Temperature and Copper Concentration Effects on the Formation of Graphene-Encapsulated Copper Nanoparticles from Kraft Lignin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Precursor Mixing
2.4. Thermal Treatment
2.5. Characterization
2.6. Statistical Analysis
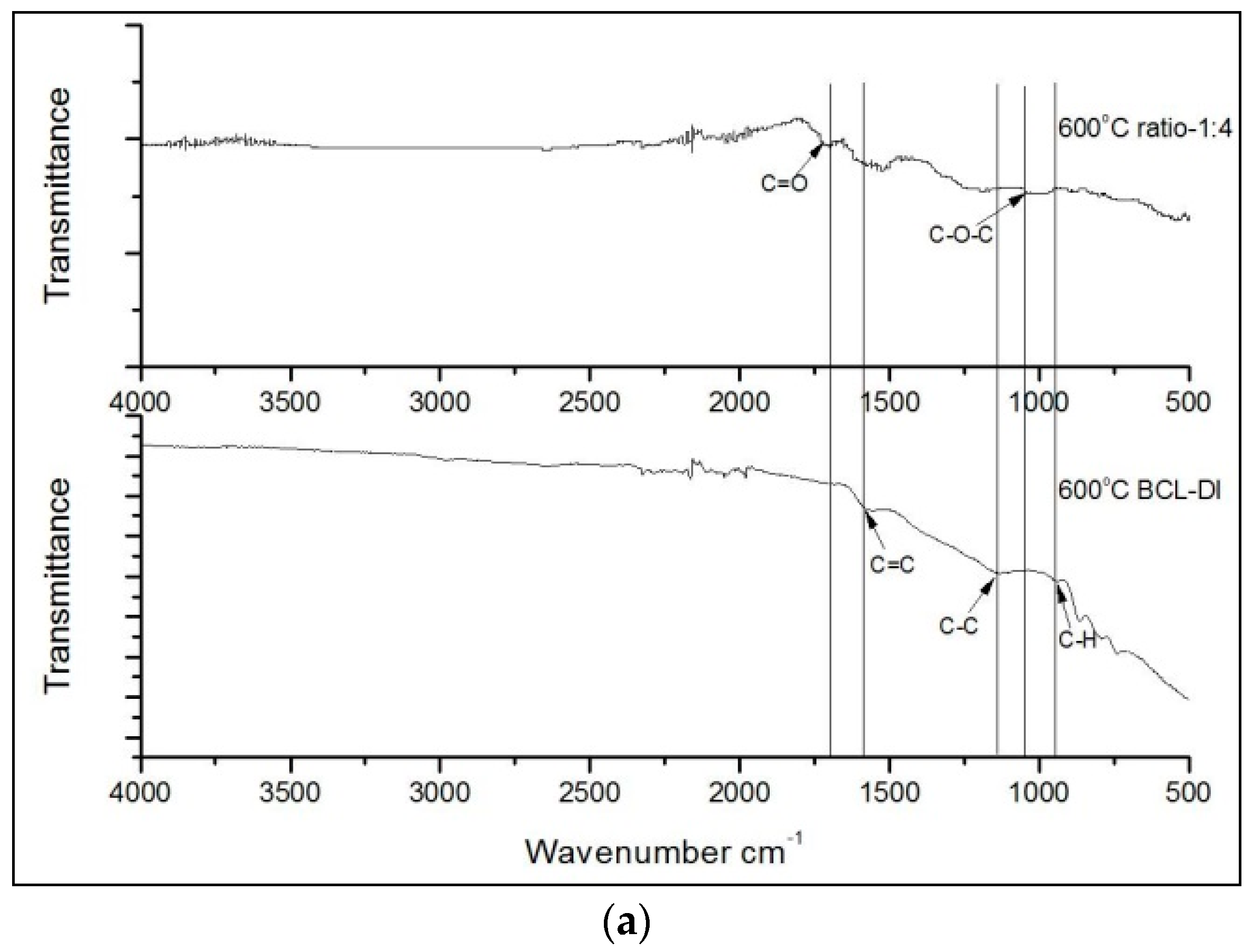
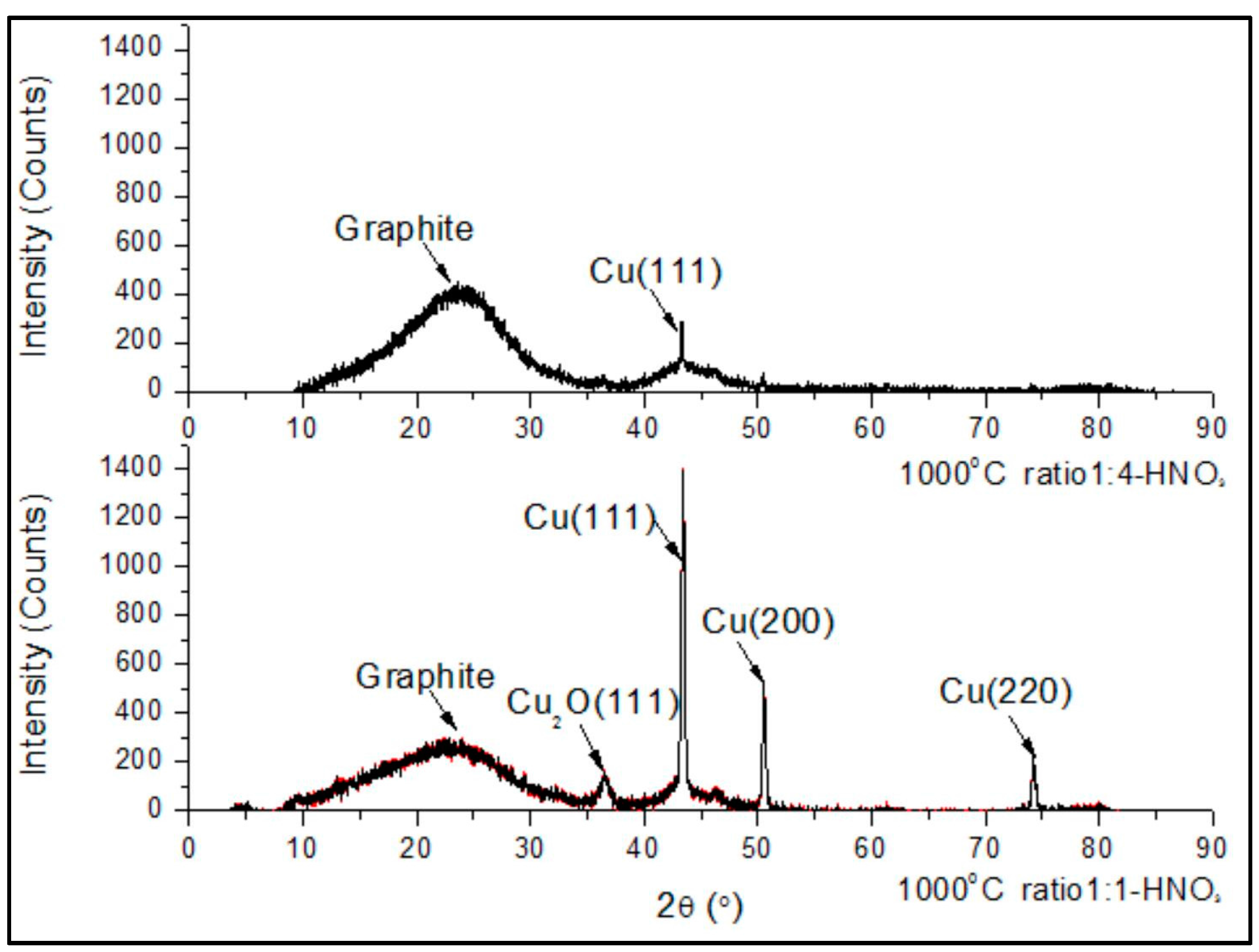
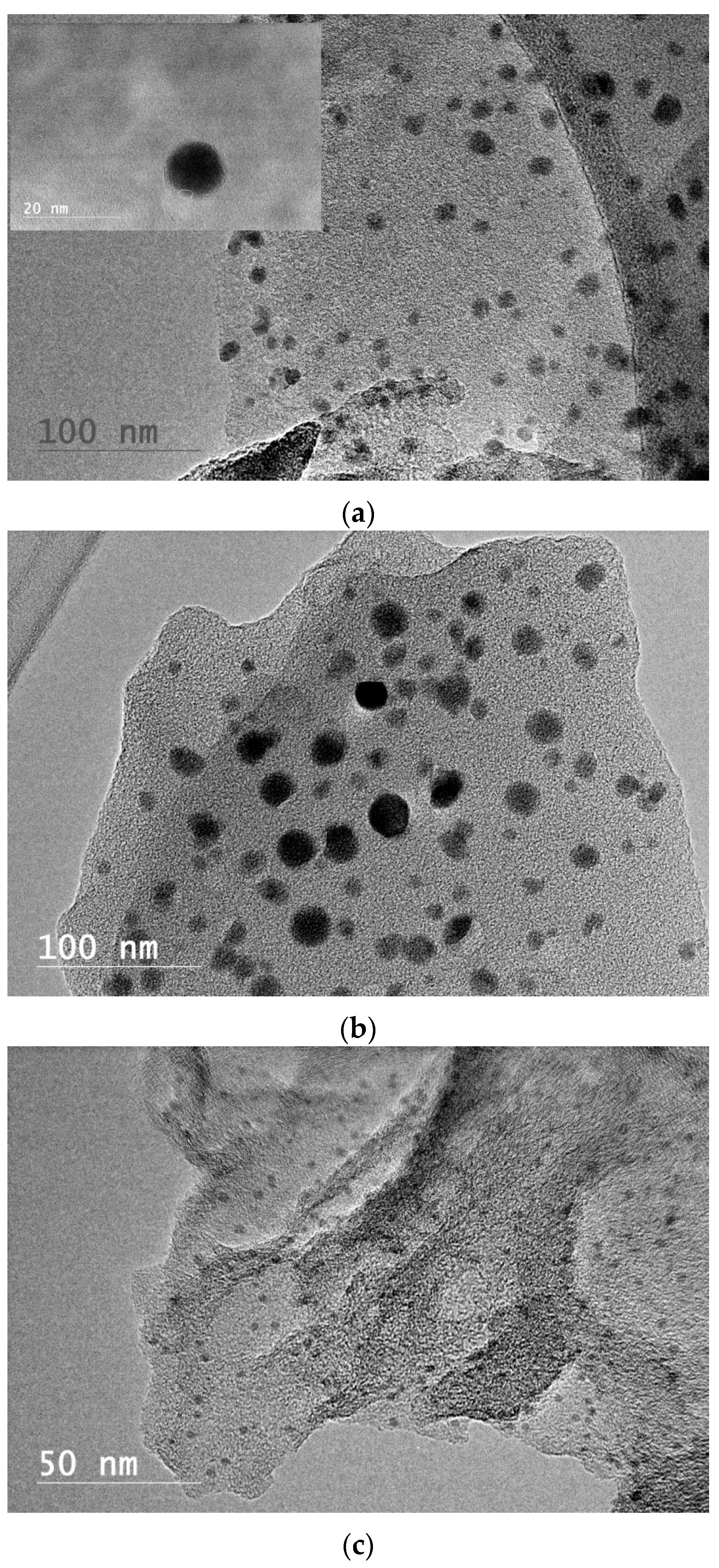
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Host, J.J.; Dravid, V.P.; Teng, M.H. Systematic study of graphite encapsulated nickel nanocrystal synthesis with formation mechanism implications. J. Mater. Res. 1998, 13, 2547–2555. [Google Scholar] [CrossRef]
- Kartal, K.N.; Green, F.; Clausen, C.A. Do the unique properties of nanometals affect leachability or efficacy against fungi and termites? Int. Biodeterior. Biodegrad. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Zheng, Q.; Cai, Z.; Ma, Z.; Gong, S. Cellulose nanofibril/reduced graphene oxide/carbon nanotube hybrid aerogels for highly flexible and all-solid-state supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Limaj, O.; Janner, D.; Etezade, D.; Abajo, F.J.G.D.; Pruneri, V.; Altug, H. Mid-infrared plasmonic biosensing with graphene. Science 2015, 349, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Jia, Z.; Yan, B.; Yu, D.; Wu, X. Hydrogen assisted growth of high quality epitaxial graphene on the C-face of 4H-SiC. Appl. Phys. Lett. 2015, 106, 013106. [Google Scholar] [CrossRef]
- Maeda, F.; Hibino, H. Molecular beam epitaxial growth of graphene using cracked ethylene. J. Cryst. Growth 2013, 378, 404–409. [Google Scholar] [CrossRef]
- Sanbonsuge, S.; Abe, S.; Handa, H.; Takahashi, R.; Imaizumi, K.; Fukidome, H.; Suemitsu, M. Improvement in Film Quality of Epitaxial Graphene on SiC(111)/Si(111) by SiH4 Pretreatment. Jpn. J. Appl. Phys. 2012, 51, 6–10. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Wang, Y.W.; Ting, C.C.; Wang, H.C.; Chen, K.Y.; Yang, C.C. Effect of Catalyst Morphology on the Quality of CVD Grown Graphene. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Seo, J.H.; Kang, B.J.; Mun, J.H.; Lim, S.K.; Cho, B.J. Effect of a surface pre-treatment on graphene growth using a SiC substrate. Microelectron. Eng. 2010, 87, 2002–2007. [Google Scholar] [CrossRef]
- Weatherup, R.S.; Baehtz, C.; Dlubak, B.; Bayer, B.C.; Kidambi, P.R.; Blume, R.; Schloegl, R.; Hofmann, S. Introducing Carbon Diffusion Barriers for Uniform, High-Quality Graphene Growth from Solid Sources. Nano Lett. 2013, 13, 4624–4631. [Google Scholar] [CrossRef] [PubMed]
- Rümmeli, M.H.; Gorantla, S.; Bachmatiuk, A.; Phieler, J.; Geißler, N.; Ibrahim, I.; Pang, J.; Eckert, J. On the Role of Vapor Trapping for Chemical Vapor Deposition (CVD) Grown Graphene over Copper. Chem. Mater. 2013, 25, 4861–4866. [Google Scholar] [CrossRef]
- Reina, A.; Thiele, S.; Jia, X.; Bhaviripudi, S.; Dresselhaus, M.S.; Schaefer, J.A.; Kong, J. Growth of large-area single- and Bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2010, 2, 509–516. [Google Scholar] [CrossRef]
- Zou, Z.; Dai, B.; Liu, Z. CVD process engineering for designed growth of graphene. Sci. Sin. Chim. 2013, 43, 1–17. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.H. Utilization Opportunities for Biorefinery Lignins: An Industrial Perspective; International Lignin Biochemical Conference: Toronto, ON, Canada, 2010. [Google Scholar]
- Fang, W.; Yang, S.; Wang, X.; Yuan, T.; Sun, R. Manufacture and application of lignin-based carbon fibers (LCFs) and lignin-based carbon nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.; Koo, J.H.; Lee, H.; Lee, S.; Choi, T.; Jung, H.; Koo, B.; Park, J.; Kim, H.; et al. Synthesis of few-layered graphene nanoballs with copper cores using solid carbon source. Appl. Mater. Interfaces 2013, 5, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Barnes, H.M.; Zhang, J.; Cai, Z. Effect of processing parameters on the synthesis of lignin-based graphene-encapsulated copper nanoparticles. Wood Fiber Sci. 2017, 49, 22–32. [Google Scholar]
- Leng, W.; Barnes, H.M.; Yan, Q.; Cai, Z.; Zhang, J. Low temperature synthesis of graphene-encapsulated copper nanoparticles from kraft lignin. Mater. Lett. 2016, 185, 131–134. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/index.html (accessed on 28 May 2017).
- Kim, J.D.; Roh, J.S.; Kim, M.S. Effect of carbonization temperature on crystalline structure and properties of isotropic pitch-based carbon fiber. Carbon Lett. 2017, 21, 51–60. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Klingeler, R.; Gemming, T.; Buchner, B.; Rummeli, M.H. Synthesis of carbon-encapsulated iron nanoparticles by pyrolysis of iron citrate and poly (vinyl alcohol): A critical evaluation of yield and selectivity. Nanotechnology 2011, 22, 315606. [Google Scholar] [CrossRef] [PubMed]
- Theivasanthi, T.; Alagar, M. Konjac Bio-Molecules Assisted, Rod-Spherical shaped Lead Nano Powder Synthesized by Electrolytic Process and Its Characterization Studies. Nano Biomed. Eng. 2012, 5, 10–19. [Google Scholar] [CrossRef]
- Mun, S.P.; Cai, Z.; Zhang, J. Fe-catalyzed thermal conversion of sodium lignoslfonate to graphene. Mater. Lett. 2013, 100, 180–183. [Google Scholar] [CrossRef]
- Li, J.; Song, H.; Chen, X.; Liang, J.; Zhang, Y. Effects of preparation parameters on formation of carbon-encapsulated iron nanoparticles. Carbon Tech. 2009, 28, 1–5. [Google Scholar]
- Chiu, C.C.; Lo, J.C.; Teng, M.H. A novel high efficiency method for the synthesis of graphite encapsulated metal (GEM) nanoparticles. Diamond Relat. Mater. 2012, 24, 179–183. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Feng, J.; Zhang, J.; Deng, X.; Zhou, B.; Zhang, H.; Xue, D.; Li, F.; Mellors, N.J.; et al. One-pot polylol synthesis of graphene decorated with size and density-tunable Fe3O4 nanoparticles for porcine pancreatic lipase immobilization. Carbon 2013, 60, 488–497. [Google Scholar] [CrossRef]
- Amaya, R.O.; Matsumoto, Y.; Guzman, M.A.P.; Lopez, M.O. In situ synthesis of Cu2O and Cu nanoparticles during the thermal reduction of copper foil-supported graphene oxide. J. Nanopart. Res. 2015, 17, 397. [Google Scholar] [CrossRef]
- Lin, T.; Wang, Y.; Bi, H.; Wan, D.; Huang, F.; Xie, X.; Jiang, M. Hydrogen flame synthesis of few-layer graphene from a solid carbon source on hexagonal boron nitride. J. Mater. Chem. 2012, 22, 2859–2862. [Google Scholar]

| Weight Ratio | Temperature (°C) | ||
|---|---|---|---|
| 600 | 800 | 1000 | |
| 0:1 | 41.47% (1.2%) 1 | 38.24% (0.7%) | 37.30% (1.4%) |
| 1:4 | 49.42% (2.6%) | 48.02% (0.3%) | 46.57% (0.5%) |
| 1:2 | - | - | 42.78% (1.3%) |
| 1:1 | - | - | 33.76% (2.1%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, W.; Barnes, H.M.; Cai, Z.; Zhang, J. Temperature and Copper Concentration Effects on the Formation of Graphene-Encapsulated Copper Nanoparticles from Kraft Lignin. Materials 2017, 10, 677. https://doi.org/10.3390/ma10060677
Leng W, Barnes HM, Cai Z, Zhang J. Temperature and Copper Concentration Effects on the Formation of Graphene-Encapsulated Copper Nanoparticles from Kraft Lignin. Materials. 2017; 10(6):677. https://doi.org/10.3390/ma10060677
Chicago/Turabian StyleLeng, Weiqi, H. Michael Barnes, Zhiyong Cai, and Jilei Zhang. 2017. "Temperature and Copper Concentration Effects on the Formation of Graphene-Encapsulated Copper Nanoparticles from Kraft Lignin" Materials 10, no. 6: 677. https://doi.org/10.3390/ma10060677




