Preparation and Supercooling Modification of Salt Hydrate Phase Change Materials Based on CaCl2·2H2O/CaCl2
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Selection
2.2. Preparation of PCMs
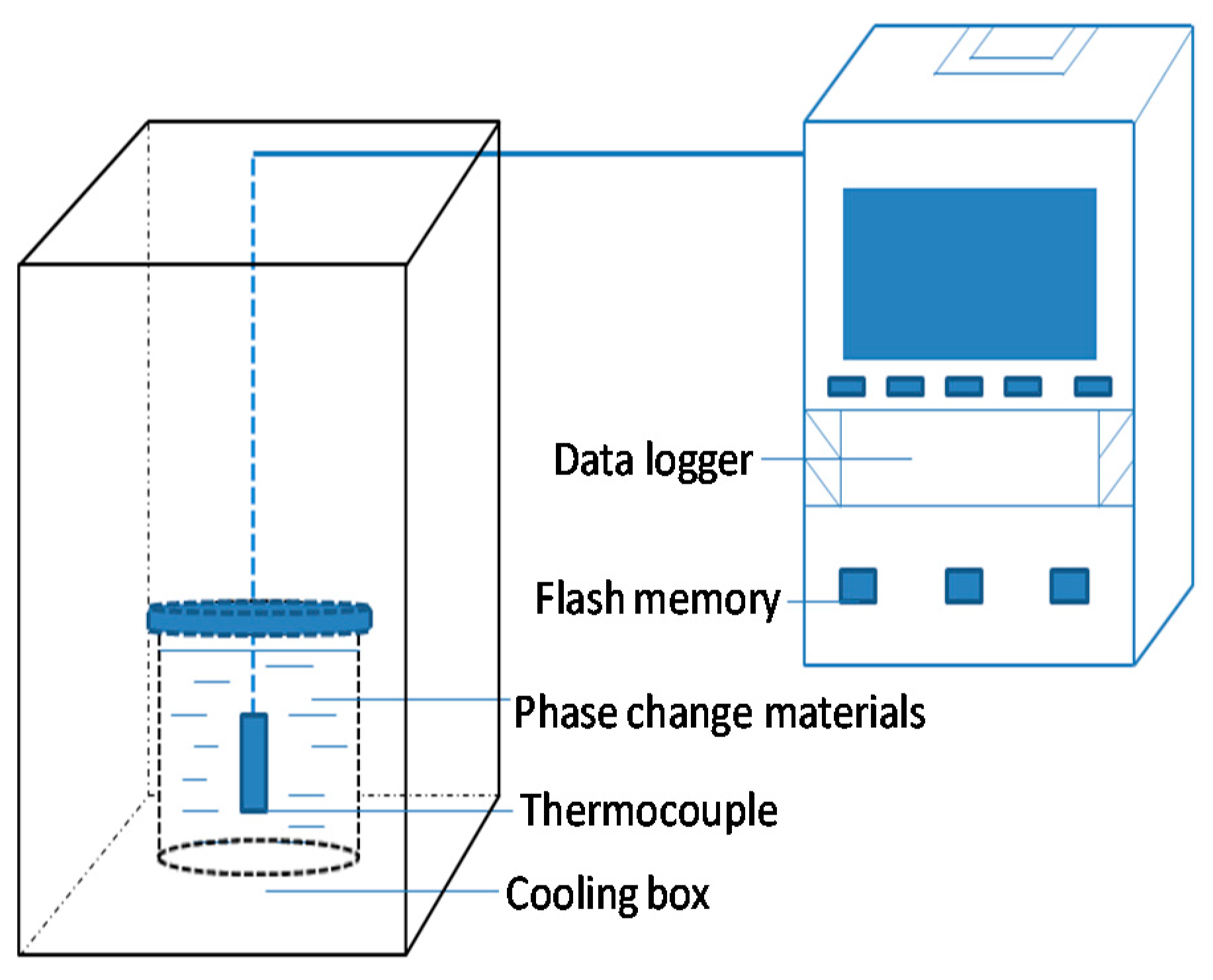
2.3. Experimental Methods and Procedure
3. Results and Discussion
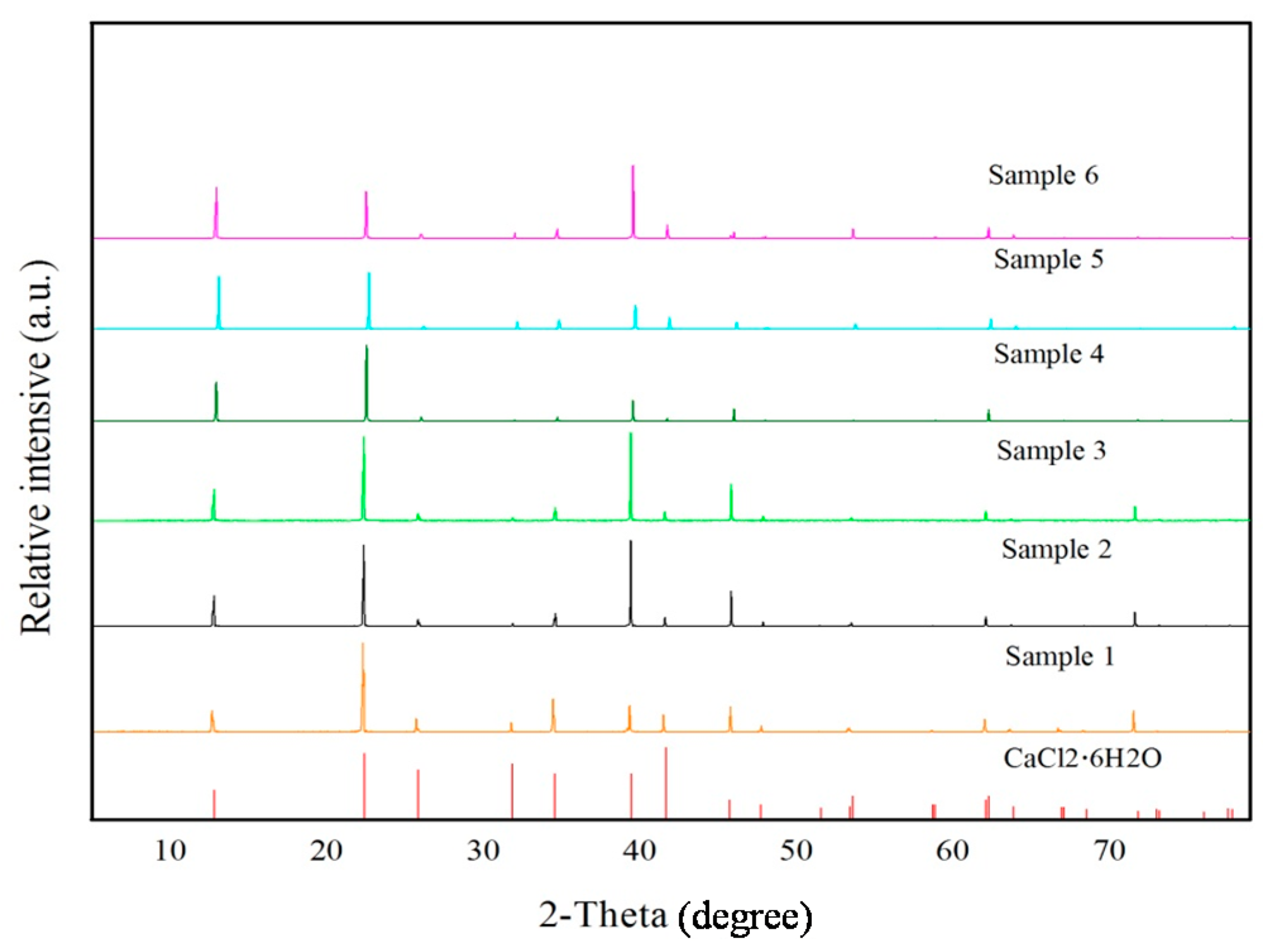
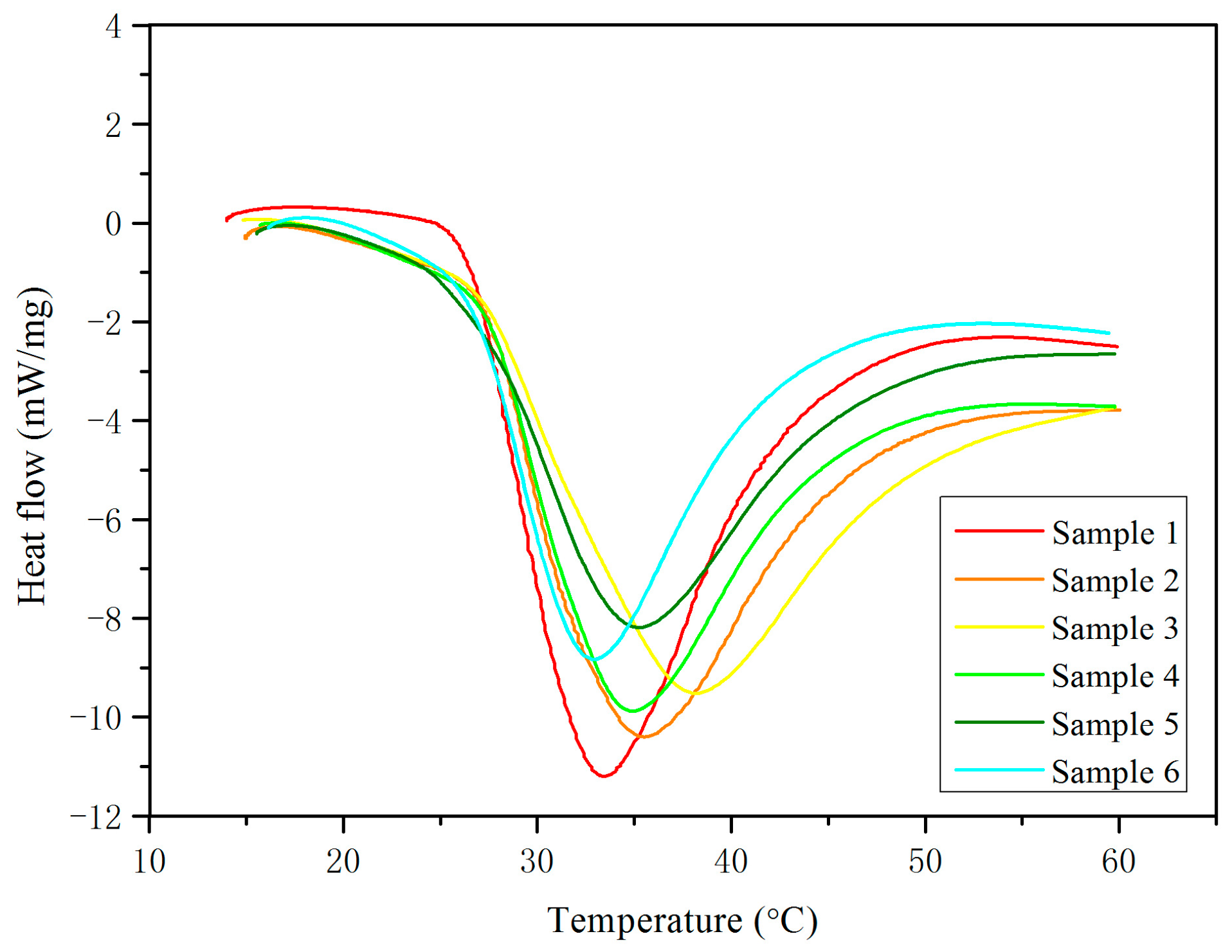
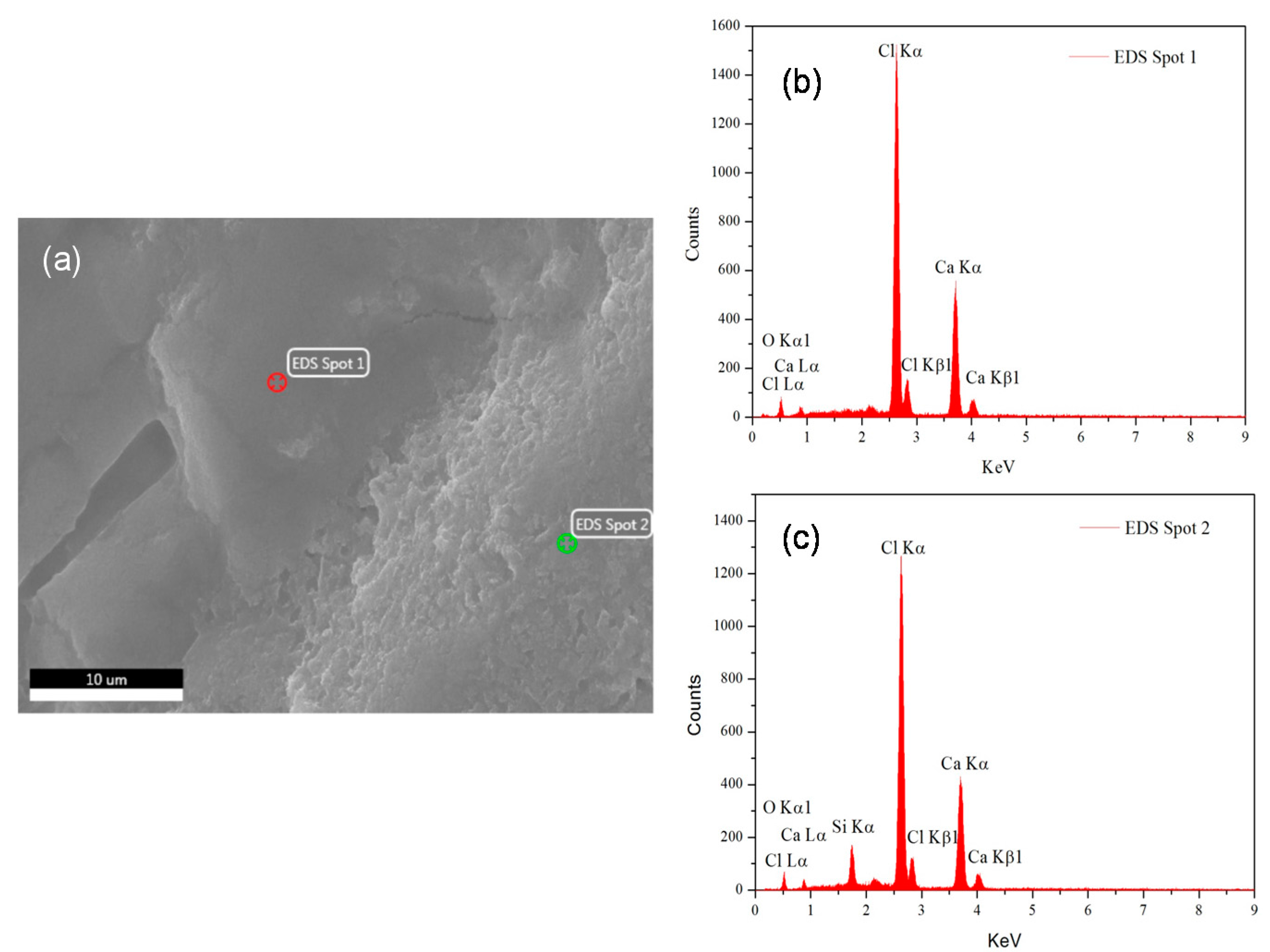
3.1. Analysis of Mineralogical, Thermal and Supercooling Phenomenon
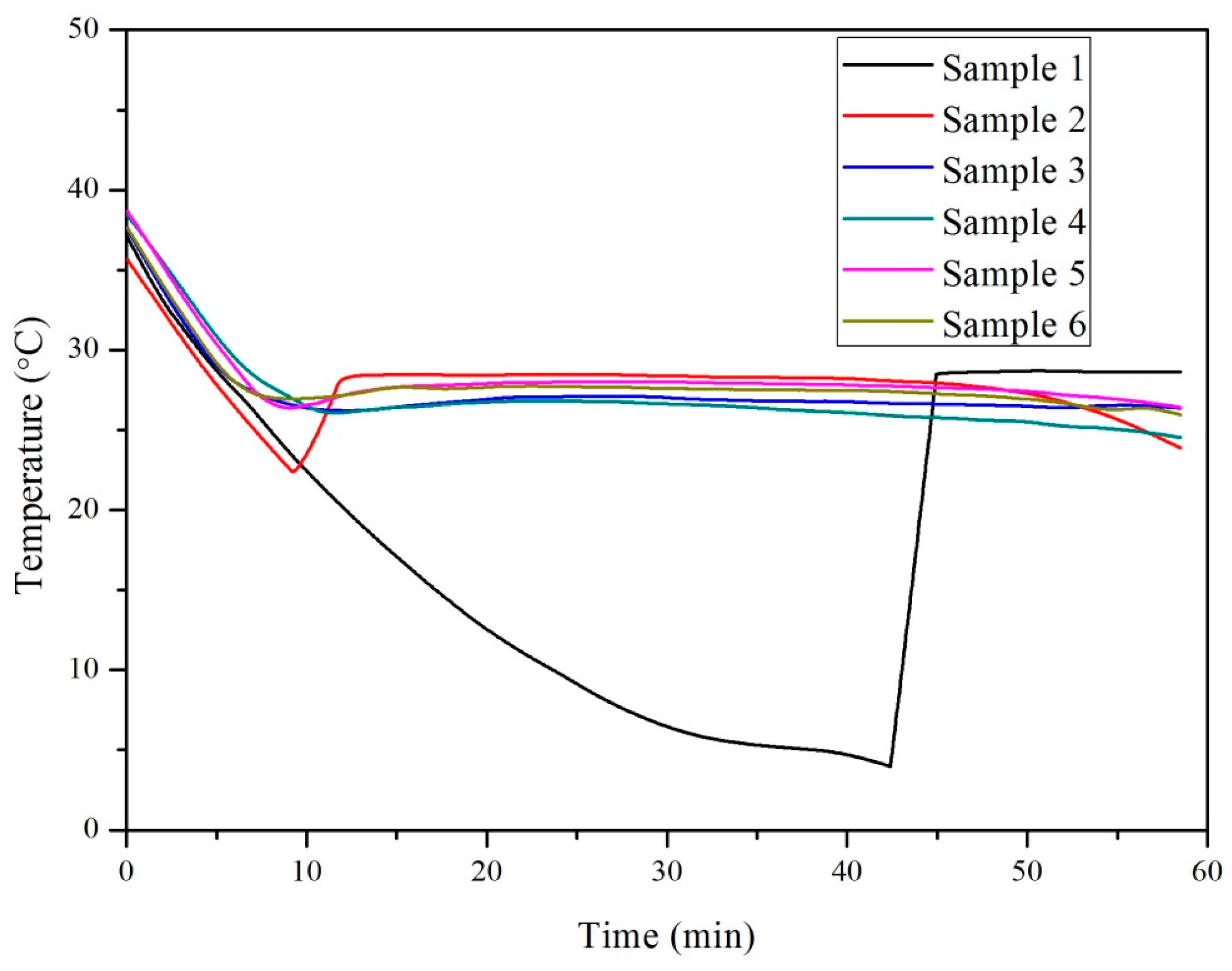
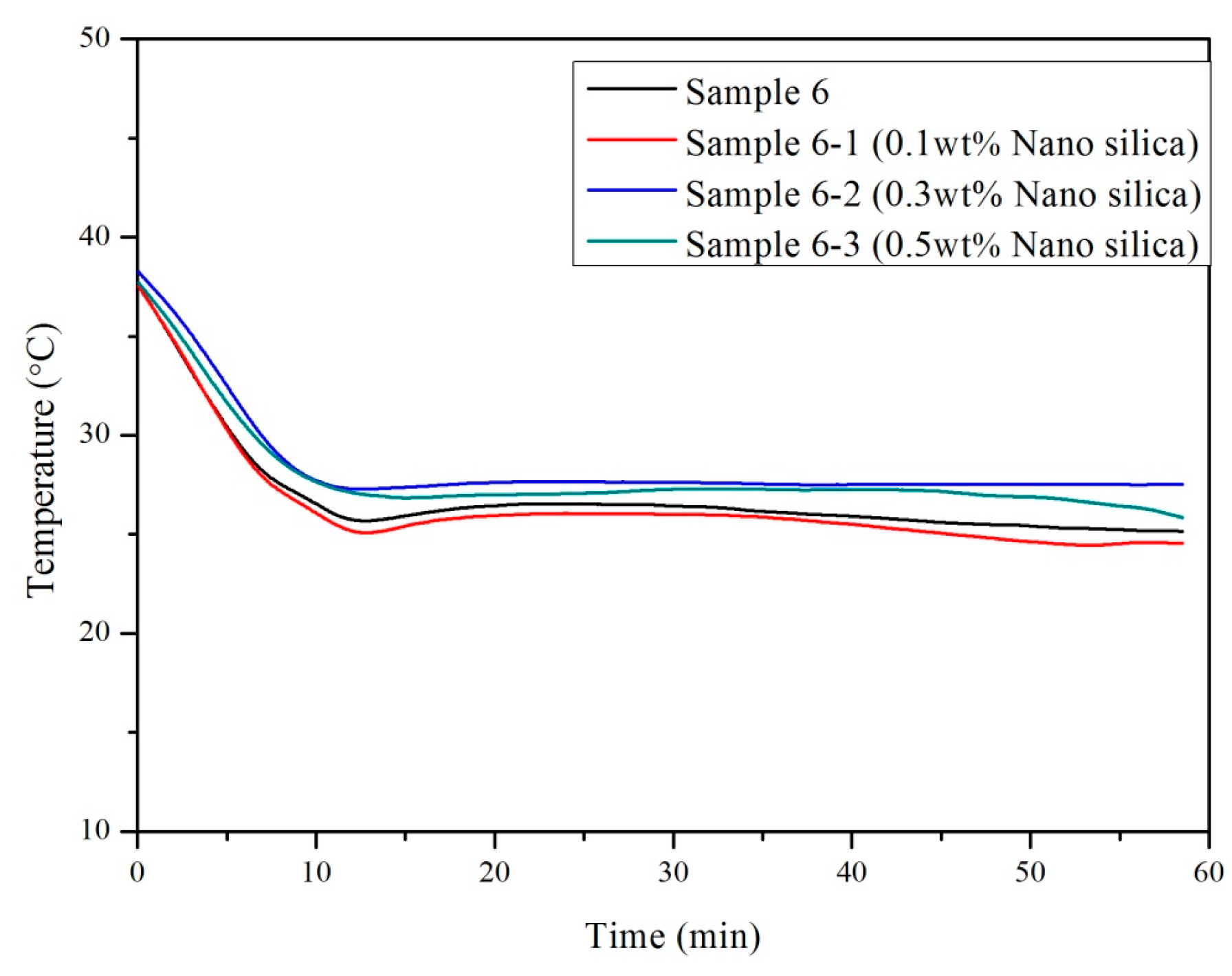
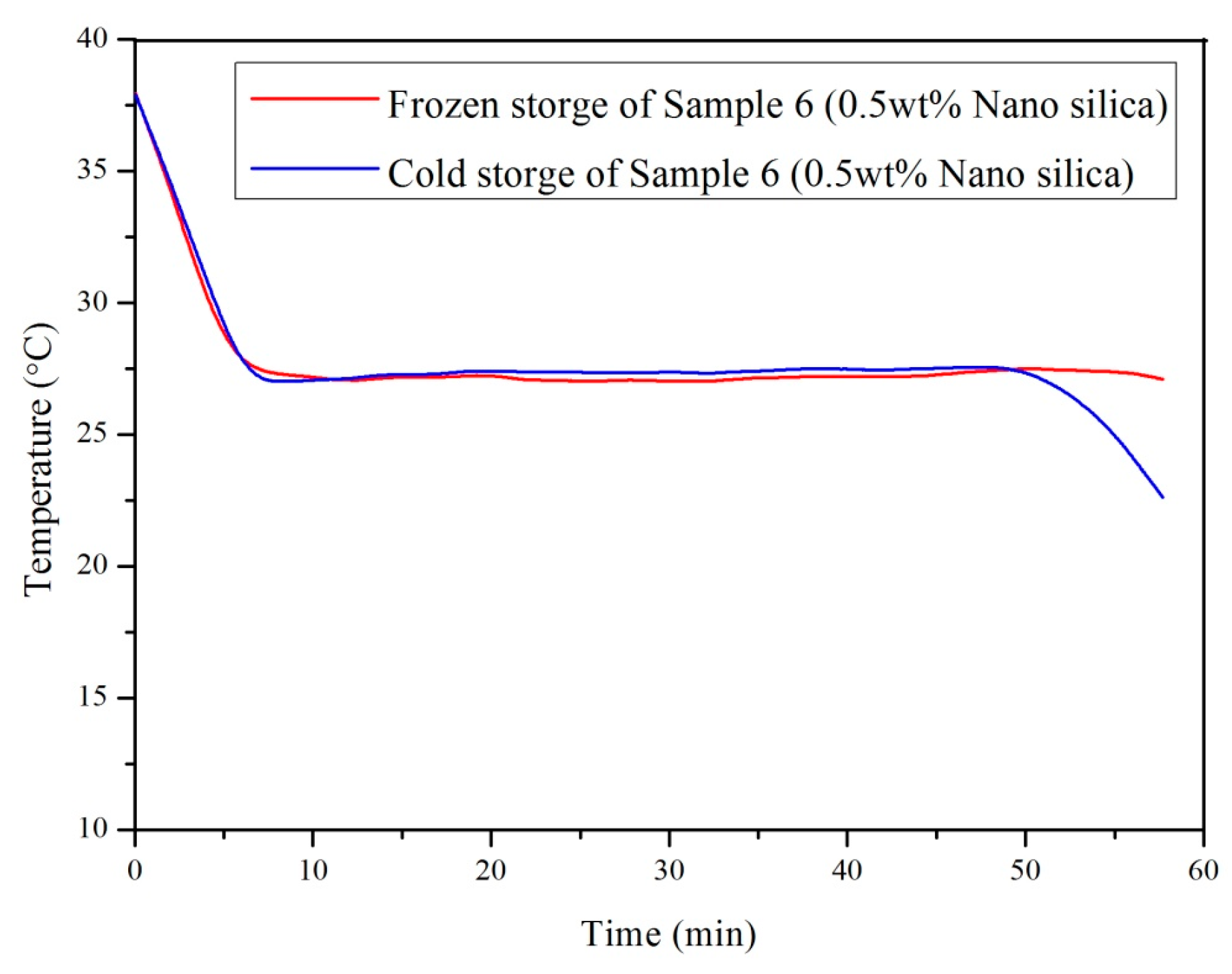
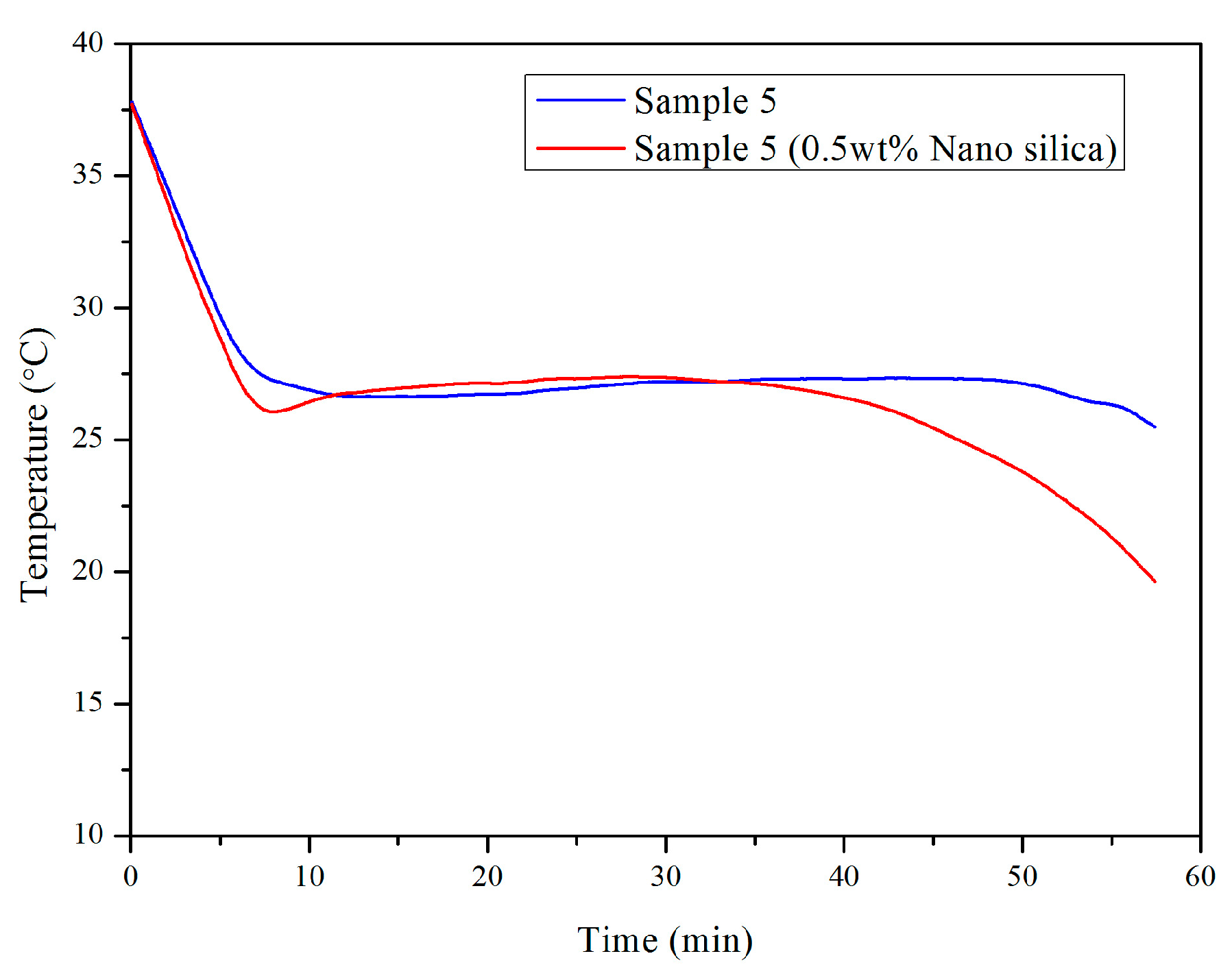
3.2. Influence of Nano-SiO2 Addition and Cooling Conditions in Reducing Supercooling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Memon, S.A.; Cui, H.; Lo, T.Y.; Li, Q. Development of structural–functional integrated concrete with macro-encapsulated PCM for thermal energy storage. Appl. Energy 2015, 150, 245–257. [Google Scholar]
- Memon, S.A.; Cui, H.Z.; Zhang, H.; Xing, F. Utilization of macro encapsulated phase change materials for the development of thermal energy storage and structural lightweight aggregate concrete. Appl. Energy 2015, 139, 43–55. [Google Scholar]
- Hawes, D.W.; Banu, D.; Feldman, D. The stability of phase change materials in concrete. Sol. Energy Mater. Sol. Cells 1992, 27, 103–118. [Google Scholar] [CrossRef]
- Lane, G.A. Phase-Change Materials for Energy-Storage Nucleation to Prevent Supercooling. Sol. Energy Mater. Sol. Cells 1992, 27, 135–160. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.A.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Shahbaz, K.; AlNashef, I.M.; Lin, R.J.; Hashim, M.A.; Mjalli, F.S.; Farid, M.M. A novel calcium chloride hexahydrate-based deep eutectic solvent as a phase change materials. Sol. Energy Mater. Sol. Cells 2016, 155, 147–154. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials: Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2016, 145, 255–286. [Google Scholar]
- Feilchenfeld, H.; Sarig, S. Calcium-Chloride Hexahydrate—A Phase-Changing Material for Energy-Storage. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 130–133. [Google Scholar]
- Agron, P.A.; Busing, W.R. Calcium and Strontium Dichloride Hexahydrates by Neutron-Diffraction. Acta Crystallogr. C 1986, 42, 141–143. [Google Scholar] [CrossRef]
- Kibria, M.A.; Anisur, M.R.; Mahfuz, M.H.; Saidur, R.; Metselaar, I.H. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Tong, M.; Liu, Y. Experimental study on thermophysical properties of nanofluids as phase-change material (PCM) in low temperature cool storage. Energy Convers. Manag. 2012, 64, 199–205. [Google Scholar] [CrossRef]
- Ma, Y.; Lei, B.Y.; Liu, Y.C.; Wu, T. Effects of additives on the subcooling behavior of Al2(SO4)3·18H2O phase transition. Appl. Therm. Eng. 2016, 99, 189–194. [Google Scholar] [CrossRef]
- Cui, W.; Yuan, Y.; Sun, L.; Cao, X.; Yang, X. Experimental studies on the supercooling and melting/freezing characteristics of nano-copper/sodium acetate trihydrate composite phase change materials. Renew. Energy 2016, 99, 1029–1037. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, D.; Li, X.; Li, H.; Lei, J. Thermal energy storage behavior of Al2O3–H2O nanofluids. Thermochim. Acta 2009, 483, 73–77. [Google Scholar] [CrossRef]
- Hu, P.; Lu, D.-J.; Fan, X.-Y.; Zhou, X.; Chen, Z.-S. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Sol. Energy Mater. Sol. Cells 2011, 95, 2645–2649. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.-Y.; Niu, J. PCM-in-water emulsion for solar thermal applications: The effects of emulsifiers and emulsification conditions on thermal performance, stability and rheology characteristics. Sol. Energy Mater. Sol. Cells 2016, 147, 211–224. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Cheng, Z.D.; Jia, L.S.; Mo, S.P.; Liu, Z.W. Ultrahigh specific surface area of graphene for eliminating subcooling of water. Appl. Energy 2014, 130, 824–829. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. Thermal cycle testing of calcium chloride hexahydrate as a possible PCM for latent heat storage. Sol. Energy Mater. Sol. Cells 2008, 92, 891–899. [Google Scholar] [CrossRef]
- Carlsson, B. Phase change behaviour of some latent heat storage media based on calcium chloride hexahydrate. Sol. Energy 2009, 83, 485–500. [Google Scholar] [CrossRef]
- Schmit, H.; Pfeffer, W.; Rathgeber, C.; Hiebler, S. Calorimetric investigation of the concentration dependent enthalpy change around semicongruent melting CaCl2·6H2O. Thermochim. Acta 2016, 635, 26–33. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Nian, H.; Ren, X.; Dong, O.; Hai, C.; Shen, Y.; Zeng, J. Phase change behavior of latent heat storage media based on calcium chloride hexahydrate composites containing strontium chloride hexahydrate and oxidation expandable graphite. Appl. Therm. Eng. 2016, 102, 38–44. [Google Scholar] [CrossRef]
- Arias, F.; Wang, X. Segregation due to motion of front of solidification in phase change materials systems and dependence with shape and dimension factors. Appl. Therm. Eng. 2015, 75, 366–370. [Google Scholar] [CrossRef]


| Temperature (°C) | 0 | 10 | 20 | 30 | 40 | 60 | 80 | 100 |
|---|---|---|---|---|---|---|---|---|
| Solubility (g) | 59.5 | 64.7 | 74.5 | 100 | 128 | 137 | 147 | 159 |
| Parameters and Items | Sample No. | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| x (g) | 50.00 | 39.58 | 29.17 | 18.75 | 8.33 | 0.00 |
| y (g) | 0.00 | 20.42 | 40.83 | 61.25 | 81.67 | 98.00 |
| z (g) | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
| x + y (g) | 50.00 | 60.00 | 70.00 | 80.00 | 90.00 | 98.00 |
| y/(x + y) (%) | 0 | 34.03 | 58.32 | 76.56 | 90.74 | 100 |
| x/(x + y + z) (%) | 50.00 | 35.98 | 24.31 | 14.42 | 5.95 | 0 |
| y/(x + y + z) (%) | 0 | 18.56 | 34.03 | 47.12 | 58.34 | 66.21 |
| Items | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | |
| Mass of samples (mg) | 12.0 | 0.2 | 17.9 | 0.2 | 27.6 | 0.4 | 17.8 | 0.2 | 23.8 | 0.3 | 14.5 | 0.3 |
| Teo (°C) | 27.1 | 0.3 | 28.1 | 0.3 | 27.9 | 0.4 | 27.8 | 0.5 | 27.4 | 0.4 | 27.4 | 0.3 |
| Tm (°C) | 33.7 | 0.5 | 36.2 | 0.4 | 38.6 | 0.5 | 35.3 | 0.3 | 35.6 | 04 | 33.1 | 0.4 |
| H (J/g) | 205.13 | 2.1 | 181.02 | 1.8 | 174.37 | 1.9 | 178.60 | 1.6 | 167.44 | 1.9 | 155.29 | 1.6 |
| Additive Volume (%) | Tm (°C) | Tn (°C) | ΔT (°C) | |||
|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | |
| 0 | 26.6 | 0.4 | 25.7 | 0.6 | 0.8 | 0.2 |
| 0.1 | 26.0 | 0.3 | 25.1 | 0.4 | 0.9 | 0.2 |
| 0.3 | 27.6 | 0.4 | 27.3 | 0.3 | 0.3 | 0.1 |
| 0.5 | 27.3 | 0.5 | 27.1 | 0.5 | 0.2 | 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Dong, Z.; Memon, S.A.; Bao, X.; Cui, H. Preparation and Supercooling Modification of Salt Hydrate Phase Change Materials Based on CaCl2·2H2O/CaCl2. Materials 2017, 10, 691. https://doi.org/10.3390/ma10070691
Xu X, Dong Z, Memon SA, Bao X, Cui H. Preparation and Supercooling Modification of Salt Hydrate Phase Change Materials Based on CaCl2·2H2O/CaCl2. Materials. 2017; 10(7):691. https://doi.org/10.3390/ma10070691
Chicago/Turabian StyleXu, Xiaoxiao, Zhijun Dong, Shazim Ali Memon, Xiaohua Bao, and Hongzhi Cui. 2017. "Preparation and Supercooling Modification of Salt Hydrate Phase Change Materials Based on CaCl2·2H2O/CaCl2" Materials 10, no. 7: 691. https://doi.org/10.3390/ma10070691




