Experiment and Artificial Neural Network Prediction of Thermal Conductivity and Viscosity for Alumina-Water Nanofluids
Abstract
:1. Introduction
2. Experimental Methods
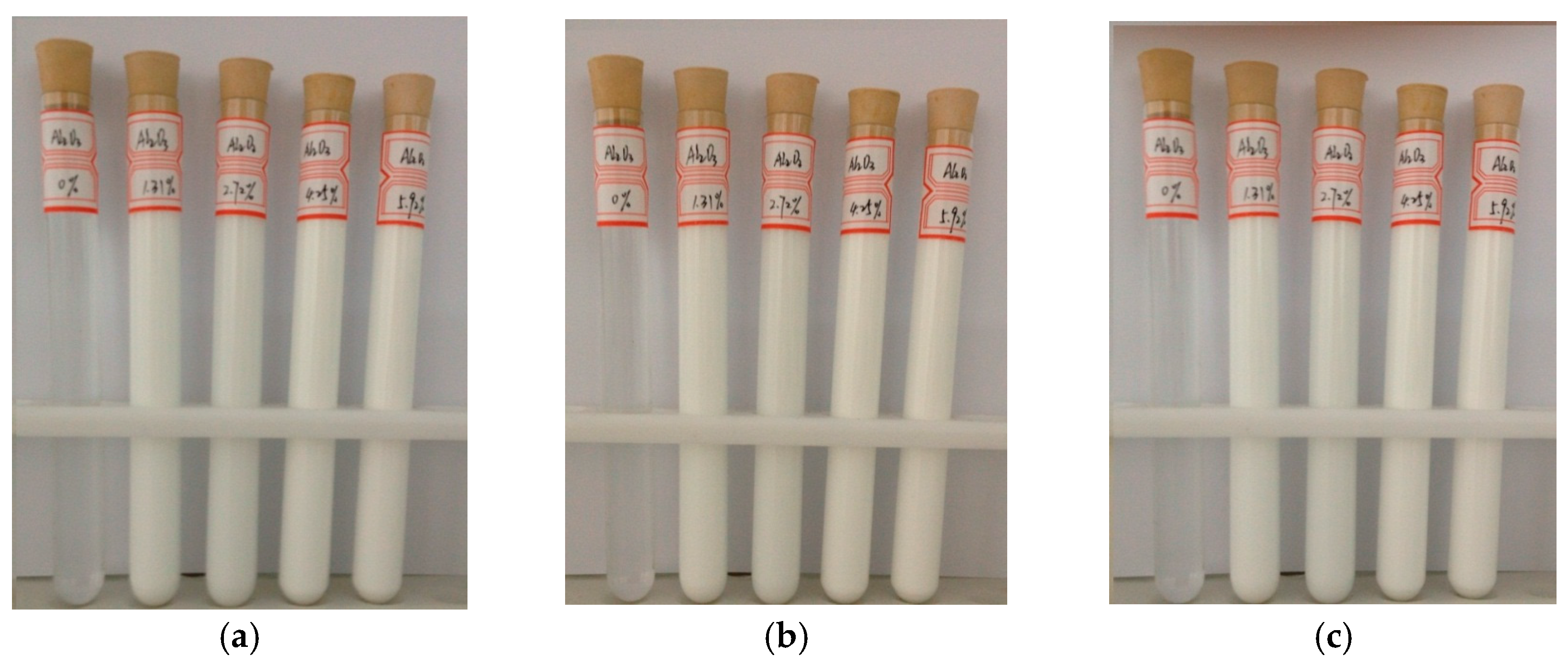
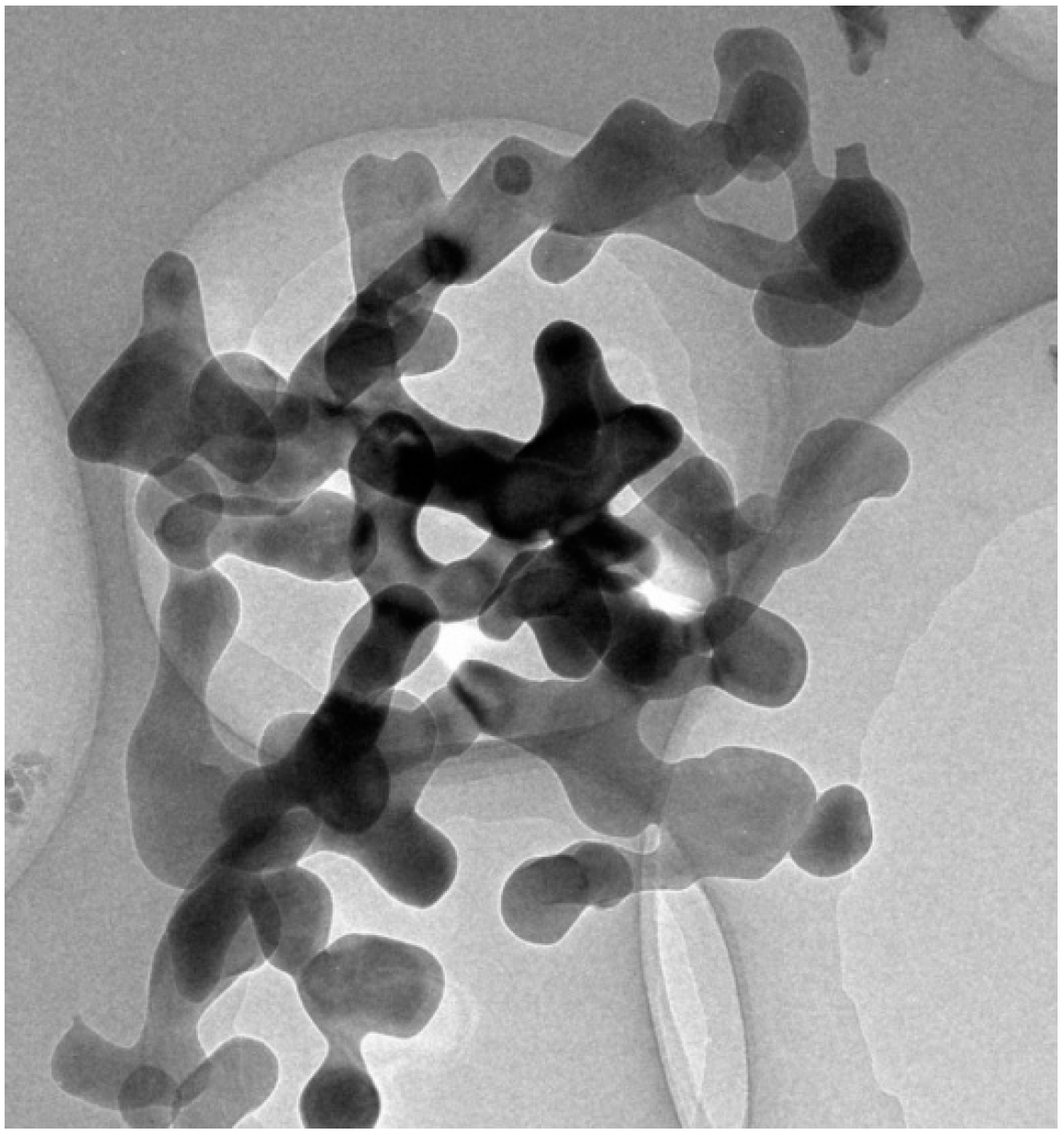
2.1. Materials and Method of Preparing Nanofluids
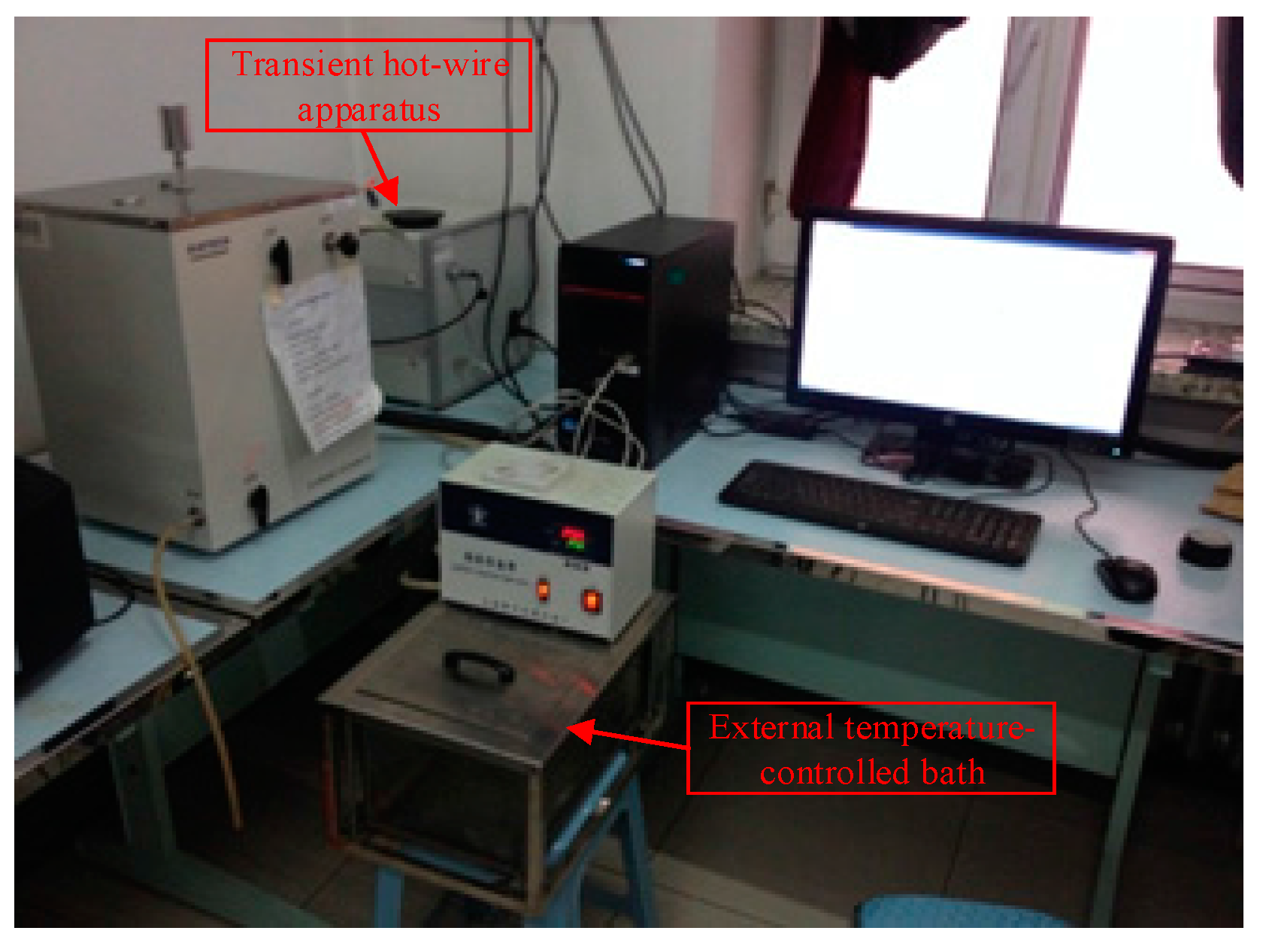
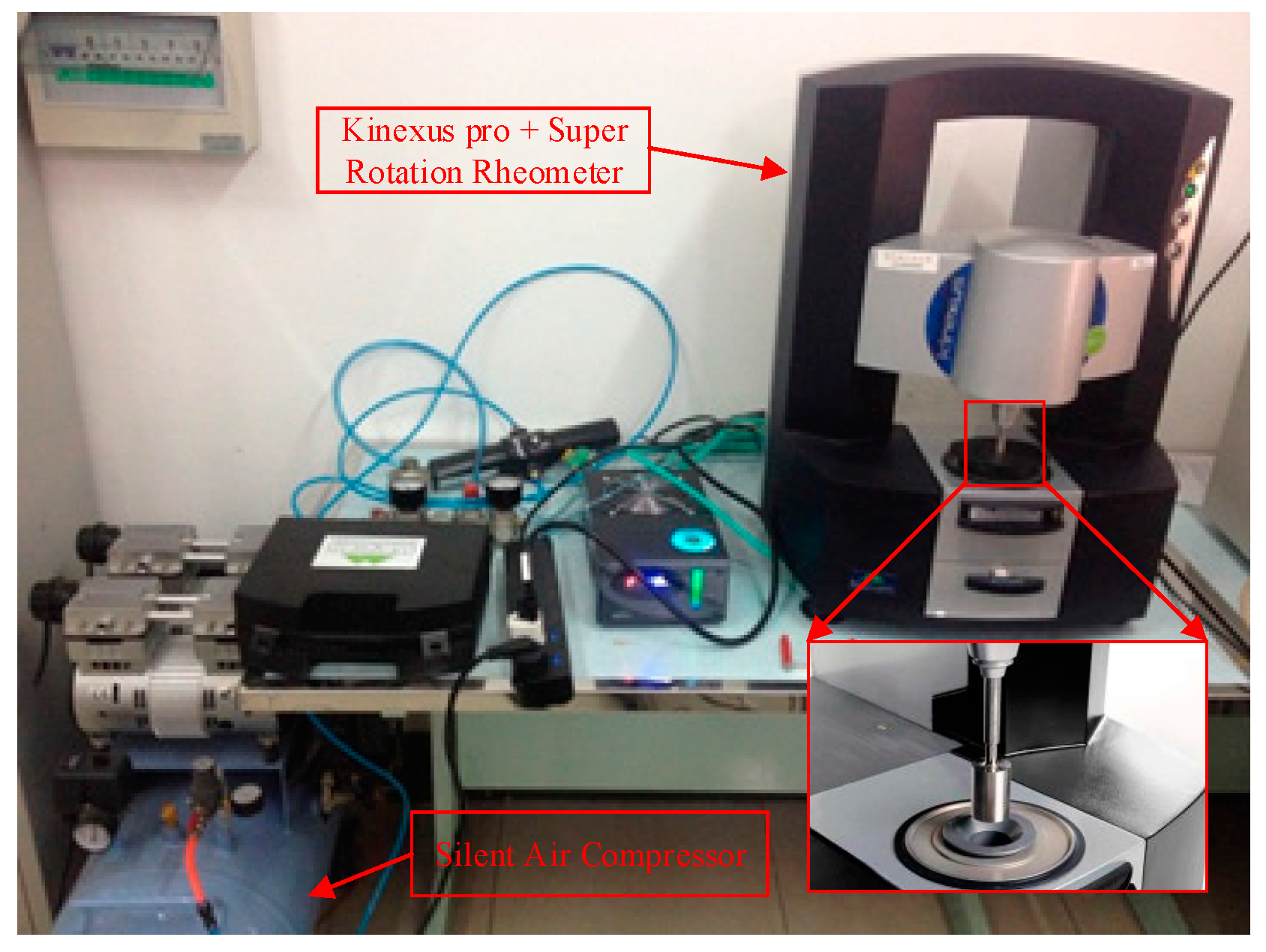
2.2. Method of Investigating the Thermal Conductivity and Viscosity
3. Modeling Method Based on ANN
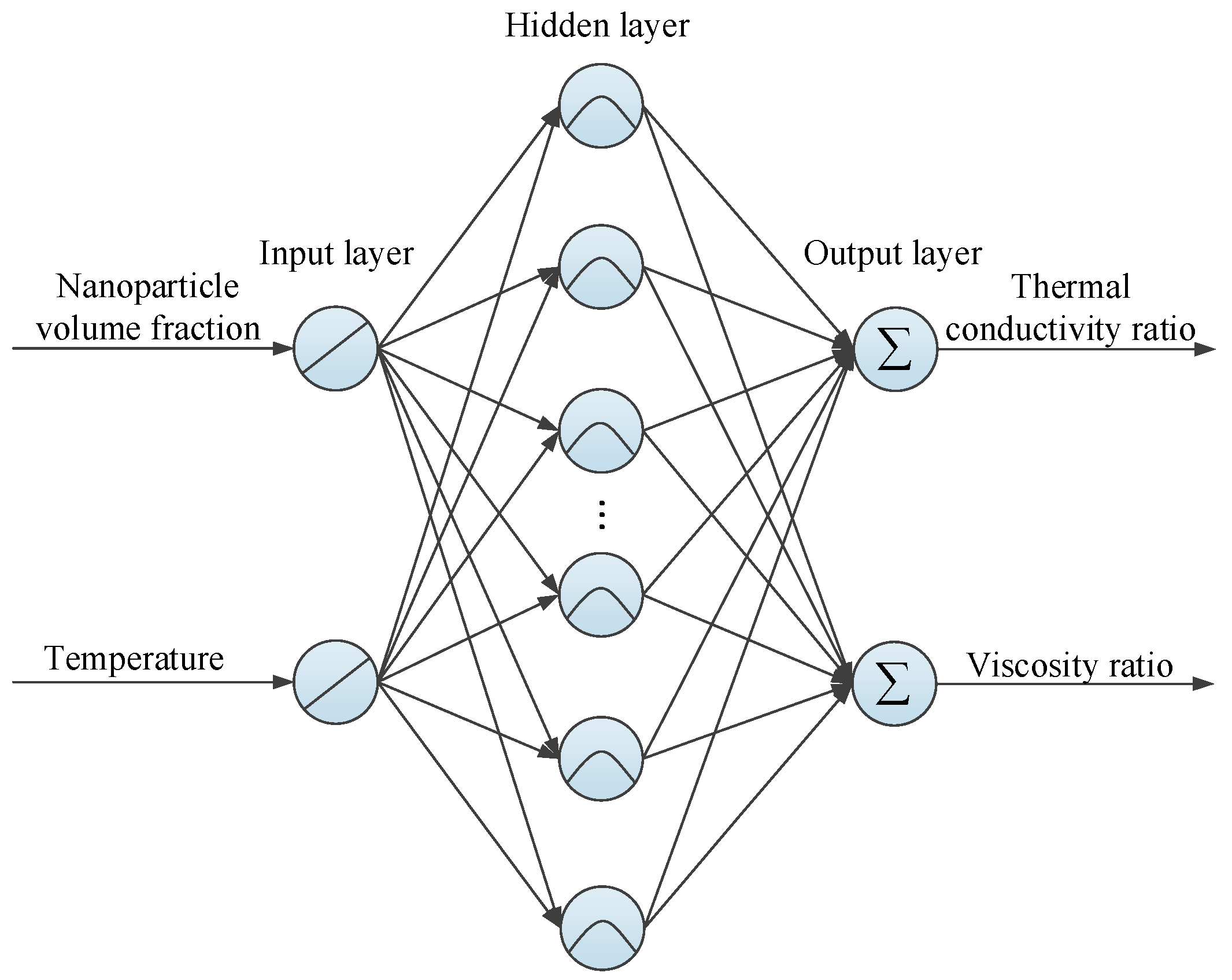
3.1. RBF Neural Network Theory
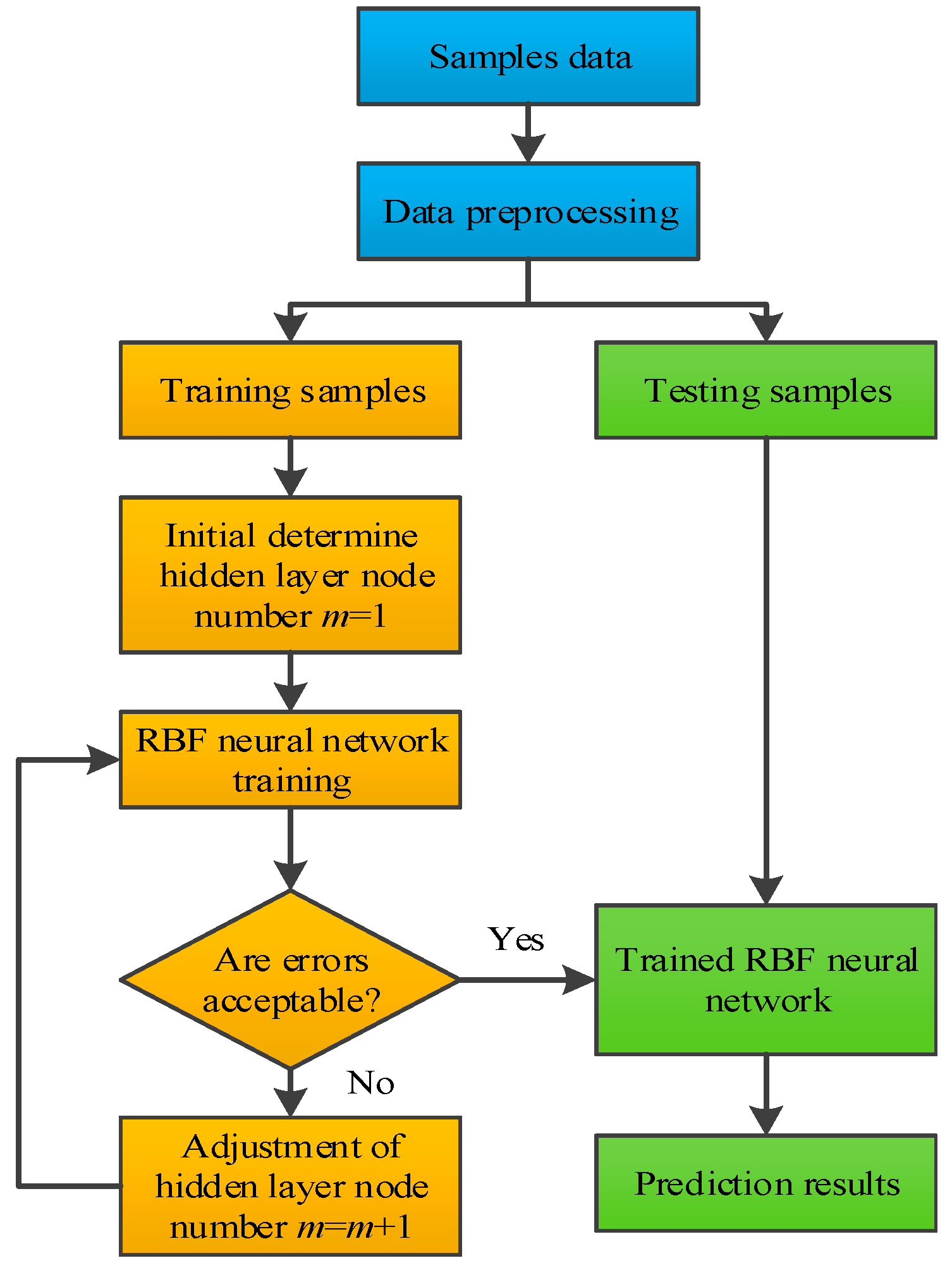
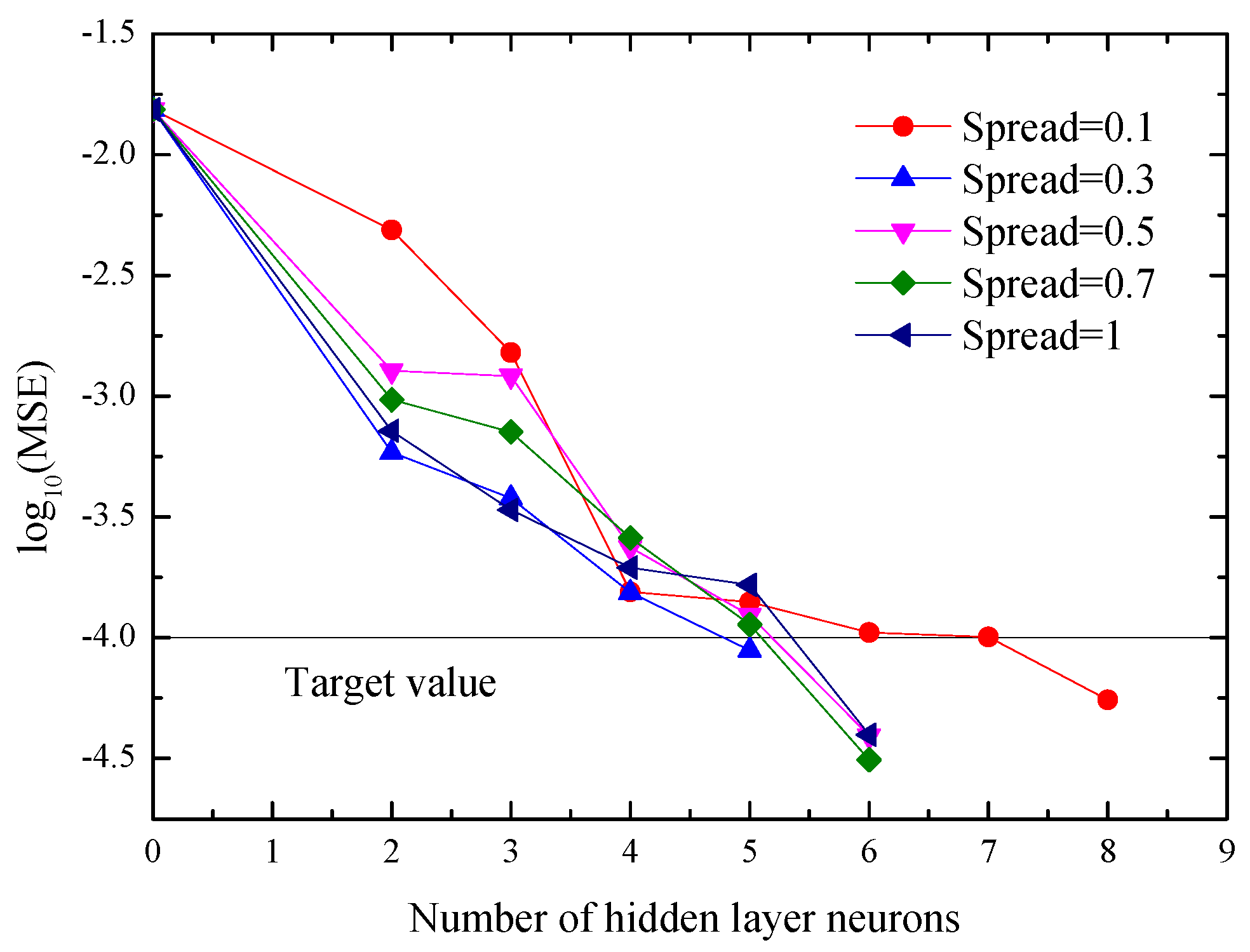
3.2. Implementing Procedure
4. Results and Discussion
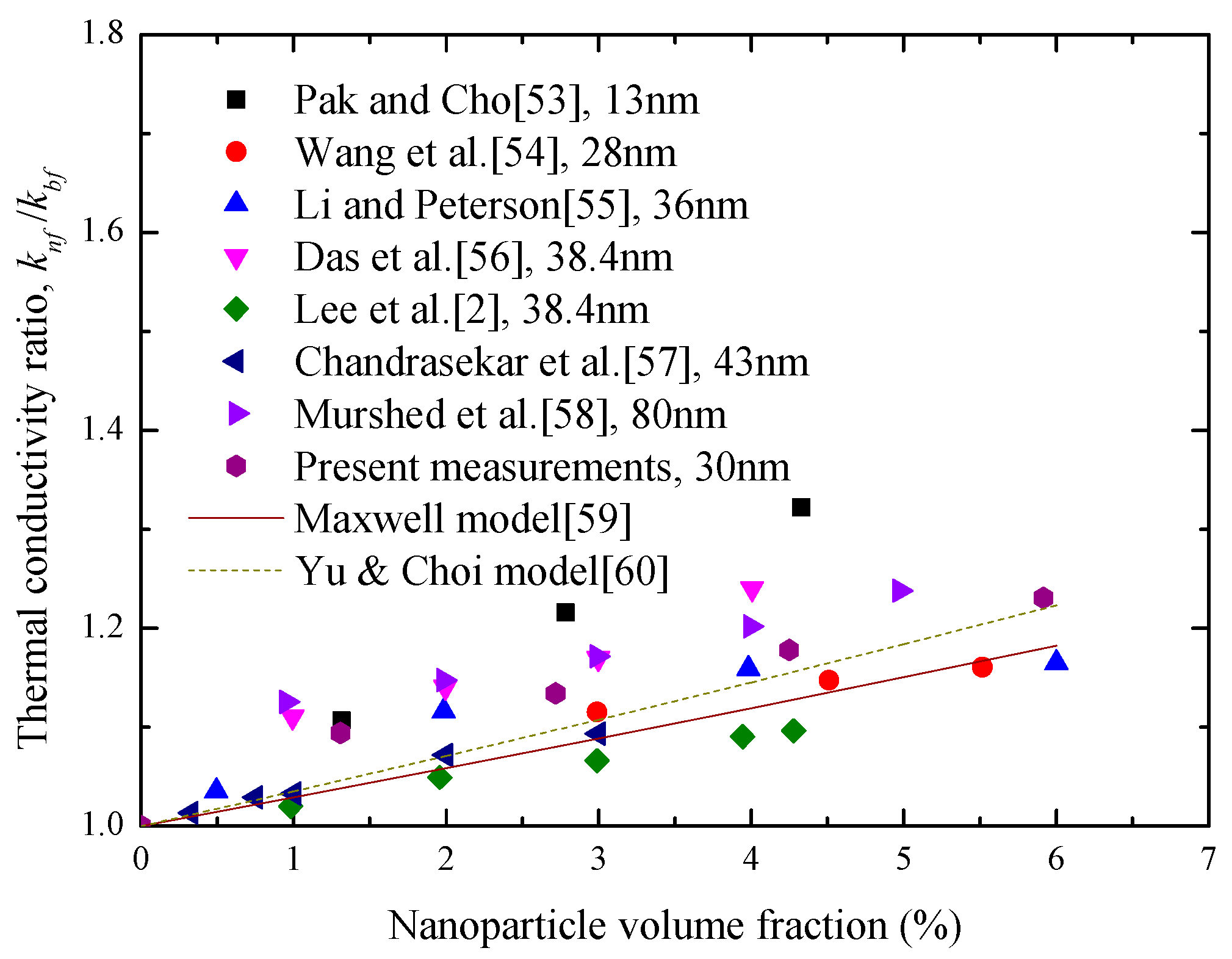
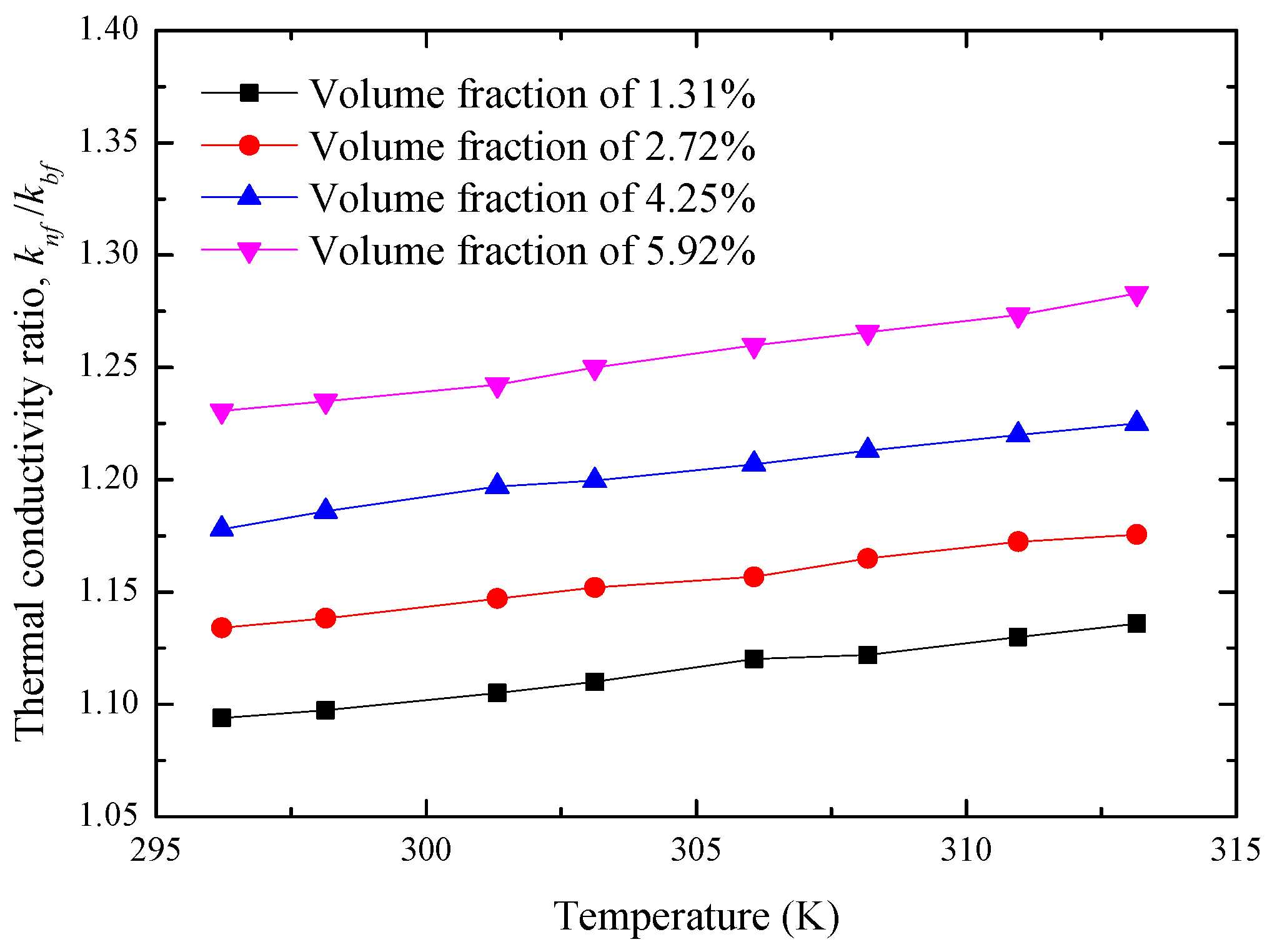
4.1. Enhancement of Thermal Conductivity
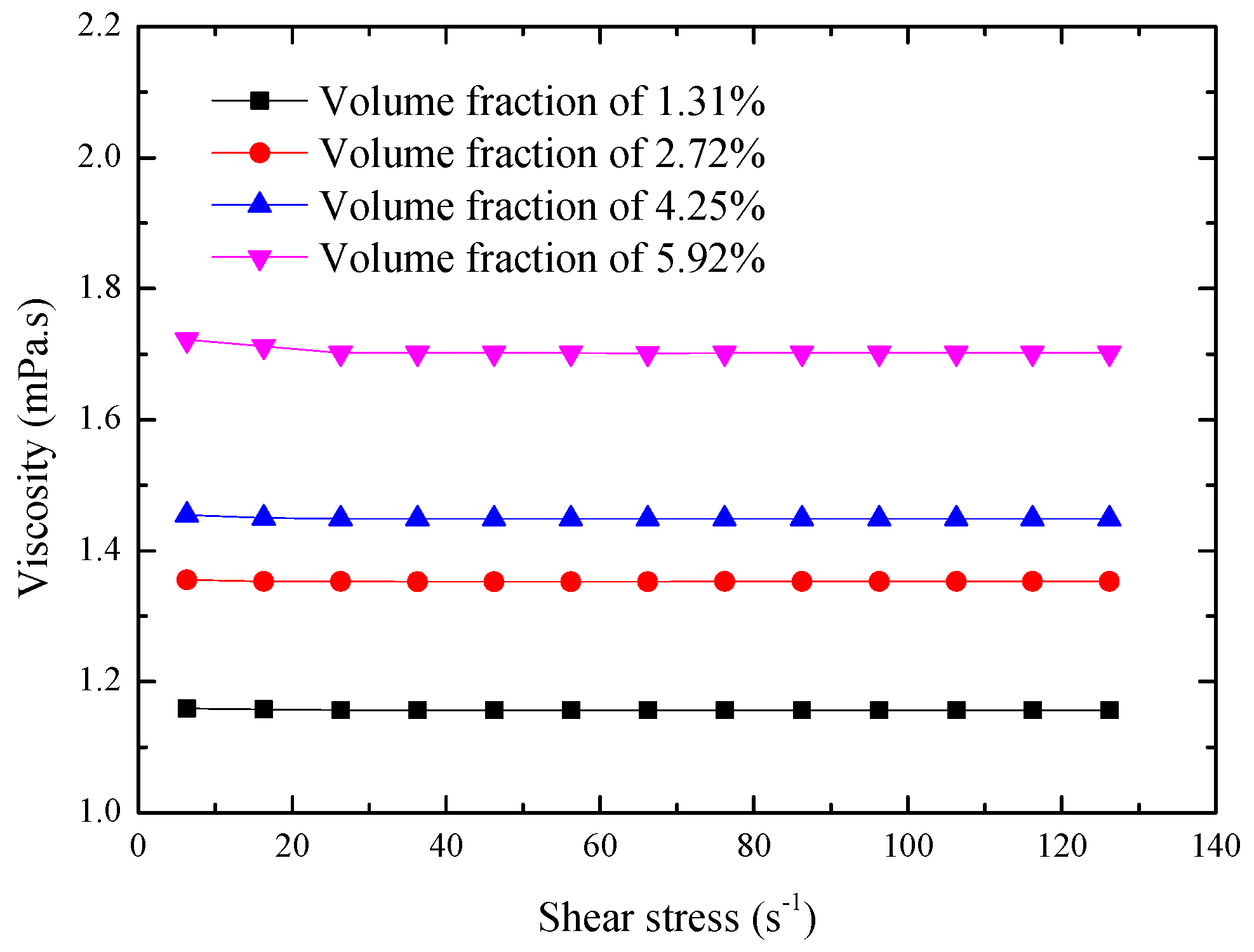
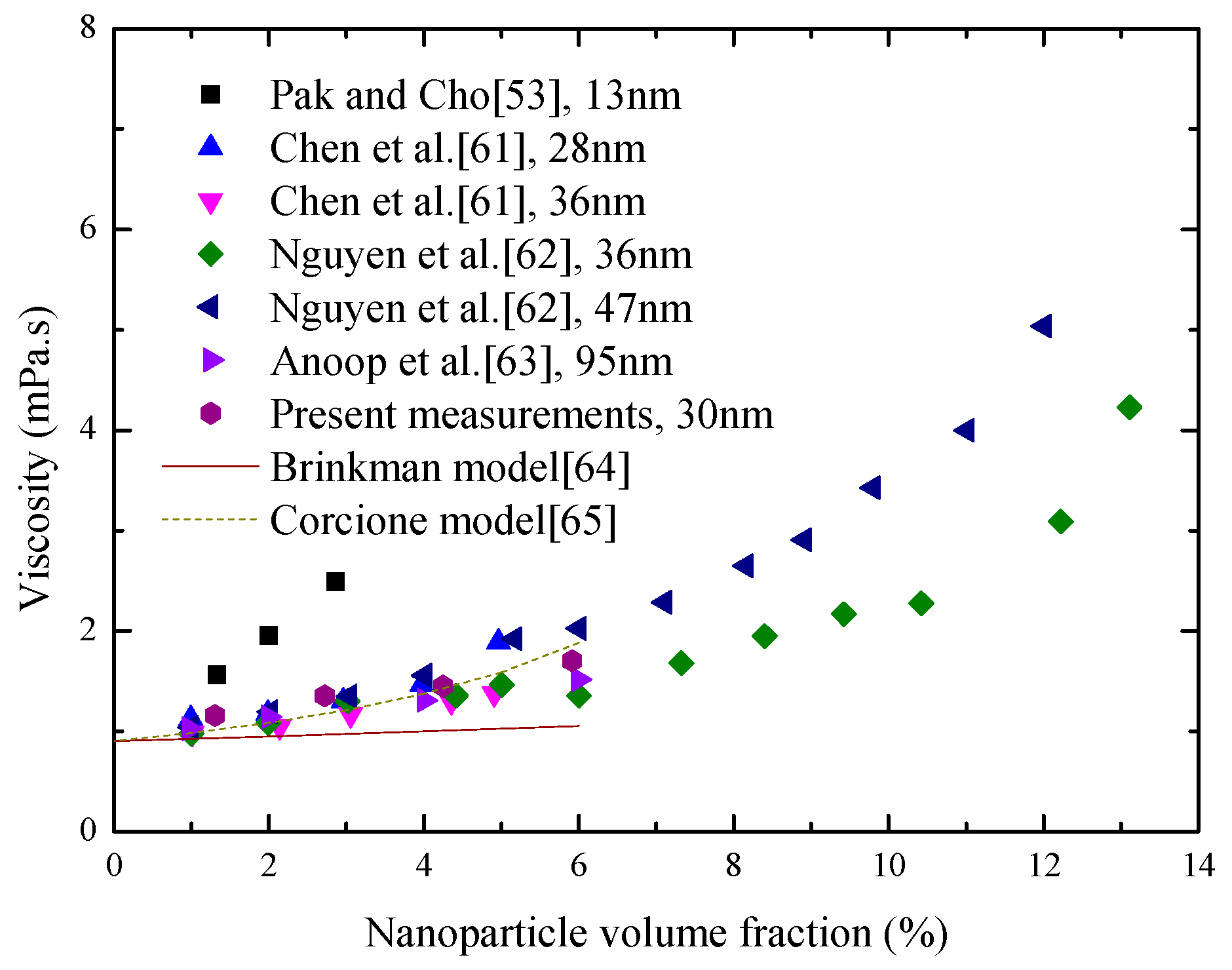
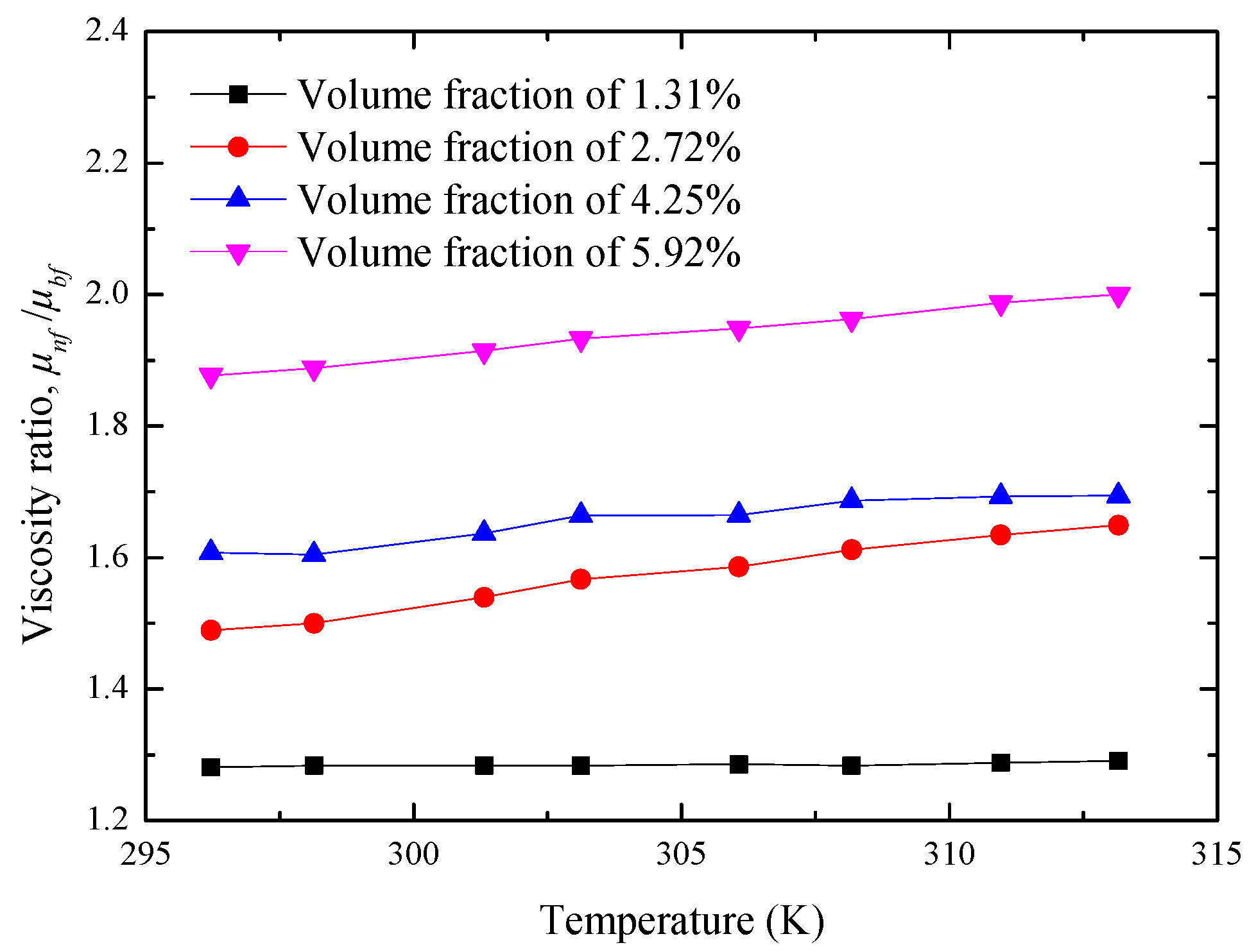
4.2. Viscosity Investigation
4.3. Predictive Analysis of RBF Neural Networks
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, N.B.; Li, S.Y.; Yang, J.L. A review on nanofluids: Data-driven modeling of thermalphysical properties and the application in automotive radiator. Renew. Sustain. Energy Rev. 2016, 66, 596–616. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.S.; Li, S.; Eastman, J.A. Measuring thermal conductivity of fluids containing oxide nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Zhao, N.B.; Yang, J.L.; Li, S.Y.; Wang, Q. Numerical investigation of laminar thermal-hydraulic performance of Al2O3–water nanofluids in offset strip fins channel. Int. Commun. Heat Mass Transf. 2016, 75, 42–51. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Thermophysical and electrokinetic properties of nanofluids-a critical review. Appl. Therm. Eng. 2008, 28, 2109–2125. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Zhao, N.B.; Yang, J.L.; Li, H.; Zhang, Z.Y.; Li, S.Y. Numerical investigations of laminar heat transfer and flow performance of Al2O3-water nanofluids in a flat tube. Int. J. Heat Mass Transf. 2016, 92, 268–282. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Saidur, R.; Amalina, A.M. Latest developments on the viscosity of nanofluids. Int. J. Heat Mass Transf. 2012, 55, 874–885. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Hossain, M.S.; Saidur, R.; Sabri, M.F.M.; Said, Z.; Hassani, S. Spotlight on available optical properties and models of nanofluids: A review. Renew. Sustain. Energy Rev. 2015, 43, 750–762. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef] [PubMed]
- Solangi, K.H.; Kazi, S.N.; Luhur, M.R.; Badarudin, A.; Amiri, A.; Sadri, R.; Zubir, M.N.M.; Gharehkhani, S.; Teng, K.H. A comprehensive review of thermo-physical properties and convective heat transfer to nanofluids. Energy 2015, 89, 1065–1086. [Google Scholar] [CrossRef]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Estellé, P. A state of the art review on viscosity of nanofluids. Renew. Sustain. Energy Rev. 2017, 76, 1134–1152. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Mohammed, H.A.; Alawi, O.A.; Samion, S. A review on preparation methods and challenges of nanofluids. Int. Commun. Heat Mass Transf. 2014, 54, 115–125. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two component systems. Ind. Eng. Chem. Fundamen. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Azmi, W.H.; Sharma, K.V.; Mamat, R.; Najafi, G.; Mohamad, M.S. The enhancement of effective thermal conductivity and effective dynamic viscosity of nanofluids—A review. Renew. Sustain. Energy Rev. 2016, 53, 1046–1058. [Google Scholar] [CrossRef]
- Liu, M.; Ding, C.; Wang, J. Modeling of thermal conductivity of nanofluids considering aggregation and interfacial thermal resistance. RSC Adv. 2016, 6, 3571–3577. [Google Scholar] [CrossRef]
- Xue, Q.; Xu, W.M. A model of thermal conductivity of nanofluids with interfacial shells. Mater. Chem. Phys. 2005, 90, 298–301. [Google Scholar] [CrossRef]
- Jiang, H.; Li, H.; Xu, Q.; Shi, L. Effective thermal conductivity of nanofluids considering interfacial nano-shells. Mater. Chem. Phys. 2014, 148, 195–200. [Google Scholar] [CrossRef]
- Cheng, N.S.; Law, A.W.K. Exponential formula for computing effective viscosity. Powder Technol. 2003, 129, 156–160. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Masoumi, N.; Sohrabi, N.; Behzadmehr, A. A new model for calculating the effective viscosity of nanofluids. J. Phys. Appl. Phys. 2009, 42, 055501. [Google Scholar] [CrossRef]
- Prasher, R.; Phelan, P.E.; Bhattacharya, P. Effect of aggregation kinetics on the thermal conductivity of nanoscale colloidal solutions (nanofluid). Nano Lett. 2006, 6, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, B.; Zou, M.; Xu, P. A new model for heat conduction of nanofluids based on fractal distributions of nanoparticles. J. Phys. Appl. Phys. 2006, 39, 4486. [Google Scholar] [CrossRef]
- Yang, K.T. Artificial neural networks (ANNs): A new paradigm for thermal science and engineering. J. Heat Transf. 2008, 130, 093001. [Google Scholar] [CrossRef]
- Ghaedi, A. Simultaneous prediction of the thermodynamic properties of aqueous solution of ethylene glycol monoethyl ether using artificial neural network. J. Mol. Liq. 2015, 207, 327–333. [Google Scholar] [CrossRef]
- Hojjat, M.; Etemad, S.G.; Bagheri, R.; Thibault, J. Thermal conductivity of non-newtonian nanofluids: Experimental data and modeling using neural network. Int. J. Heat Mass Transf. 2011, 54, 1017–1023. [Google Scholar] [CrossRef]
- Longo, G.A.; Zilio, C.; Ceseracciu, E.; Reggiani, M. Application of artificial neural network (ANN) for the prediction of thermal conductivity of oxide-water nanofluids. Nano Energy 2012, 1, 290–296. [Google Scholar] [CrossRef]
- Mehrabi, M.; Sharifpur, M.; Meyer, J.P. Adaptive neuro-fuzzy modeling of the thermal conductivity of alumina-water nanofluids. In Proceedings of the ASME 2012 Third International Conference on Micro/Nanoscale Heat and Mass Transfer, Atlanta, GA, USA, 3–6 March 2012; pp. 155–161. [Google Scholar]
- Mehrabi, M.; Sharifpur, M.; Meyer, J.P. Application of the FCM-based Neuro-fuzzy inference system and genetic algorithm-polynomial neural network approaches to modelling the thermal conductivity of alumina-water nanofluids. Int. Commun. Heat Mass Transf. 2012, 39, 971–977. [Google Scholar] [CrossRef]
- Ariana, M.A.; Vaferi, B.; Karimi, G. Prediction of thermal conductivity of alumina water-based nanofluids by artificial neural networks. Powder Technol. 2015, 278, 1–10. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Bahiraei, M.; Toghraie, D.; Mahian, O.; Wongwises, S. Thermal conductivity modeling of MgO/EG nanofluids using experimental data and artificial neural network. J. Therm. Anal. Calorim. 2014, 118, 287–294. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Naderi, A.; Alirezaie, A.; Karimipour, A.; Wongwises, S.; Goodarzi, M.; Dahari, M. Modeling of thermal conductivity of ZnO-EG using experimental data and ANN methods. Int. Commun. Heat Mass Transf. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Esfe, M.H.; Wongwises, S.; Naderi, A.; Asadi, A.; Safaei, M.R.; Rostamian, H.; Dahari, M.; Karimipour, A. Thermal conductivity of Cu/TiO2-water/EG hybrid nanofluid: Experimental data and modeling using artificial neural network and correlation. Int. Commun. Heat Mass Transf. 2015, 66, 100–104. [Google Scholar] [CrossRef]
- Esfe, M.H.; Afrand, M.; Wongwises, S.; Naderi, A.; Asadi, A.; Rostami, S.; Akbari, M. Applications of feed forward multilayer perceptron artificial neural networks and empirical correlation for prediction of thermal conductivity of Mg(OH)2-EG using experimental data. Int. Commun. Heat Mass Transf. 2015, 67, 46–50. [Google Scholar] [CrossRef]
- Esfe, M.H.; Rostamian, H.; Afrand, M.; Karimipour, A.; Hassani, M. Modeling and estimation of thermal conductivity of MgO-water/EG (60:40) by artificial Neural network and correlation. Int. Commun. Heat Mass Transf. 2015, 68, 98–103. [Google Scholar] [CrossRef]
- Esfe, M.H.; Naderi, A.; Akbari, M.; Afrand, M.; Karimipour, A. Evaluation of thermal conductivity of COOH-functionalized MWCNTs/water via temperature and solid volume fraction by using experimental data and ANN methods. J. Therm. Anal. Calorim. 2015, 121, 1273–1278. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Sina, N.; Afrand, M.; Rostami, S. Designing an artificial neural network to predict thermal conductivity and dynamic viscosity of ferromagnetic nanofluid. Int. Commun. Heat Mass Transf. 2015, 68, 50–57. [Google Scholar] [CrossRef]
- Vakili, M.; Yahyaei, M.; Kalhor, K. Thermal conductivity modeling of graphene nanoplatelets/deionized water nanofluid by MLP neural network and theoretical modeling using experimental results. Int. Commun. Heat Mass Transf. 2016, 74, 11–17. [Google Scholar] [CrossRef]
- Yousefi, F.; Karimi, H.; Papari, M.M. Modeling viscosity of nanofluids using diffusional neural networks. J. Mol. Liq. 2012, 175, 85–90. [Google Scholar] [CrossRef]
- Mehrabi, M.; Sharifpur, M.; Meyer, J.P. Viscosity of nanofluids based on an artificial intelligence model. Int. Commun. Heat Mass Transf. 2013, 43, 16–21. [Google Scholar] [CrossRef]
- Zhao, N.B.; Li, S.Y.; Wang, Z.T.; Cao, Y.P. Prediction of viscosity of nanofluids using artificial neural networks. In Proceedings of the ASME 2014 International Mechanical Engineering Congress & Exposition, Montreal, QC, Canada, 14–20 November 2014. [Google Scholar] [CrossRef]
- Zhao, N.B.; Wen, X.Y.; Yang, J.L.; Li, S.Y.; Wang, Z.T. Modeling and prediction of viscosity of water-based nanofluids by radial basis function neural networks. Powder Technol. 2015, 281, 173–183. [Google Scholar] [CrossRef]
- Heidari, E.; Sobati, M.A.; Movahedirad, S. Accurate prediction of nanofluid viscosity using a multilayer perceptron artificial neural network (MLP-ANN). Chemom. Intell. Lab. Syst. 2016, 155, 73–85. [Google Scholar] [CrossRef]
- Yang, L.; Du, K.; Niu, X.; Li, Y.; Zhang, Y. An experimental and theoretical study of the influence of surfactant on the preparation and stability of ammonia-water nanofluids. Int. J. Refrig. 2011, 34, 1741–1748. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Malvern. Available online: http://www.malvern.com/en/products/product-range/kinexus-range/kinexus-pro-plus/ (accessed on 15 April 2017).
- Aladag, B.; Halelfadl, S.; Doner, N.; Maré, T.; Duret, S.; Estellé, P. Experimental investigations of the viscosity of nanofluids at low temperatures. Appl. Energy 2012, 97, 876–880. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; He, Y.; Hu, Y.; Zhu, J.; Jiang, B. Experimental investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluids. Appl. Therm. Eng. 2015, 88, 363–368. [Google Scholar] [CrossRef]
- Turnbull, D.; Elkan, C. Fast recognition of musical genres using RBF networks. IEEE Trans. Knowl. Data Eng. 2005, 17, 580–584. [Google Scholar] [CrossRef]
- Iliyas, S.A.; Elshafei, M.; Habib, M.A.; Adeniran, A.A. RBF neural network inferential sensor for process emission monitoring. Control Eng. Pract. 2013, 21, 962–970. [Google Scholar] [CrossRef]
- Chen, S.; Cowan, C.F.N.; Grant, P.M. Orthogonal least squares learning algorithm for radial basis function networks. IEEE Trans. Neural Netw. 1991, 2, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal conductivity of nanoparticle-fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Li, C.H.; Peterson, G.P. The effect of particle size on the effective thermal conductivity of Al2O3-water nanofluids. J. Appl. Phys. 2007, 101, 44312. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Suresh, S.; Chandra, B.A. Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid. Exp. Therm. Fluid Sci. 2010, 34, 210–216. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2-water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Oxford, UK, 1981. [Google Scholar]
- Yu, W.; Choi, S.U.S. The role of interfacial layer in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; He, Y.; Tan, C. Rheological behaviour of ethylene glycol based titania nanofluids. Chem. Phys. Lett. 2007, 444, 333–337. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, S.; Angue, M.H. Temperature and particle-size dependent viscosity data for water-based nanofluids-hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Anoop, K.B.; Kabelac, S.; Sundararajan, T.; Das, S.K. Rheological and flow characteristics of nanofluids: Influence of electroviscous effects and particle agglomeration. J. Appl. Phys. 2009, 106, 034909. [Google Scholar] [CrossRef]
- Brinkman, H.C. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Corcione, M. Empirical correlating equations for predicting the effective thermal conductivity and dynamic viscosity of nanofluids. Energy Convers. Manag. 2011, 52, 789–793. [Google Scholar] [CrossRef]

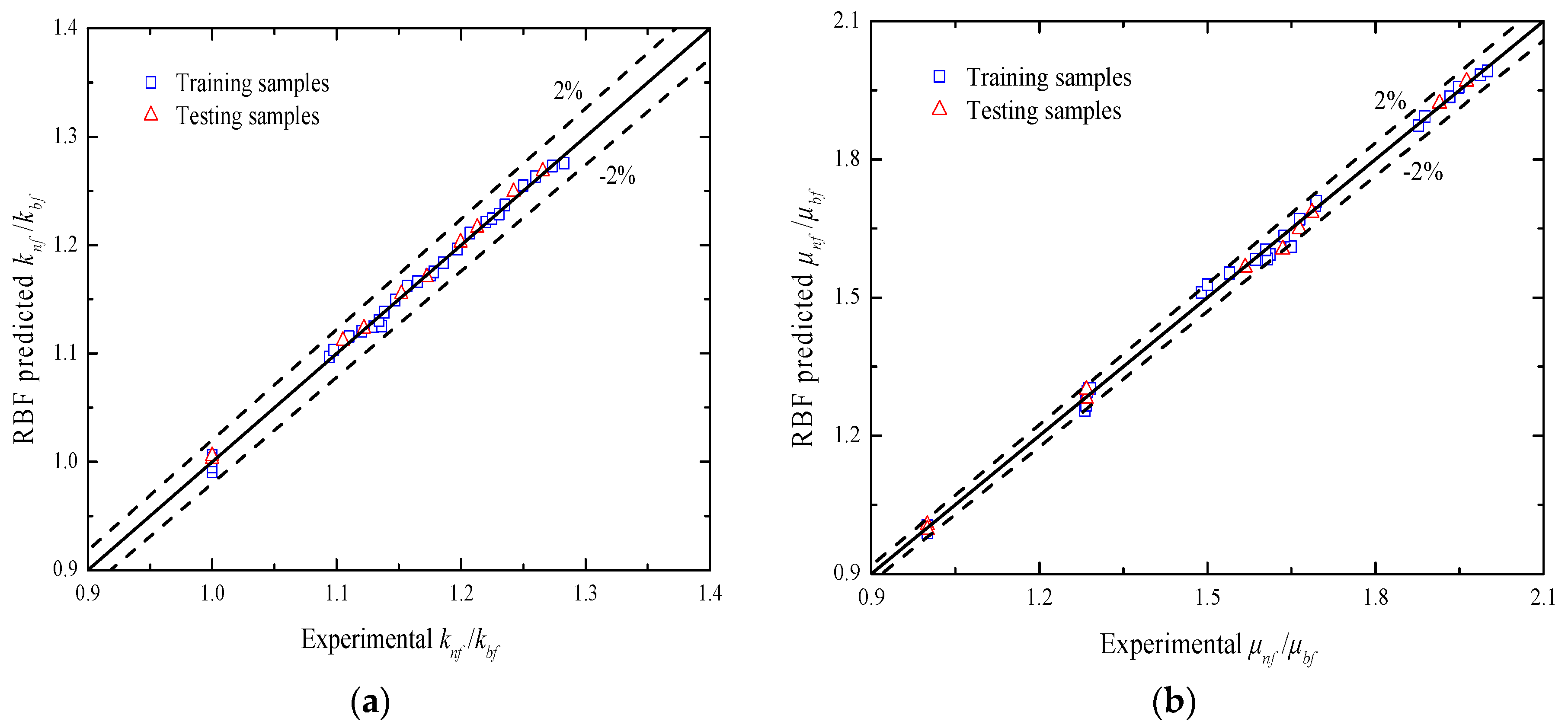
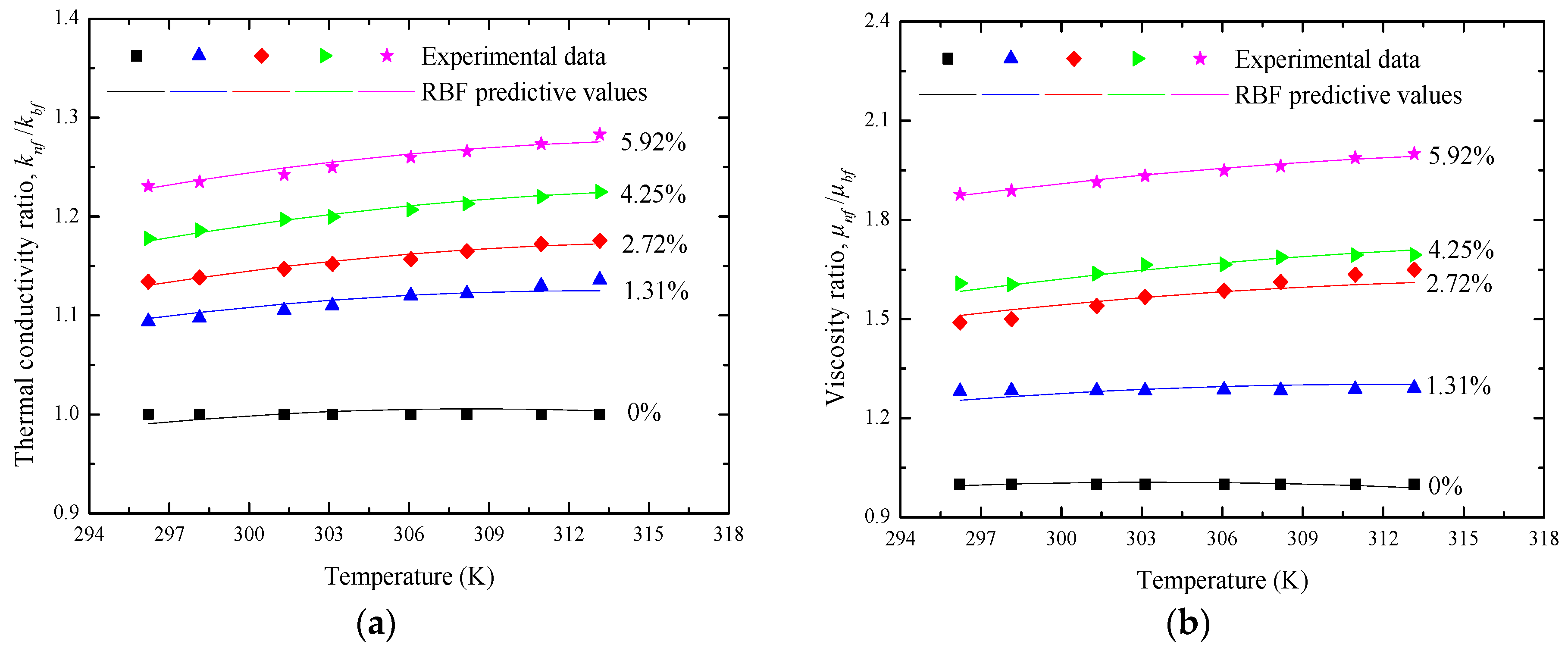
| Temperature (K) | Reference Values (W/(m·K)) | Measure Values (W/(m·K)) | Deviation (%) |
|---|---|---|---|
| 288 | 0.5916 | 0.595 | 0.5747 |
| 293 | 0.6003 | 0.6019 | 0.2665 |
| 298 | 0.6088 | 0.6111 | 0.3778 |
| 303 | 0.6173 | 0.6185 | 0.1944 |
| 308 | 0.6245 | 0.6252 | 0.1121 |
| 313 | 0.6318 | 0.6292 | −0.4115 |
| 318 | 0.6379 | 0.6374 | −0.0784 |
| Temperature (K) | Reference Values (mPa·s) | Measure Values (mPa·s) | Deviation (%) |
|---|---|---|---|
| 288 | 1.1426 | 1.1517 | −0.7955 |
| 293 | 1.0094 | 0.9998 | 0.9517 |
| 298 | 0.8938 | 0.8943 | −0.0658 |
| 303 | 0.8029 | 0.7958 | 0.8791 |
| 308 | 0.7226 | 0.7182 | 0.6106 |
| 313 | 0.6634 | 0.6693 | −0.8866 |
| 318 | 0.6008 | 0.6043 | −0.5876 |
| Parameters | Evaluation Criteria | Spread | ||||
|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 0.7 | 1 | ||
| knf/kbf | RMSE | 8.572 × 10−3 | 6.797 × 10−3 | 4.140 × 10−3 | 4.346 × 10−3 | 4.043 × 10−3 |
| MAPE(%) | 0.5177 | 0.4803 | 0.2872 | 0.3197 | 0.2866 | |
| SSE | 2.939 × 10−3 | 1.848 × 10−3 | 6.857 × 10−4 | 7.556 × 10−4 | 6.538 × 10−4 | |
| R2 | 0.999944 | 0.999965 | 0.999987 | 0.999986 | 0.999988 | |
| μnf/μbf | RMSE | 1.423 × 10−2 | 2.311 × 10−2 | 1.624 × 10−2 | 1.381 × 10−2 | 1.658 ×10−2 |
| MAPE(%) | 0.5618 | 1.3862 | 0.8233 | 0.7169 | 0.8280 | |
| SSE | 8.094 × 10−3 | 2.137 × 10−2 | 1.055 × 10−2 | 7.634 × 10−3 | 1.100 × 10−2 | |
| R2 | 0.999913 | 0.999770 | 0.999887 | 0.999918 | 0.999882 | |
| Neuron | Hidden Layer | Output Layer | ||||
|---|---|---|---|---|---|---|
| Weights wij and Biases | Weights wjk and Biases | |||||
| Nanoparticle Volume Fraction | Temperature | Biases | Thermal Conductivity | Viscosity | Biases | |
| 1 | 0.7184 | 1 | 1.1894 | −61.8011 | −143.4012 | −4.7283 |
| 2 | 0 | 1 | 1.1894 | −4.4132 | −18.1112 | −6.1187 |
| 3 | 1 | 1 | 1.1894 | 24.4808 | 49.7229 | |
| 4 | 0.4595 | 1 | 1.1894 | 92.2675 | 218.6915 | |
| 5 | 0.2208 | 0.9930 | 1.1894 | −76.4725 | −178.5244 | |
| 6 | 0 | 0.9841 | 1.1894 | 36.6988 | 86.1722 | |
| 7 | 0.7184 | 1 | 1.1894 | −61.8011 | −143.4012 | |
| 8 | 0 | 1 | 1.1894 | −4.4132 | −18.1112 | |
| Parameters | Evaluation Criteria | Training Samples | Testing Samples |
|---|---|---|---|
| knf/kbf | RMSE | 4.464 × 10−3 | 3.974 × 10−3 |
| MAPE (%) | 0.3230 | 0.3098 | |
| SSE | 5.977 × 10−4 | 1.579 × 10−4 | |
| R2 | 0.999985 | 0.999988 | |
| μnf/μbf | RMSE | 1.419 × 10−2 | 1.263 × 10−2 |
| MAPE (%) | 0.7472 | 0.6261 | |
| SSE | 6.040 × 10−3 | 1.594 × 10−3 | |
| R2 | 0.999913 | 0.999932 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Li, Z. Experiment and Artificial Neural Network Prediction of Thermal Conductivity and Viscosity for Alumina-Water Nanofluids. Materials 2017, 10, 552. https://doi.org/10.3390/ma10050552
Zhao N, Li Z. Experiment and Artificial Neural Network Prediction of Thermal Conductivity and Viscosity for Alumina-Water Nanofluids. Materials. 2017; 10(5):552. https://doi.org/10.3390/ma10050552
Chicago/Turabian StyleZhao, Ningbo, and Zhiming Li. 2017. "Experiment and Artificial Neural Network Prediction of Thermal Conductivity and Viscosity for Alumina-Water Nanofluids" Materials 10, no. 5: 552. https://doi.org/10.3390/ma10050552





