Effects of Poly(cyclohexanedimethylene terephthalate) on Microstructures, Crystallization Behavior and Properties of the Poly(ester ether) Elastomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Characterization
3. Results and Discussion
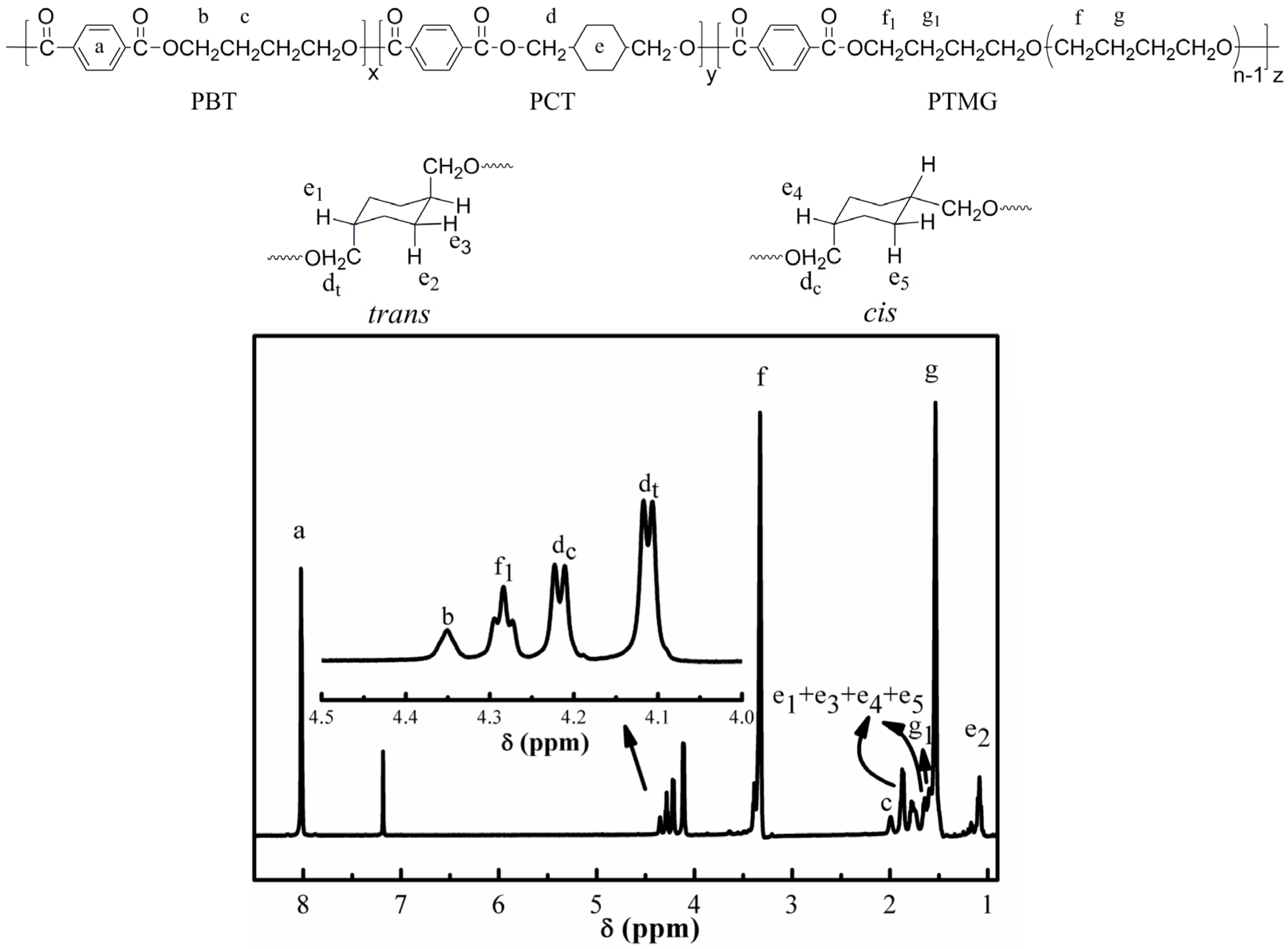
3.1. Structure and Molecular Weight Analysis
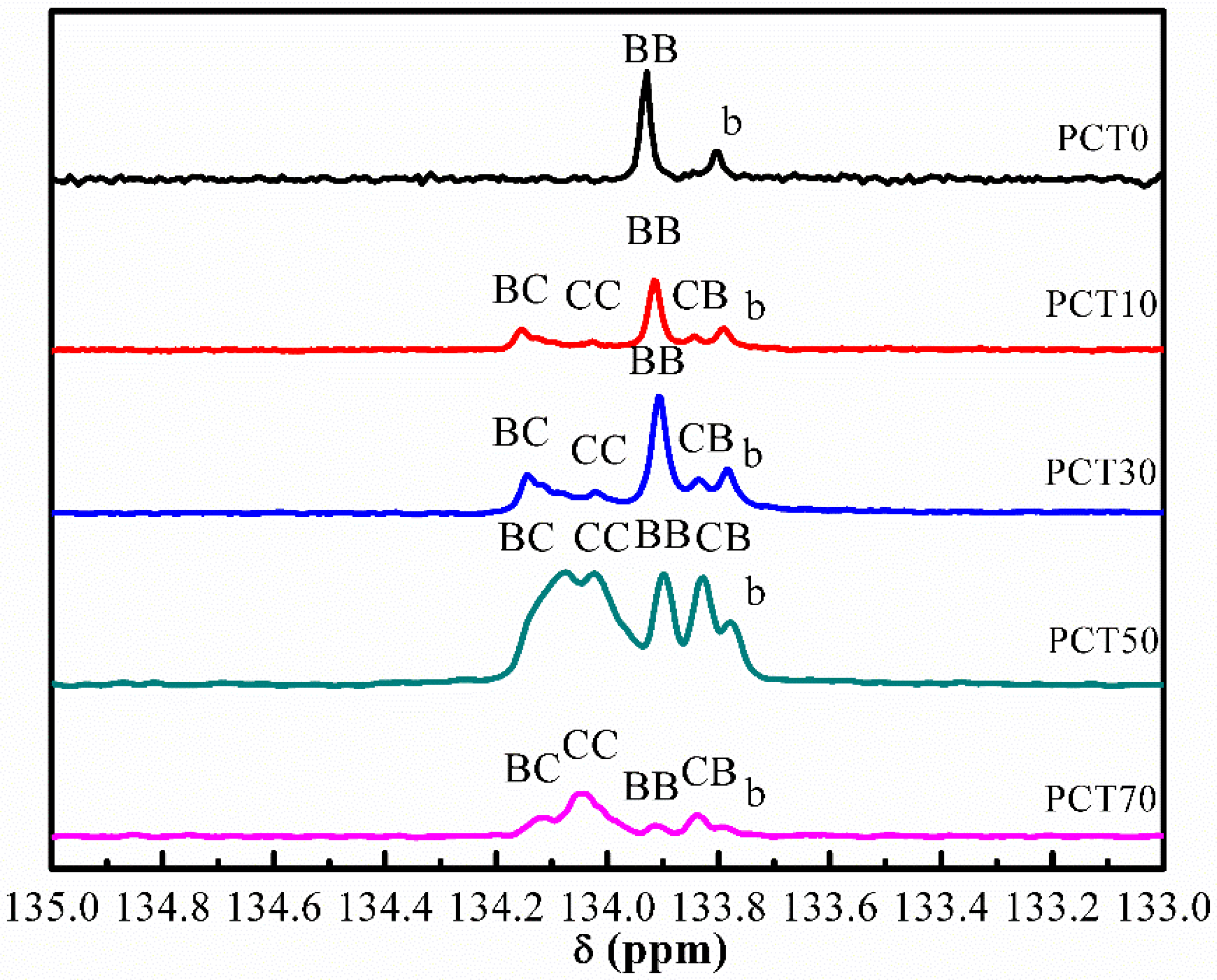
3.2. Sequence Distribution Analysis
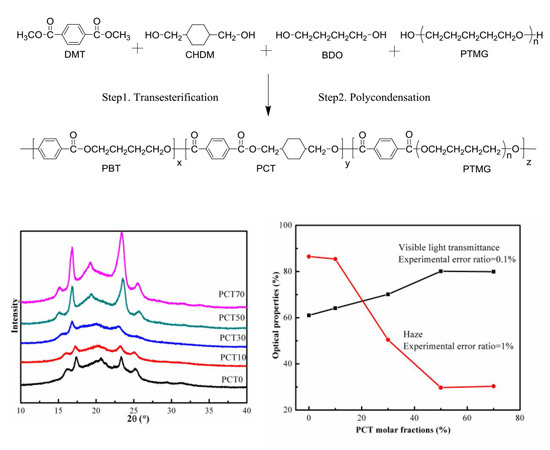
3.3. WAXD Analysis
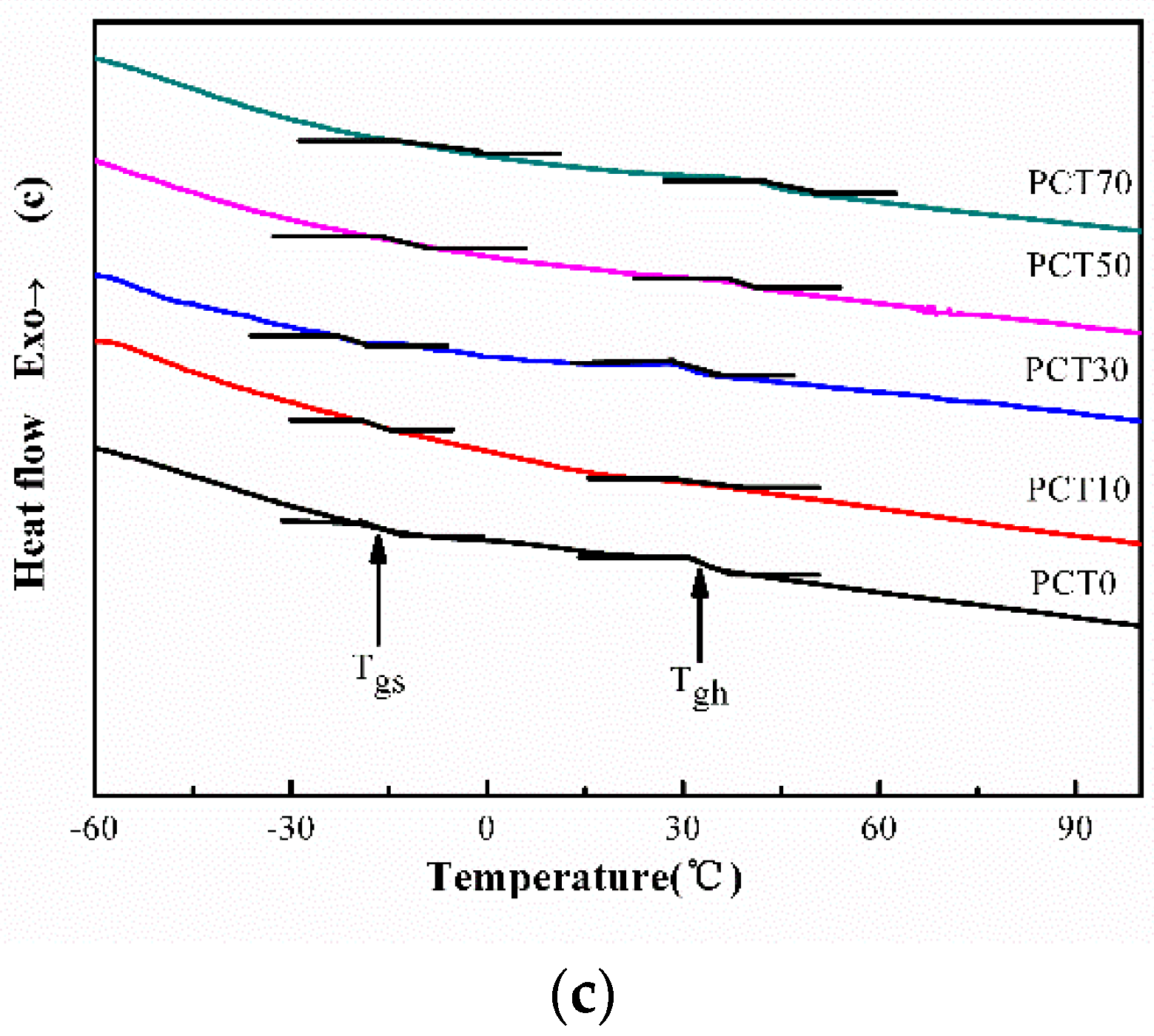
3.4. DSC Analysis
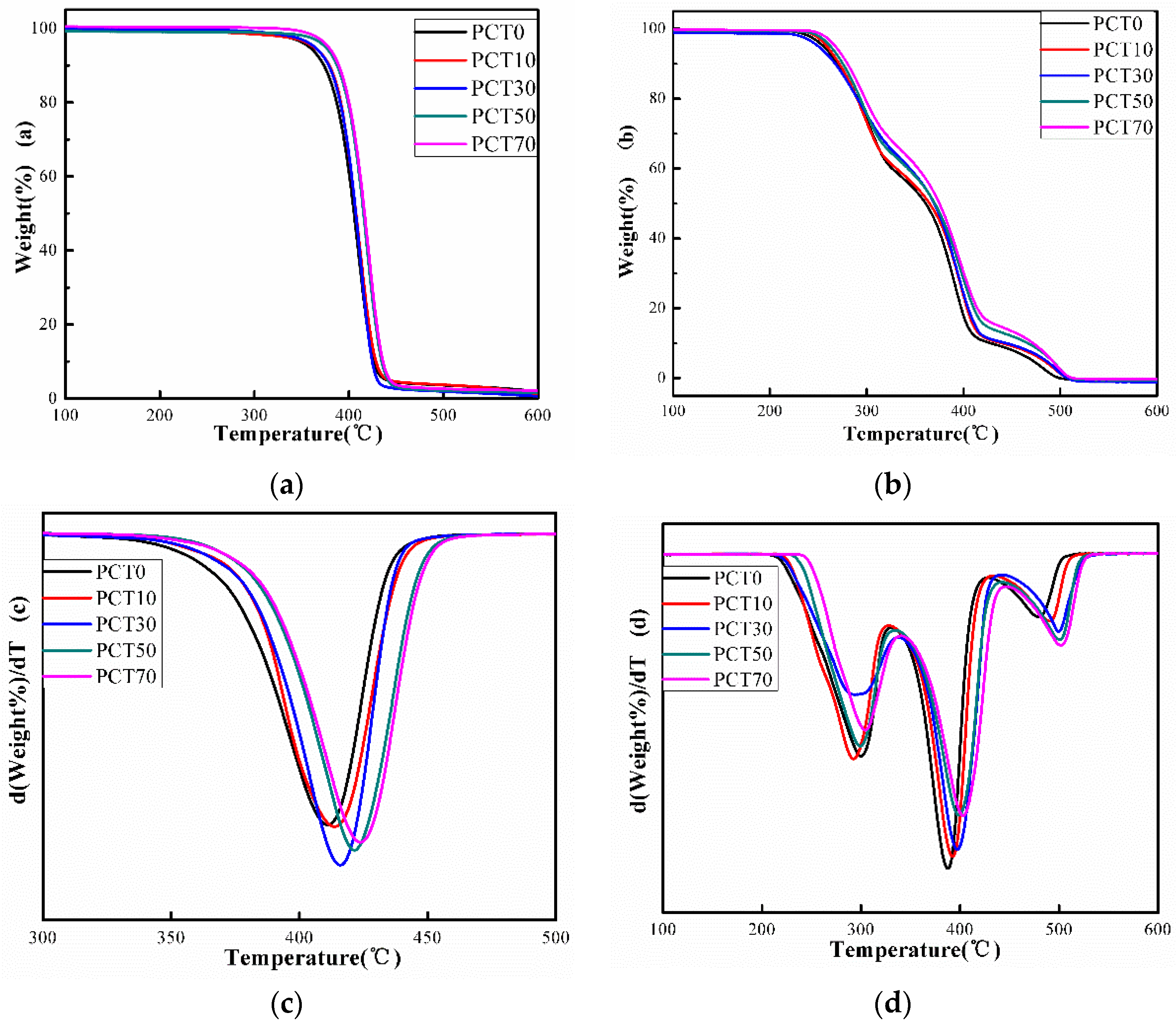
3.5. TGA Analysis
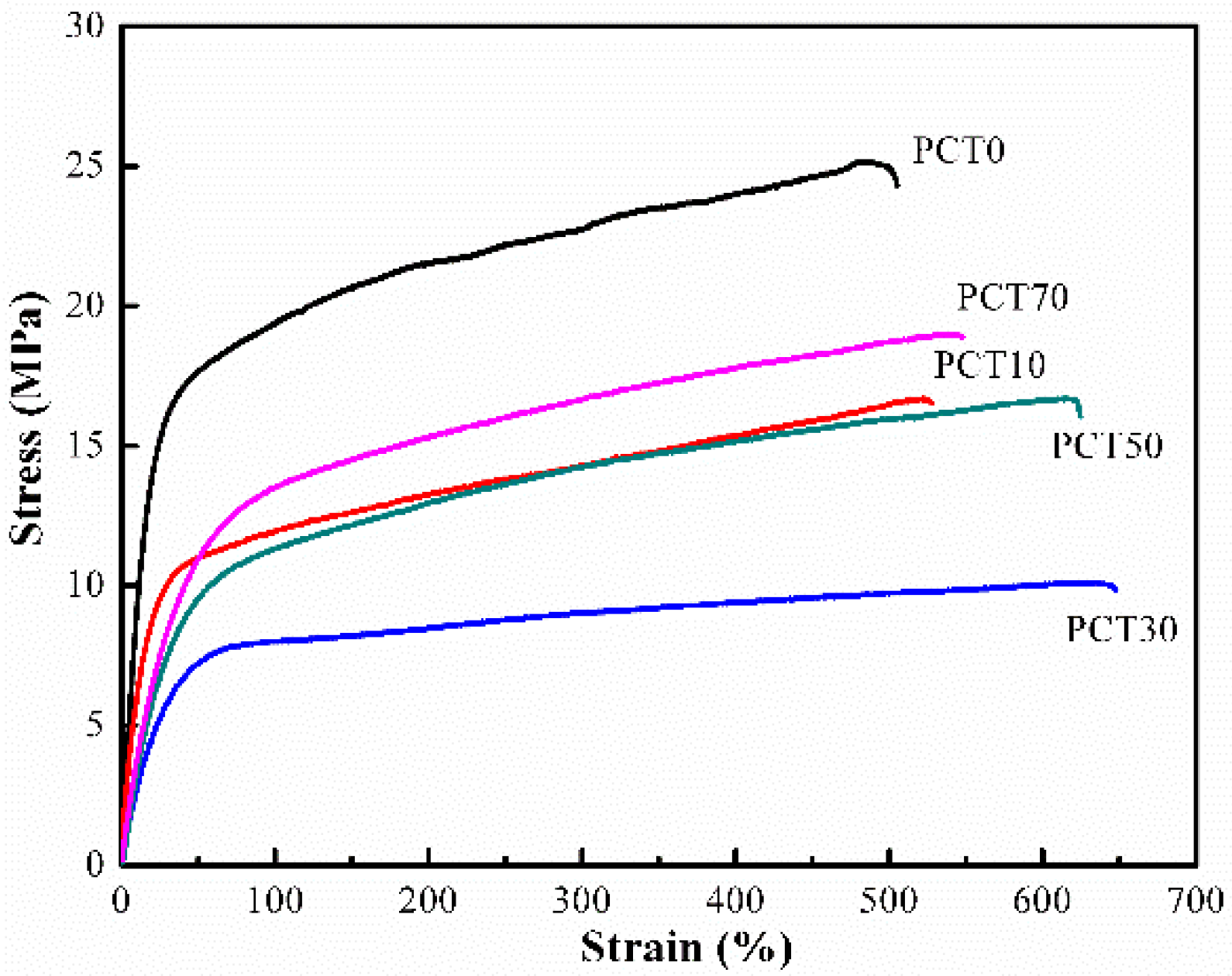
3.6. Mechanical Properties
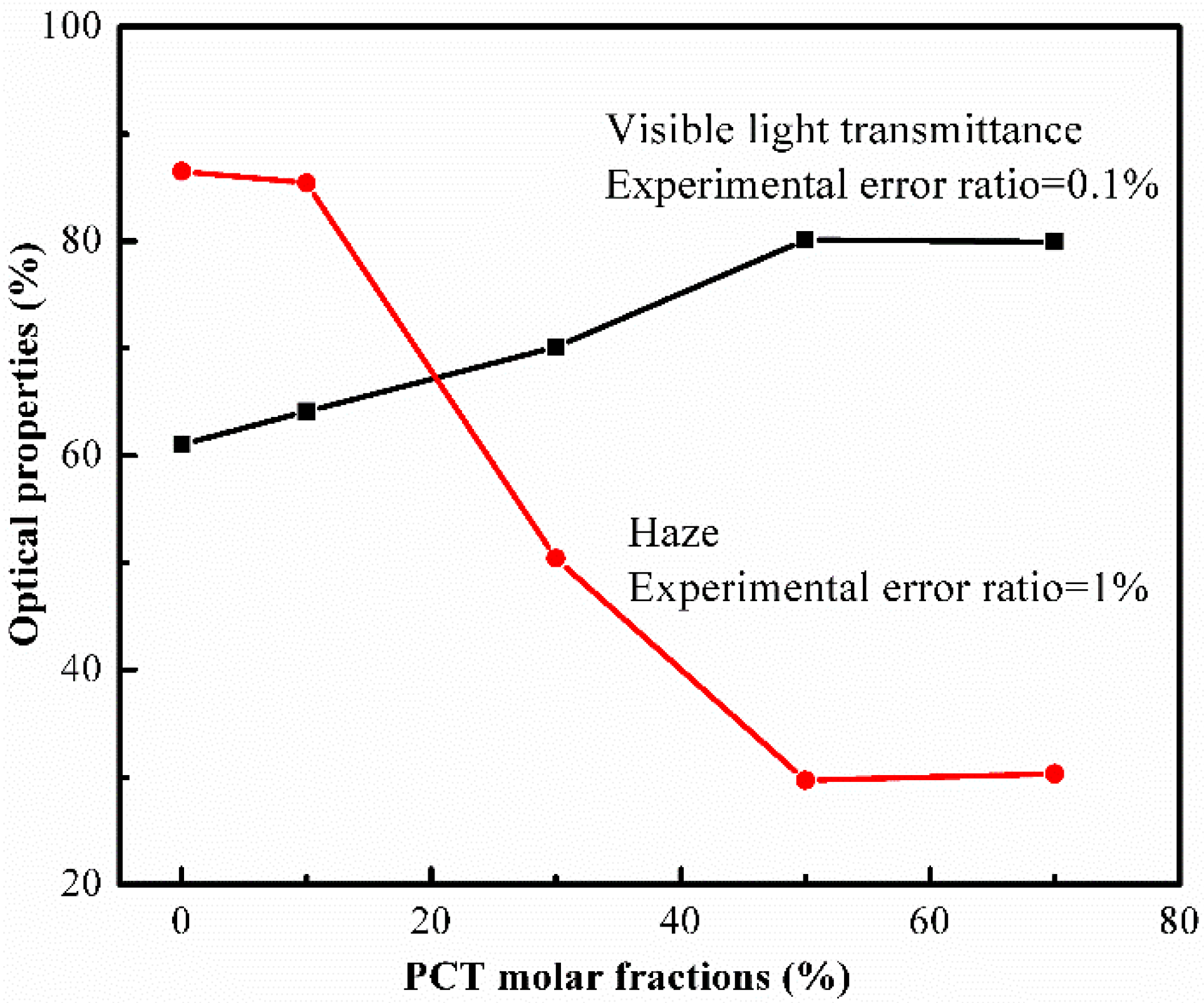
3.7. Optical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orimo, Y.; Hotta, A. Stress-strain behavior, elastic recovery, fracture points, and time-temperature superposition of an oot-possessing triblock copolymer. Macromolecules 2011, 44, 5310–5317. [Google Scholar] [CrossRef]
- Tomita, S.; Wataoka, I.; Igarashi, N.; Shimizu, N.; Takagi, H.; Sasaki, S.; Sakurai, S. Strain-induced deformation of glassy spherical microdomains in elastomeric triblock copolymer films: Time-resolved 2D-SAXS measurements under stretched state. Macromolecules 2017, 50, 677–686. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Piesowicz, E.; Szymczyk, A. Phase separation and elastic properties of poly(trimethylene terephthalate)-block-poly(ethylene oxide) copolymers. Polymers 2016, 8, 237. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, Q.K.; Yu, J.P.; Wu, Y.X.; Shen, Z.; Fan, X.H. Synthesis and properties of a new high-temperature liquid crystalline thermoplastic elastomer based on mesogen-jacketed liquid crystalline polymer. Polymer 2017, 108, 50–57. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, L.; Chen, Y. Organic/inorganic nanoobjects with controlled shapes from gelable triblock copolymers. Polymer 2010, 51, 2809–2817. [Google Scholar] [CrossRef]
- Eberl, A.; Heumann, S.; Kotek, R.F.; Mitsche, S. Enzymatic hydrolysis of ptt polymers and oligomers. J. Biotechnol. 2008, 135, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, K.; Chen, Y. Functionalization of shaped polymeric nanoobjects via bulk co-self-assembling gelable block copolymers with silane coupling agents. Polymer 2011, 52, 3681–3686. [Google Scholar] [CrossRef]
- Gabriëlse, W.; Maria Soliman, A.; Dijkstra, K. Microstructure and phase behavior of block copoly(ether ester) thermoplastic elastomers. Macromolecules 2001, 34, 1685–1693. [Google Scholar] [CrossRef]
- Szymczyk, A. Structure and properties of new polyester elastomers composed of poly(trimethylene terephthalate) and poly(ethylene oxide). Eur. Polym. J. 2009, 45, 2653–2664. [Google Scholar] [CrossRef]
- Celebi, H.; Bayram, G.; Dogan, A. Effect of zno type and concentration on the mophology, thermal, and mechanical properties of poly(ether ester)/ZnO composites. J. Appl. Polym. Sci. 2013, 129, 3417–3424. [Google Scholar] [CrossRef]
- Szymczyk, A.; Nastalczyk, J.; Sablong, R.J.; Roslaniec, Z. The influence of soft segment length on structure and properties of poly(trimethylene terephthalate)-block-poly(tetramethylene oxide) segmented random copolymers. Polym. Adv. Technol. 2011, 22, 72–83. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Wang, J.; Na, H.; Zhu, J. Incorporation of 1,4-cyclohexanedicarboxylic acid into poly(butylene terephthalate)-b-poly(tetramethylene glycol) to alter thermal properties without compromising tensile and elastic properties. RSC Adv. 2015, 5, 94091–94098. [Google Scholar] [CrossRef]
- Kint, D.P.R.; Muñoz-Guerra, S. Modification of the thermal properties and crystallization behaviour of poly(ethylene terephthalate) by copolymerization. Polym. Int. 2003, 52, 321–336. [Google Scholar] [CrossRef]
- Rosales, A.M.; Murnen, H.K.; Zuckermann, R.N.; Segalman, R.A. Control of crystallization and melting behavior in sequence specific polypeptoids. Macromolecules 2010, 43, 5627–5636. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Feng, Q.; Cui, F. Preparation and properties of poly(butylene terephthalate-co-cyclohexanedimethylene terephthalate)-b-poly(ethylene glycol) segmented random copolymers. Polym. Degrad. Stab. 2004, 85, 559–570. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; You, H. Photodegradation behavior and mechanism of poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) (PETG) random copolymers: Correlation with copolymer composition. RSC Adv. 2016, 6, 102778–102790. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, G.; Li, G.; Wu, Z.; Zhang, J. Poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) random copolymers: Effect of copolymer composition and microstructure on the thermal properties and crystallization behavior. RSC Adv. 2015, 5, 60570–60580. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J. Surface hydrophilic modification of acrylonitrile-butadiene-styrene terpolymer by poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate): Preparation, characterization, and properties studies. Appl. Surf. Sci. 2016, 388, 133–140. [Google Scholar] [CrossRef]
- Rao, Y.Q.; Greener, J.; Avila-Orta, C.A.; Hsiao, B.S.; Blanton, T.N. The relationship between microstructure and toughness of biaxially oriented semicrystalline polyester films. Polymer 2008, 49, 2507–2514. [Google Scholar] [CrossRef]
- Jung, I.K.; Lee, K.H.; Chin, I.J.; Yoon, J.S.; Kim, M.N. Properties of biodegradable copolyesters of succinic acid-1,4-butanediol/succinic acid-1,4-cyclohexanedimethanol. J. Appl. Polym. Sci. 2015, 72, 553–561. [Google Scholar] [CrossRef]
- Sun, Y.M.; Wang, C.S. Preparation and characterization of poly(ethylene-1,4-cyclohexanedimethylene arylate). Eur. Polym. J. 1999, 35, 1087–1096. [Google Scholar] [CrossRef]
- Liu, Y.; Ranucci, E.; Lindblad, M.S.; Albertsson, A.C. New biodegradable polymers from renewable sources: Polyester-carbonates based on 1,3-propylene-co-1,4-cyclohexanedimethylene succinate. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2508–2519. [Google Scholar] [CrossRef]
- Ki, H.C.; Park, O.O. Synthesis, characterization and biodegradability of the biodegradable aliphatic-aromatic random copolyesters. Polymer 2001, 42, 1849–1861. [Google Scholar] [CrossRef]
- Celli, A.; Marchese, P.; Sisti, L.; Dumand, D.; Sullalti, S.; Totaro, G. Effect of 1,4-cyclohexylene units on thermal properties of poly(1,4-cyclohexylenedimethylene adipate) and similar aliphatic polyesters. Polym. Int. 2013, 62, 1210–1217. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Capuccini, C.; Fini, M.; Mihailescu, I.N.; Ristoscu, C.; Sima, F.; Torricelli, P. Biofunctional alendronate-hydroxyapatite thin films deposited by matrix assisted pulsed laser evaporation. Biomaterials 2009, 30, 6168–6177. [Google Scholar] [CrossRef] [PubMed]
- Ernault, E.; Richaud, E.; Fayolle, B. Thermal-oxidation of epoxy/amine followed by glass transition temperature changes. Polym. Degrad. Stab. 2017, 138, 82–90. [Google Scholar] [CrossRef]
- Vasanthan, N.; Manne, N.J.; Krishnama, A. Effect of molecular orientation on the cold crystallization of amorphous-crystallizable polymers: The case of poly(trimethylene terephthalate). Ind. Eng. Chem. Res. 2013, 52, 17920–17926. [Google Scholar] [CrossRef]
- Shi, X.Q.; Aimi, K.; Ito, H.; Ando, S.; Kikutani, T. Characterization on mixed-crystal structure of poly(butylene terephthalate/succinate/adipate) biodegradable copolymer fibers. Polymer 2005, 46, 751–760. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Umemoto, S.; Kikutani, T.; Okui, N. Co-crystallization behaviour and melting-point depression in poly(ethylene terephthalate-co-1,4-cyclohexylene dimethylene terephthalate) random copolyesters. Polymer 1994, 35, 117–122. [Google Scholar] [CrossRef]
- Bessadok, A.; Roudesli, S.; Marais, S.; Follain, N.; Lebrun, L. Alfa fibres for unsaturated polyester composites reinforcement: Effects of chemical treatments on mechanical and permeation properties. Compos. Part A Appl. Sci. Manuf. 2009, 40, 184–195. [Google Scholar] [CrossRef]
- Saint-Loup, R.; Robin, J.J.; Boutevin, B. Synthesis of poly(ethylene terephthalate)- block -poly(tetramethylene oxide) copolymer by direct polyesterification of reactive oligomers. Macromol. Chem. Phys. 2003, 204, 970–982. [Google Scholar] [CrossRef]
- Sandhya, T.E.; Ramesh, C.; Sivaram, S. Copolyesters based on poly(butylene terephthalate)s containing cyclohexyl and cyclopentyl ring: Effect of molecular structure on thermal and crystallization behavior. Macromolecules 2007, 40, 6906–6915. [Google Scholar] [CrossRef]
- Yamadera, R.; Murano, M. The determination of randomness in copolyesters by high resolution nuclear magnetic resonance. J. Polym. Sci. Part A Polym. Chem. 1967, 5, 2259–2268. [Google Scholar] [CrossRef]
- Li, W.D.; Zeng, J.B.; Lou, X.J.; Zhang, J.J.; Wang, Y.Z. Aromatic-aliphatic random and block copolyesters: Synthesis, sequence distribution and thermal properties. Polym. Chem. 2012, 3, 1344–1353. [Google Scholar] [CrossRef]
- Matsuda, H.; Nagasaka, B.; Asakura, T. Sequence analysis of poly(ethylene/1,4-cyclohexanedimethylene terephthalate) copolymer using 1H and 13C NMR. Polymer 2003, 44, 4681–4687. [Google Scholar] [CrossRef]
- Diao, L.; Su, K.; Li, Z.; Ding, C. Furan-based co-polyesters with enhanced thermal properties: Poly(1,4-butylene-co-1,4-cyclohexanedimethylene-2,5-furandicarboxylic acid). RSC Adv. 2016, 6, 27632–27639. [Google Scholar] [CrossRef]
- Zhao, H.B.; Wang, X.L.; Guan, Y.; Wang, X.L.; Chen, L.; Wang, Y.Z. Block self-cross-linkable poly(ethylene terephthalate) copolyester via solid-state polymerization: Crystallization, cross-linking, and flame retardance. Polymer 2015, 70, 68–76. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Finelli, L.; Munari, A.; Rosa, M.D. Biodegradable aliphatic copolyesters containing peg-like sequences for sustainable food packaging applications. Polym. Degrad. Stab. 2014, 105, 96–106. [Google Scholar] [CrossRef]
- Briber, R.M.; Thomas, E.L. Crystallization behaviour of random block copolymers of poly(butylene terephthalate) and poly(tetramethylene ether glycol). Polymer 1985, 26, 8–16. [Google Scholar] [CrossRef]
- Li, R.K.Y.; Tjong, S.C.; Xie, X.L. The structure and physical properties of in situ composites based on semiflexible thermotropic liquid crystalline copolyesteramide and poly(butylene terephthalate). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 403–414. [Google Scholar] [CrossRef]
- Boye, C.A. X-ray diffraction studies of poly(1,4-cyclohexylenedimethylene terephthalate). J. Polym. Sci. 1961, 55, 275–284. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.; Kwon, D. Thermal degradation of poly(ethyleneglycol). Polym. Degrad. Stab. 1995, 47, 203–208. [Google Scholar] [CrossRef]
- Lotti, N.; Colonna, M.; Fiorini, M.; Finelli, L.; Berti, C. Poly(butylene terephthalate) modified with ethoxylated bisphenol s with increased glass transition temperature and improved thermal stability. Polymer 2011, 52, 904–911. [Google Scholar] [CrossRef]



| Samples | DMT (mol) | CHDM (mol) | BDO (mol) | PTMG (g) |
|---|---|---|---|---|
| PCT0 | 0.5 | - | 0.7 | 100 |
| PCT10 | 0.5 | 0.052 | 0.648 | 100 |
| PCT30 | 0.5 | 0.153 | 0.547 | 100 |
| PCT50 | 0.5 | 0.255 | 0.445 | 100 |
| PCT70 | 0.5 | 0.355 | 0.345 | 100 |
| Samples | MPTMG (%) | MPBT (%) | MPCT (%) | trans/cis | Mn | Mw | PDI |
|---|---|---|---|---|---|---|---|
| PCT0 | 20.2 | 79.1 | - | - | 1.8 × 104 | 3.7 × 104 | 2.08 |
| PCT10 | 19.8 | 69.6 | 10.6 | 69.8/30.2 | 1.7 × 104 | 3.6 × 104 | 2.12 |
| PCT30 | 19.7 | 49.5 | 30.8 | 70.3/29.7 | 1.7 × 104 | 3.5 × 104 | 2.11 |
| PCT50 | 20.1 | 28 | 51.9 | 71.6/28.4 | 1.6 × 104 | 3.3 × 104 | 2.07 |
| PCT70 | 20.3 | 7.6 | 72.1 | 69.1/30.9 | 1.5 × 104 | 3.1 × 104 | 2.06 |
| Samples | Fraction of Dyad | Molar Fraction of Diester | Average Sequence Length | Degree of Randomness | ||||
|---|---|---|---|---|---|---|---|---|
| fBB | fBC + fCB | fCC | PB | PC | LnB | LnC | DR | |
| PCT0 | - | - | - | - | - | - | - | - |
| PCT10 | 0.578 | 0.382 | 0.04 | 0.778 | 0.222 | 4.1 | 1.2 | 1.05 |
| PCT30 | 0.456 | 0.441 | 0.103 | 0.677 | 0.323 | 3.1 | 1.5 | 1.01 |
| PCT50 | 0.192 | 0.483 | 0.325 | 0.434 | 0.566 | 1.8 | 2.3 | 0.98 |
| PCT70 | 0.112 | 0.462 | 0.426 | 0.343 | 0.657 | 1.5 | 2.8 | 1.02 |
| Samples | Crystal Plane | 2θ (°) | d (Å) | β (°) | L (Å) |
|---|---|---|---|---|---|
| PCT0 | 01 | 16.27 | 5.46 | 0.59 | 151.14 |
| 010 | 17.39 | 5.10 | 0.43 | 207.69 | |
| 11 | 20.65 | 4.29 | 0.96 | 93.47 | |
| 100 | 23.33 | 3.80 | 0.61 | 147.77 | |
| 11 | 25.20 | 3.52 | 0.64 | 141.34 | |
| PCT10 | 01 | 16.06 | 5.51 | 0.61 | 146.15 |
| 010 | 17.25 | 5.13 | 0.45 | 198.42 | |
| 11 | 20.36 | 4.35 | 0.98 | 91.52 | |
| 100 | 23.19 | 3.83 | 0.67 | 134.50 | |
| 11 | 25.07 | 3.54 | 0.66 | 137.02 | |
| PCT30 | - | - | - | - | - |
| 010 | 16.80 | 5.27 | 0.47 | 189.87 | |
| 11 | 20.16 | 4.40 | 1.01 | 88.78 | |
| 100 | 22.96 | 3.87 | 0.82 | 109.8 | |
| - | - | - | - | - | |
| PCT50 | 011 | 15.13 | 5.85 | 0.53 | 168.02 |
| 010 | 16.83 | 5.27 | 0.46 | 193.99 | |
| 10 | 19.36 | 4.58 | 0.77 | 116.31 | |
| 100 | 23.56 | 3.77 | 0.66 | 136.64 | |
| 11 | 25.72 | 3.46 | 0.66 | 137.20 | |
| PCT70 | 011 | 15.17 | 5.83 | 0.47 | 189.49 |
| 010 | 16.83 | 5.26 | 0.45 | 198.31 | |
| 10 | 19.24 | 4.61 | 0.73 | 122.66 | |
| 100 | 23.39 | 3.79 | 0.64 | 140.86 | |
| 11 | 25.50 | 3.49 | 0.65 | 139.24 |
| Samples | Cooling | Heating | |||||
|---|---|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J·g−1) | Tgs (°C) | Tgh (°C) | Tm (°C) | ΔHm (J·g−1) | WC (%) | |
| PCT0 | 147.6 | 22.6 | −16.1 | 33.8 | 187.8 | 22.8 | 19.9 |
| PCT10 | 118.1 | 17.0 | −16.7 | 26.4 | 158.8 | 17.7 | 15.9 |
| PCT30 | 83.9 | 12.0 | −17.0 | 23.5 | 150.6 | 12.1 | 10.5 |
| PCT50 | 147.9 | 13.7 | −11.9 | 42.9 | 206.5 | 14.0 | 15.0 |
| PCT70 | 198.0 | 15.6 | −12.7 | 45.5 | 245.2 | 15.7 | 18.6 |
| Samples | Elastic Modulus (E) | Tensile Strength σmax (MPa) | Elongation at Break εb (%) |
|---|---|---|---|
| PCT0 | 101.7 (±9.8) | 25.1 (±3.7) | 505.3 (±49.8) |
| PCT10 | 71.2 (±7.4) | 16.6 (±1.8) | 527.7 (±47.2) |
| PCT30 | 33.2 (±3.6) | 10.1 (±1.2) | 647.7 (±61.3) |
| PCT50 | 35.2 (±3.3) | 16.7 (±1.7) | 624.4 (±63.7) |
| PCT70 | 43.4 (±4.7) | 19.0 (±2.1) | 547.9 (±50.7) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.-C.; Zhao, H.; Hao, T.-H.; Hu, G.-H.; Jiang, T.; Zhang, Q.-C. Effects of Poly(cyclohexanedimethylene terephthalate) on Microstructures, Crystallization Behavior and Properties of the Poly(ester ether) Elastomers. Materials 2017, 10, 694. https://doi.org/10.3390/ma10070694
Feng Y-C, Zhao H, Hao T-H, Hu G-H, Jiang T, Zhang Q-C. Effects of Poly(cyclohexanedimethylene terephthalate) on Microstructures, Crystallization Behavior and Properties of the Poly(ester ether) Elastomers. Materials. 2017; 10(7):694. https://doi.org/10.3390/ma10070694
Chicago/Turabian StyleFeng, Yi-Cheng, Hui Zhao, Tong-Hui Hao, Guo-Hua Hu, Tao Jiang, and Qun-Chao Zhang. 2017. "Effects of Poly(cyclohexanedimethylene terephthalate) on Microstructures, Crystallization Behavior and Properties of the Poly(ester ether) Elastomers" Materials 10, no. 7: 694. https://doi.org/10.3390/ma10070694