile, flexural, and rheological properties. J. Compos. Mater. 2011, 46, 1029–1039. [Google Scholar] [CrossRef]
- Nan, C.-W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Hofmann, D.; Keinath, M.; Thomann, R.; Mülhaupt, R. Thermoplastic Carbon/Polyamide 12 Composites Containing Functionalized Graphene, Expanded Graphite, and Carbon Nanofillers. Macromol. Mater. Eng. 2014, 299, 1329–1342. [Google Scholar] [CrossRef]
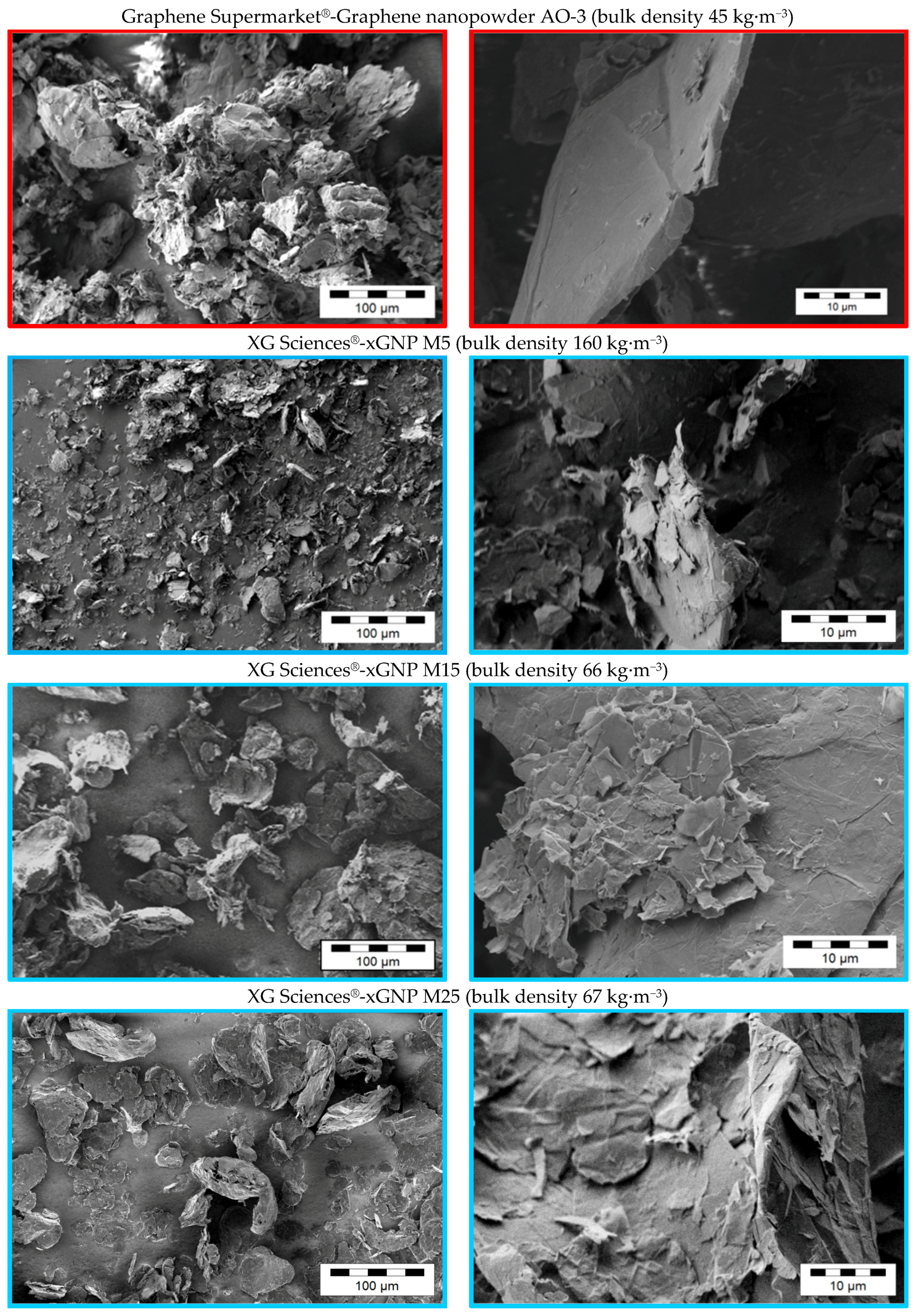

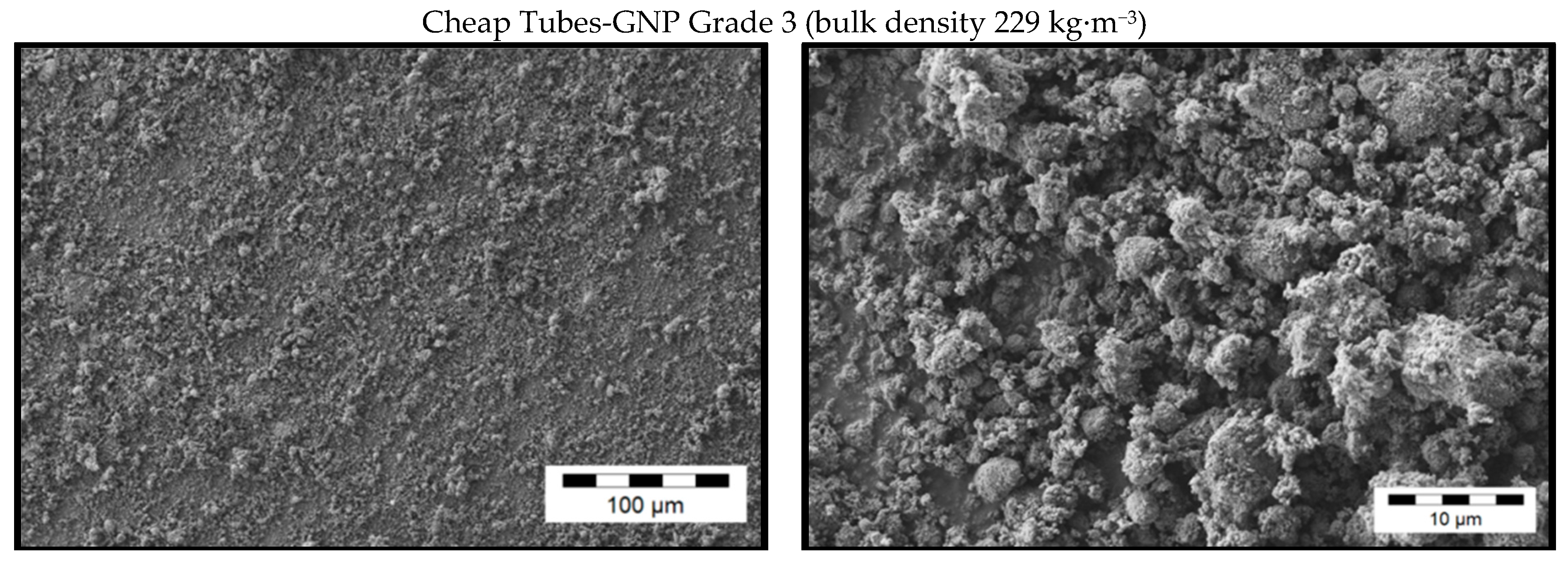
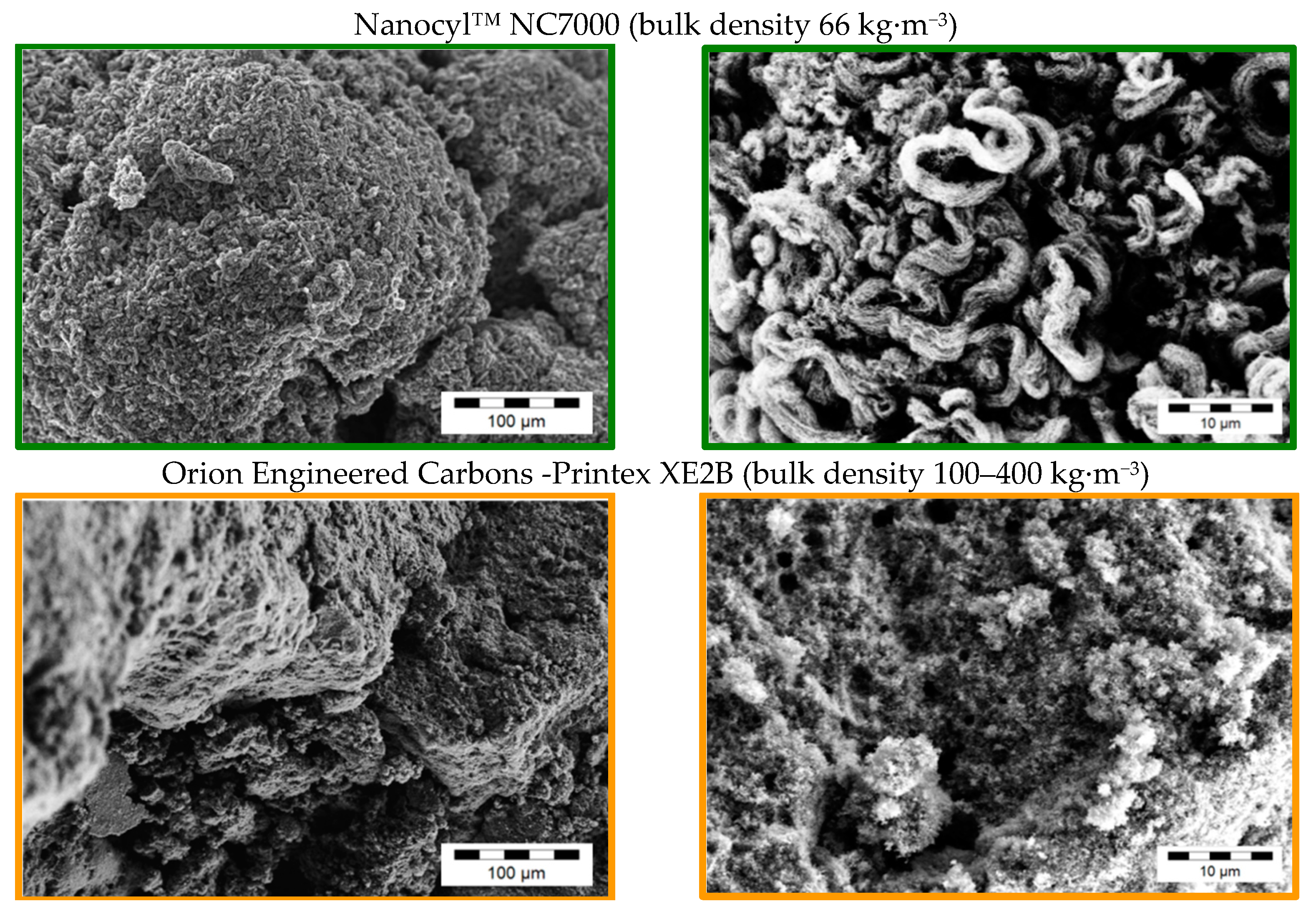
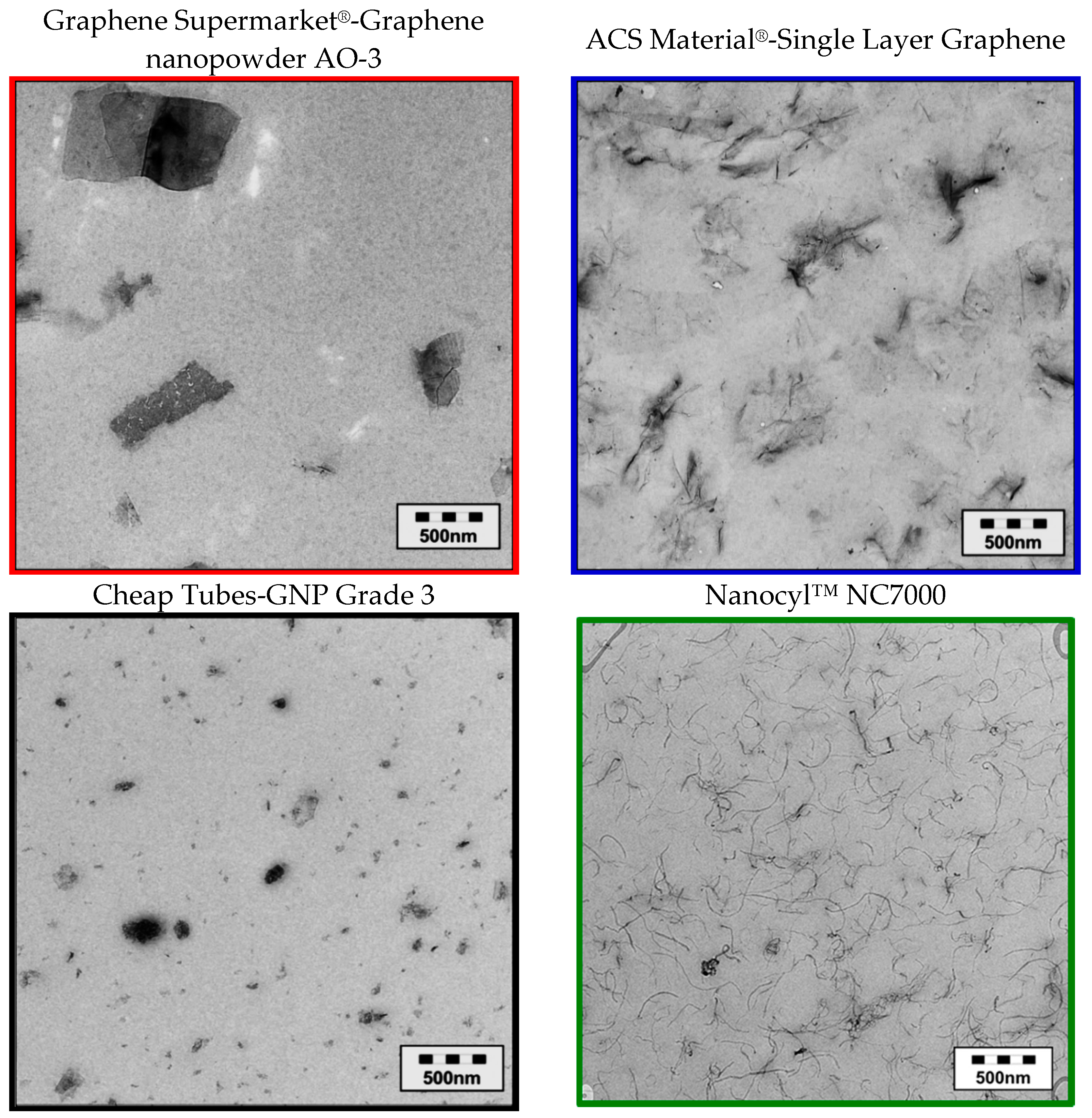
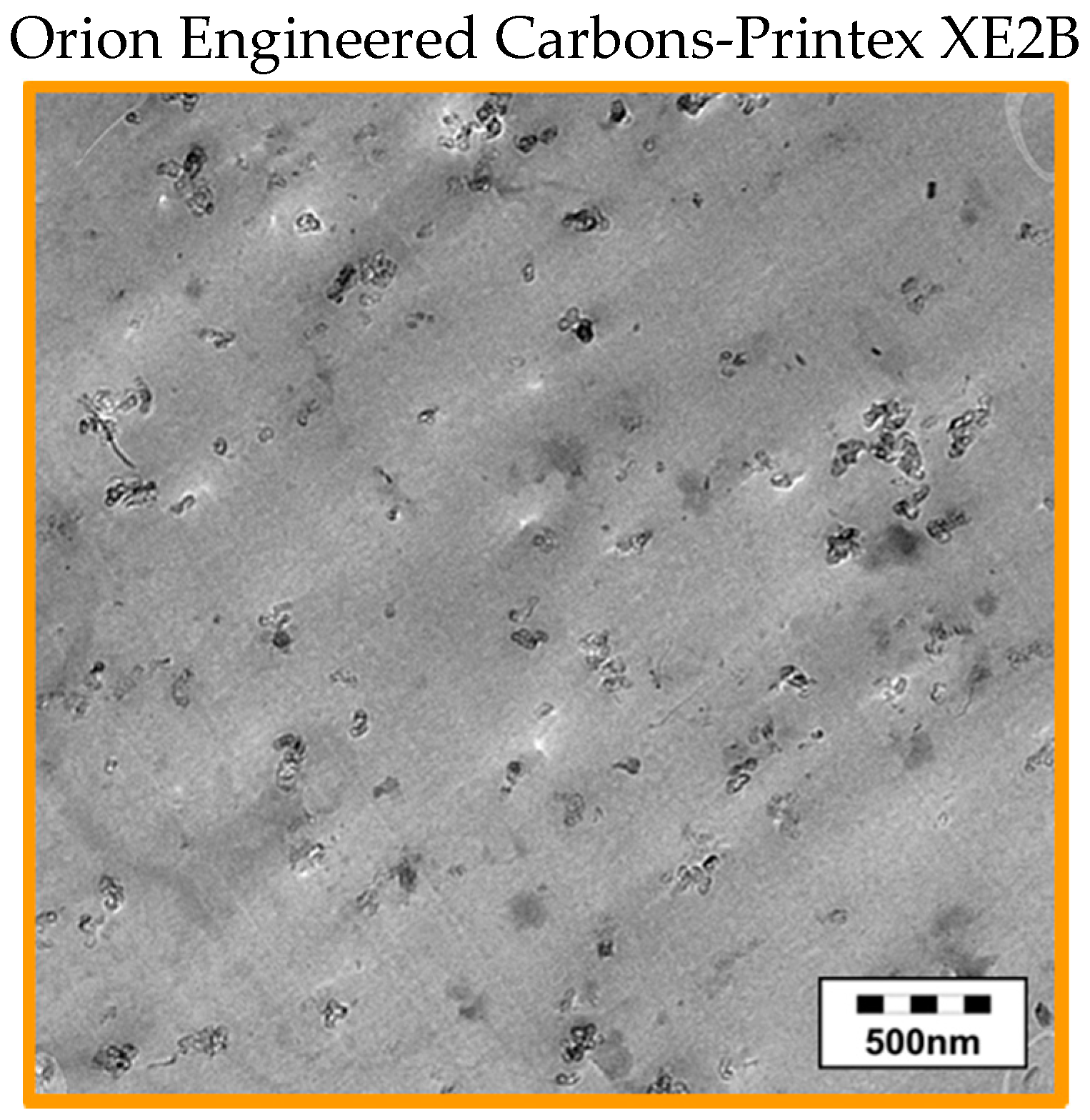
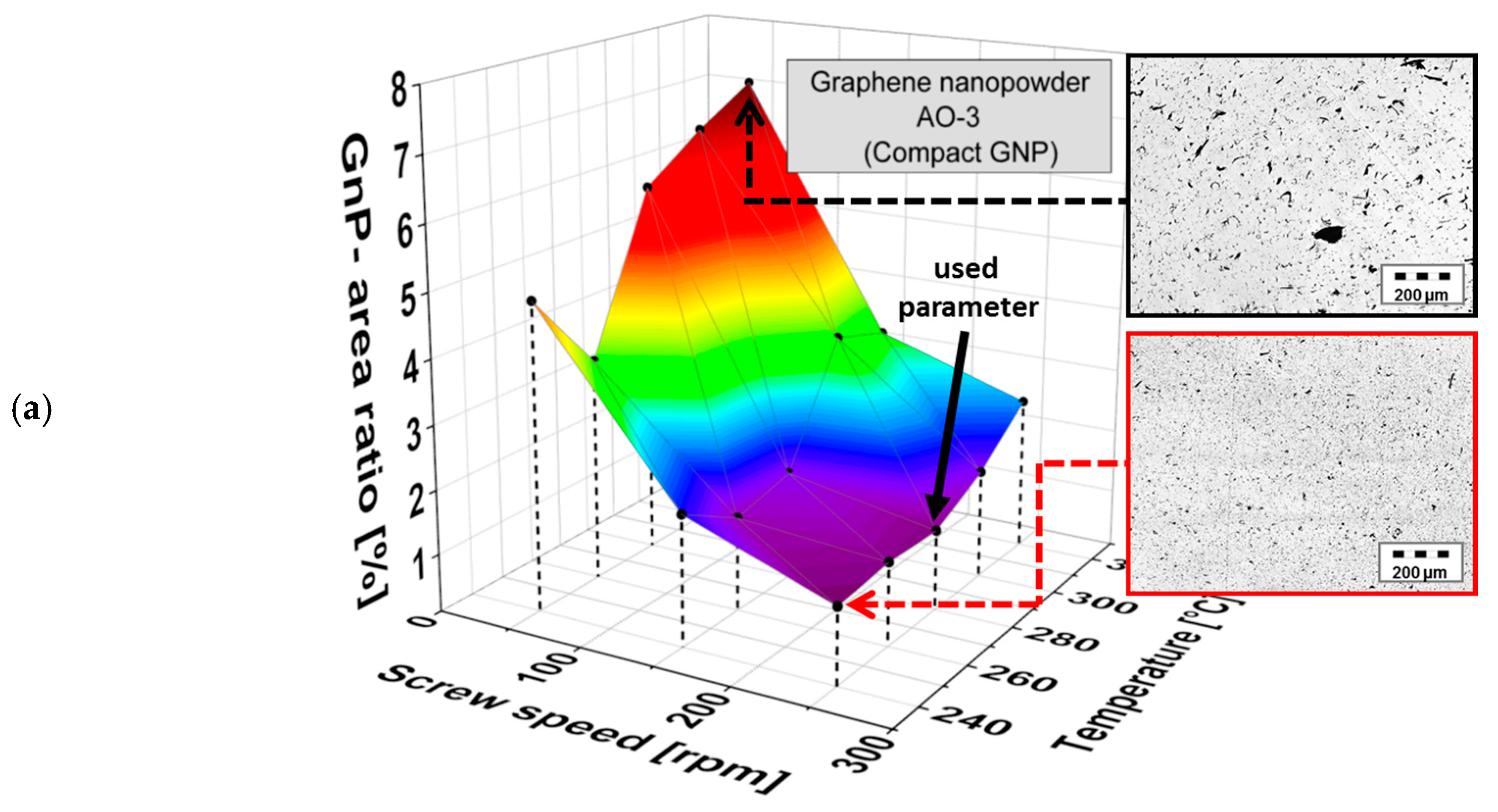
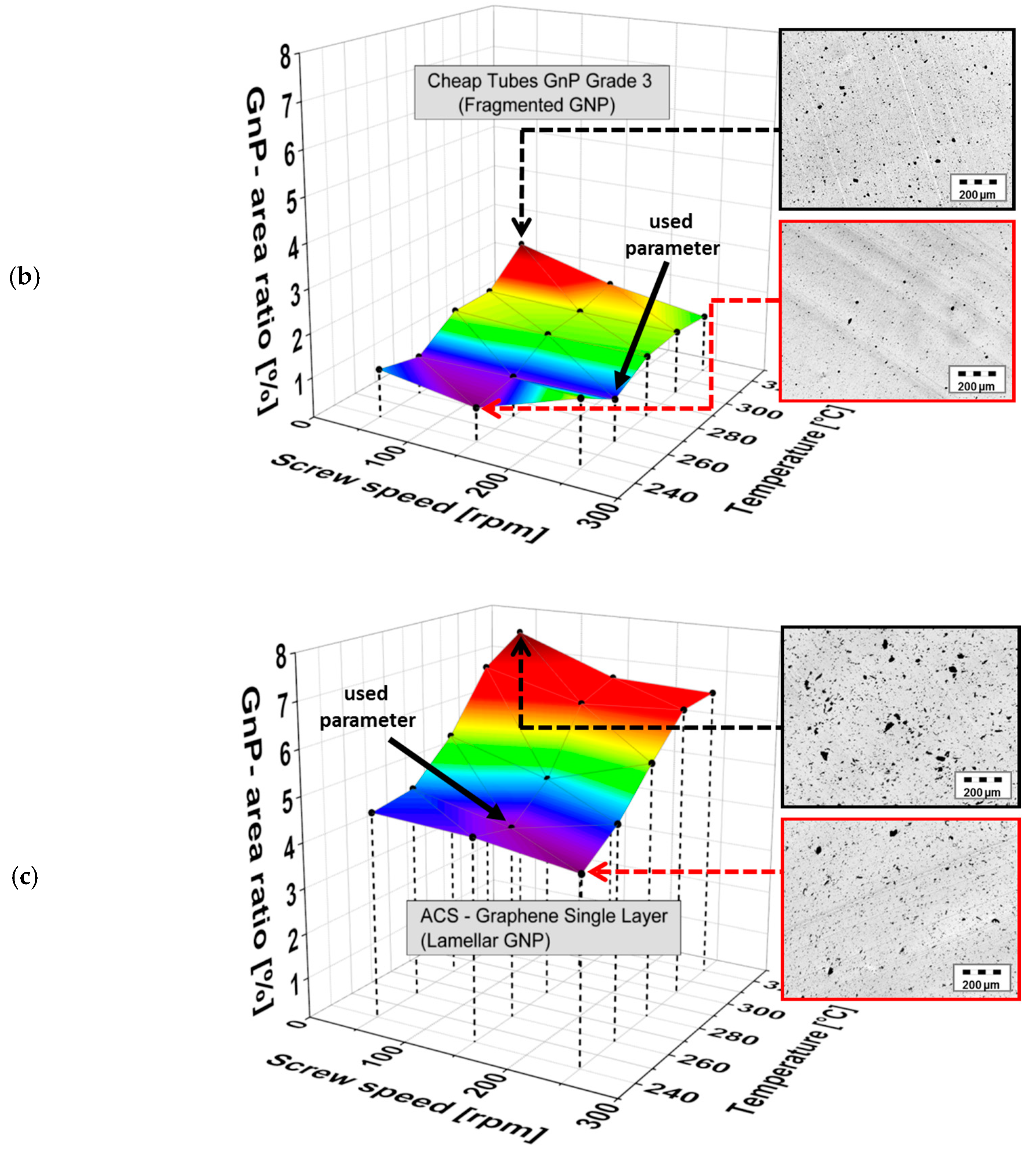




| Material as Named by the Producer/Producer | Morphology | Average Particle Size d50 [µm] | Thickness/Diameter | Electrical Conductivity [S∙cm−1] | Specific Surface [m2∙g−1] | Bulk Density [kg∙m−3] |
|---|---|---|---|---|---|---|
| Graphene nanopowder AO-3 Graphene Supermarket® | compact | 50 *3 | 12 nm [42] | 14 *2 | 80 [42] | 45 *1 |
| xGNP M5 XG Sciences | compact | 5 *3 | 6–8 nm [43] | 34 *2 | 120–150 [43] | 160 *1 |
| xGNP M15 XG Sciences | compact | 15 *3 | 6–8 nm [43] | 35 *2 | 120–150 [43] | 66 *1 |
| xGNP M25 XG Sciences | compact | 25 *3 | 6–8 nm [43] | 22 *2 | 120–150 [43] | 67 *1 |
| Single Layer Graphene ACS Material® | lamellar | 66 *3 | 1–5 layer [44] | 4 *2 | 650–750 [44] | 5 *1 |
| EXG 98 300 Graphit Kropfmühl | lamellar | 305 *3 | - | 3 *2 | >300 [45] | 1 *1 |
| GNPs *0 Grade 3 Cheap Tubes | fragmented | 2 *3 | 8 nm [46] | 5 *2 | 600–750 [46] | 229 *1 |
| Nanocyl™ NC7000 (CNT) Nanocyl S.A | fibre | >675 [47] | Ø 9.5 nm [48] | 15 *2 | 250–300 [48] | 66 [47] |
| Printex XE2B (CB) Orion Engineered Carbons | spherical | 60 *3 | Ø 30–35 nm [49] | 20 *2 | 1000 [49] | 100–400 [49] |
| Characterization Method | Dimension | Molding Parameters | Hot Press |
|---|---|---|---|
| electrical conductivity DC | round Ø 60 mm; thickness 0.3 mm | 250 °C, 1.5 min, 50 kN | PW 40 EH |
| thermal conductivity | round Ø 25 mm; thickness 5 mm | 250 °C, 1.5 min, 50 kN | PW 20 |
| DMTA | 45 mm × 10 mm; thickness 1 mm | 250 °C, 1.5 min, 50 kN | PW 40 EH |
| gas permeation | round Ø 120 mm; thickness 0.2 mm | 250 °C, 1.5 min, 50 kN | PW 40 EH |
| tensile test | round Ø 60 mm; thickness 0.5 mm | 250 °C, 1.5 min, 50 kN | PW 40 EH |
| Sample composition | Mixing Time [min] | Temperature [°C] | Screw Speed [min−1] |
|---|---|---|---|
| PC + Graphene nanopowder AO-3 | 5 | 280 | 250 |
| PC + ACS-Single Layer Graphene | 5 | 260 | 150 |
| PC + Cheap Tubes GNP Grade 3 | 5 | 260 | 250 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.T.; Hilarius, K.; Liebscher, M.; Lellinger, D.; Alig, I.; Pötschke, P. Effect of Graphite Nanoplate Morphology on the Dispersion and Physical Properties of Polycarbonate Based Composites. Materials 2017, 10, 545. https://doi.org/10.3390/ma10050545
Müller MT, Hilarius K, Liebscher M, Lellinger D, Alig I, Pötschke P. Effect of Graphite Nanoplate Morphology on the Dispersion and Physical Properties of Polycarbonate Based Composites. Materials. 2017; 10(5):545. https://doi.org/10.3390/ma10050545
Chicago/Turabian StyleMüller, Michael Thomas, Konrad Hilarius, Marco Liebscher, Dirk Lellinger, Ingo Alig, and Petra Pötschke. 2017. "Effect of Graphite Nanoplate Morphology on the Dispersion and Physical Properties of Polycarbonate Based Composites" Materials 10, no. 5: 545. https://doi.org/10.3390/ma10050545