Effect of Ultrasonic Nano-Crystal Surface Modification (UNSM) on the Passivation Behavior of Aged 316L Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
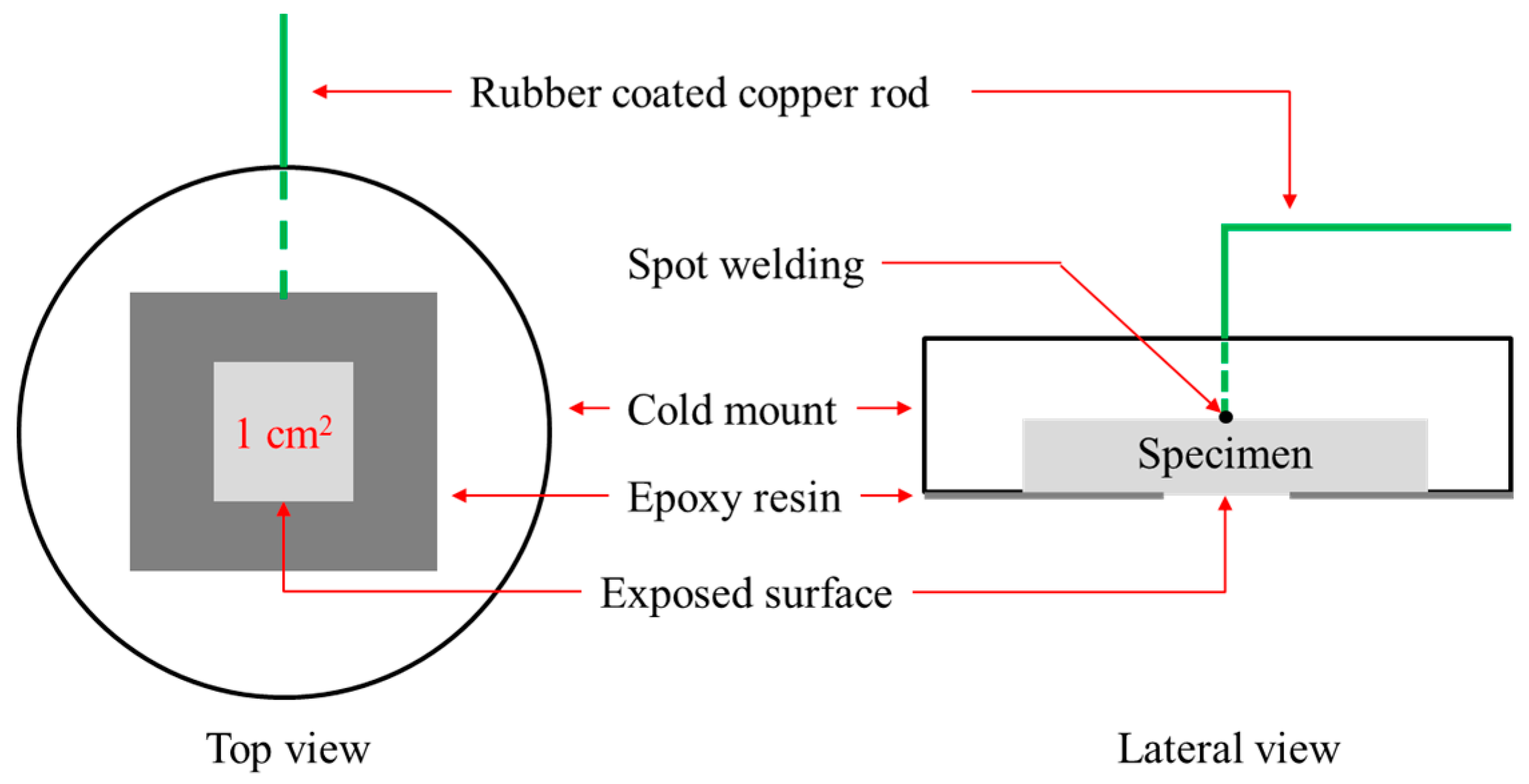
2.1 Specimen
2.2. Corrosion Test
2.2.1. Anodic Polarization Test
2.2.2. AC Impedance Measurement
2.2.3. Mott-Schottky Test
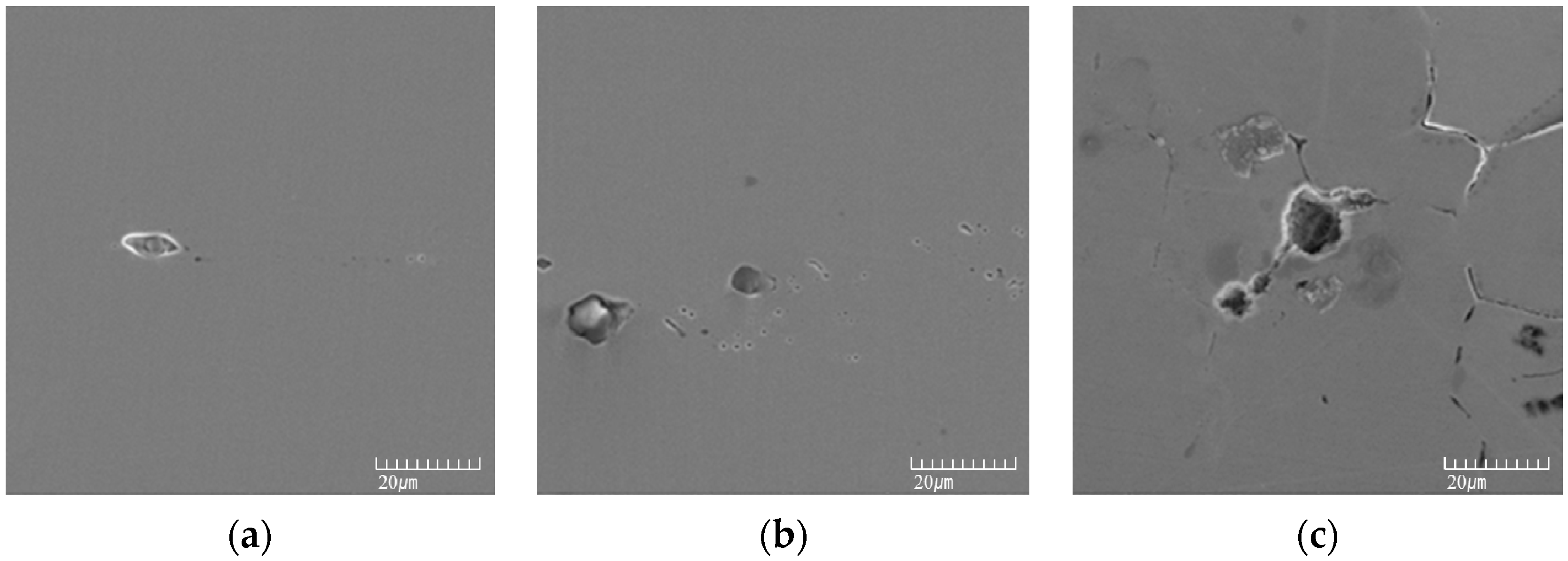
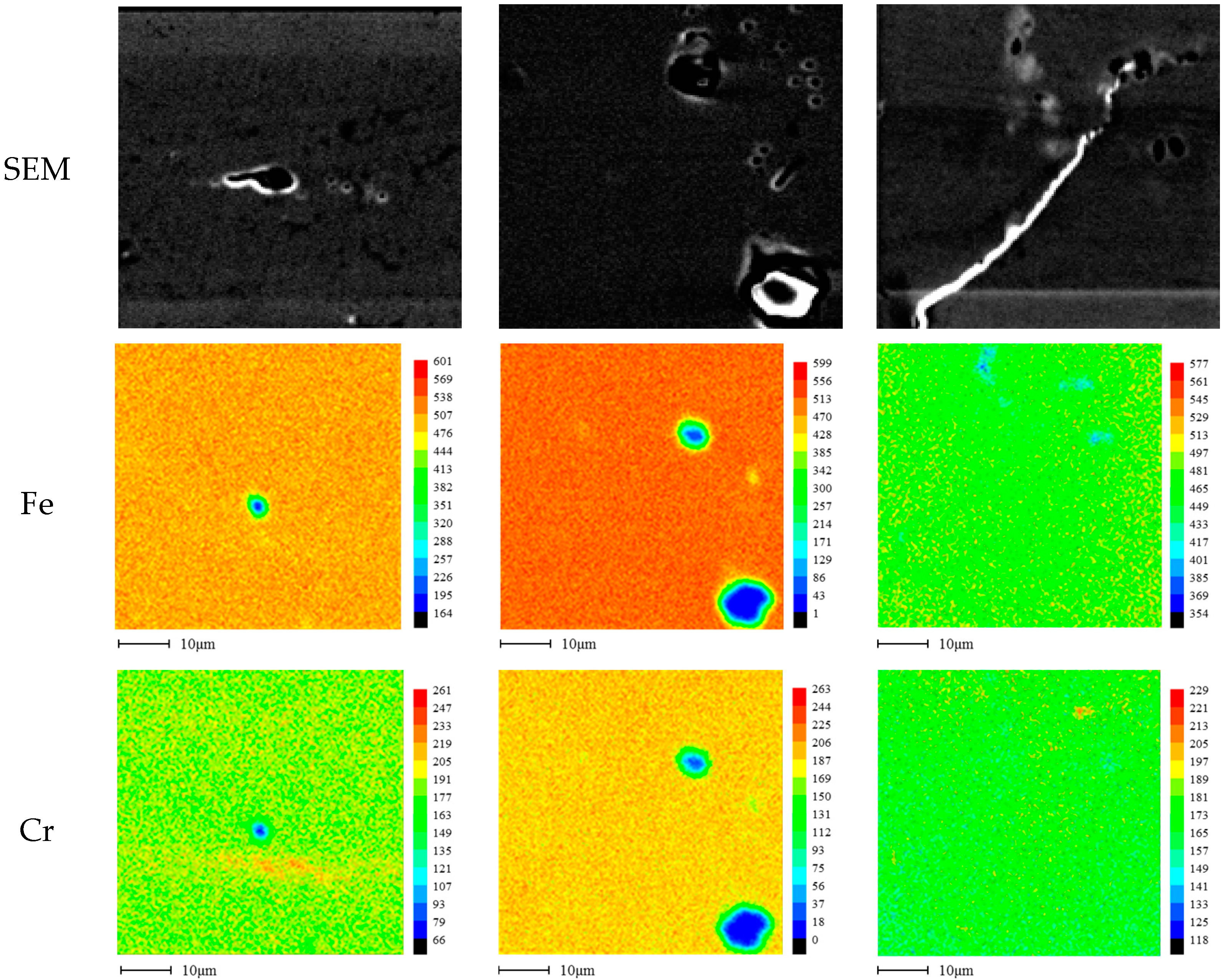
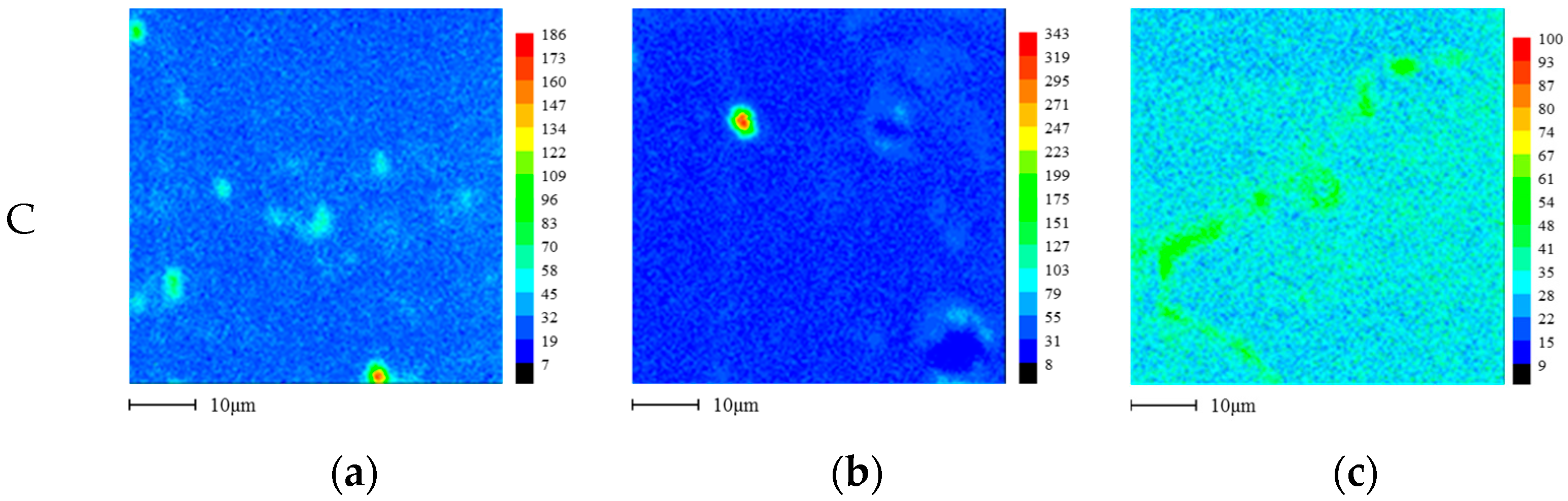
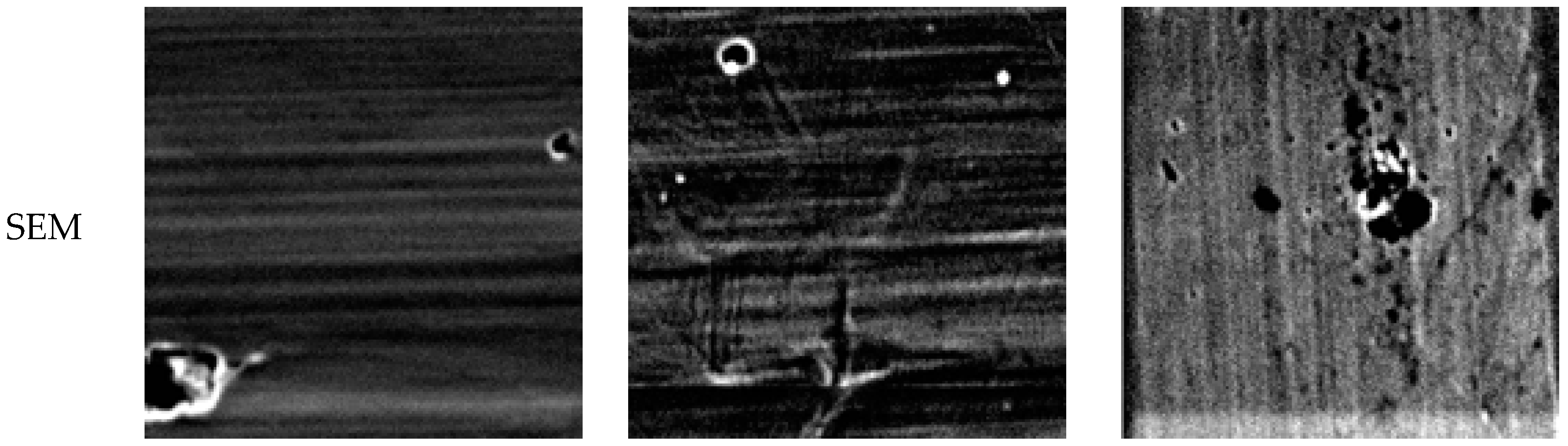
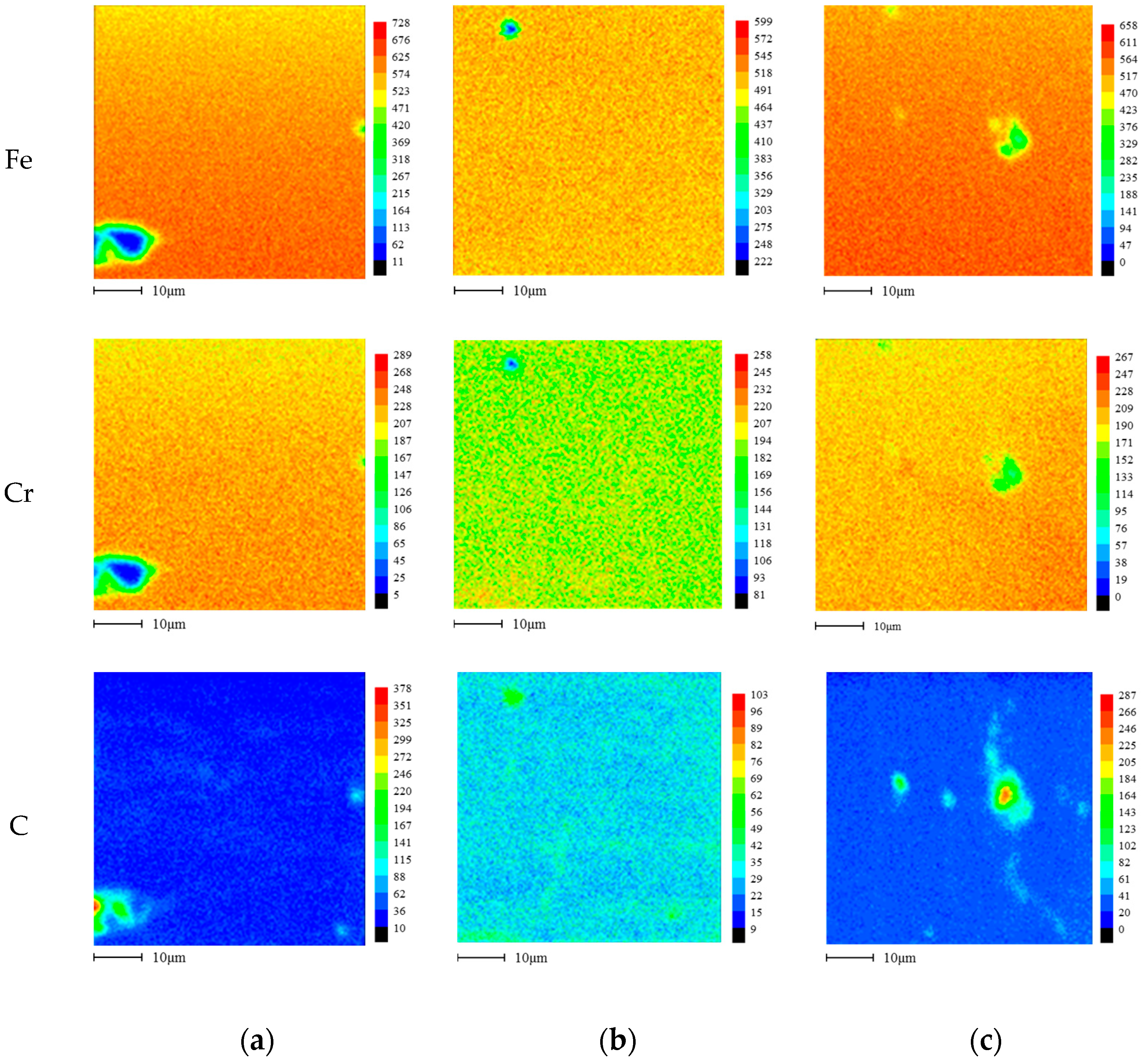
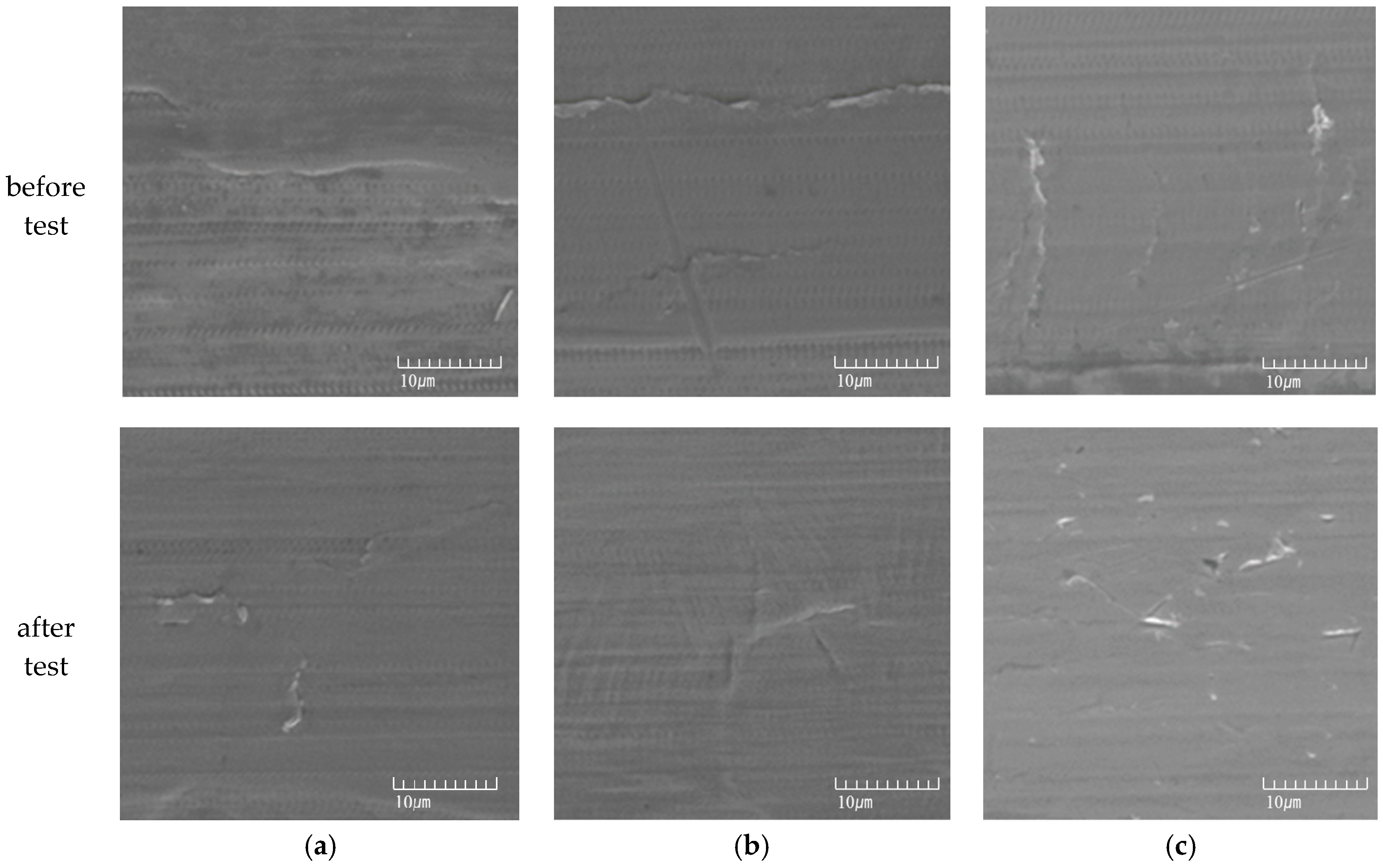
2.3. Microstructure Analysis
3. Results
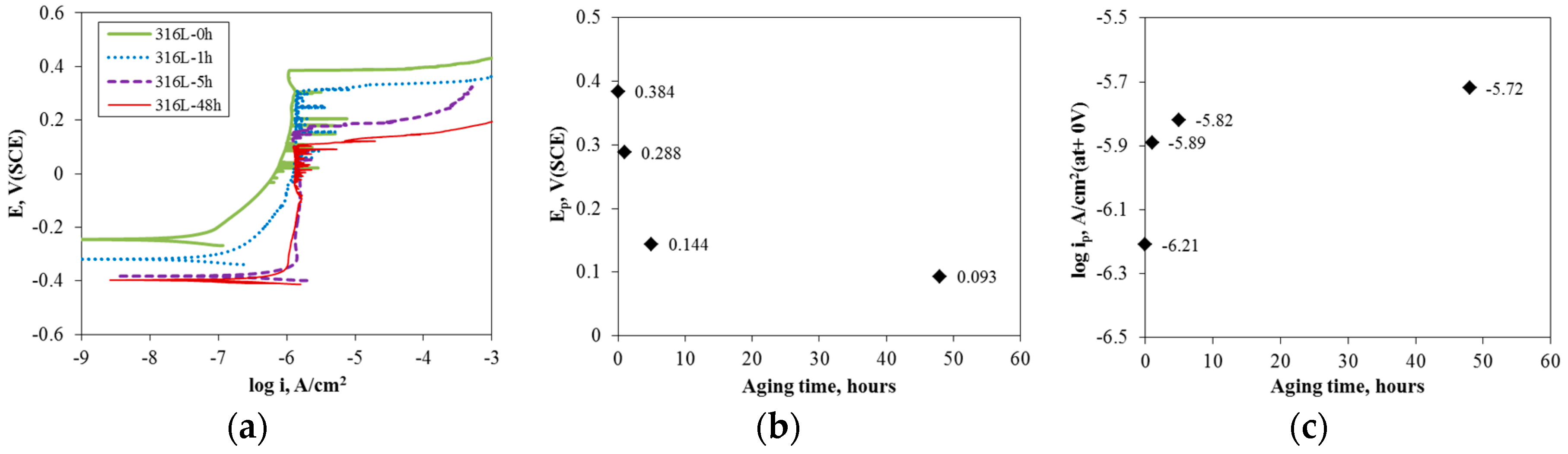
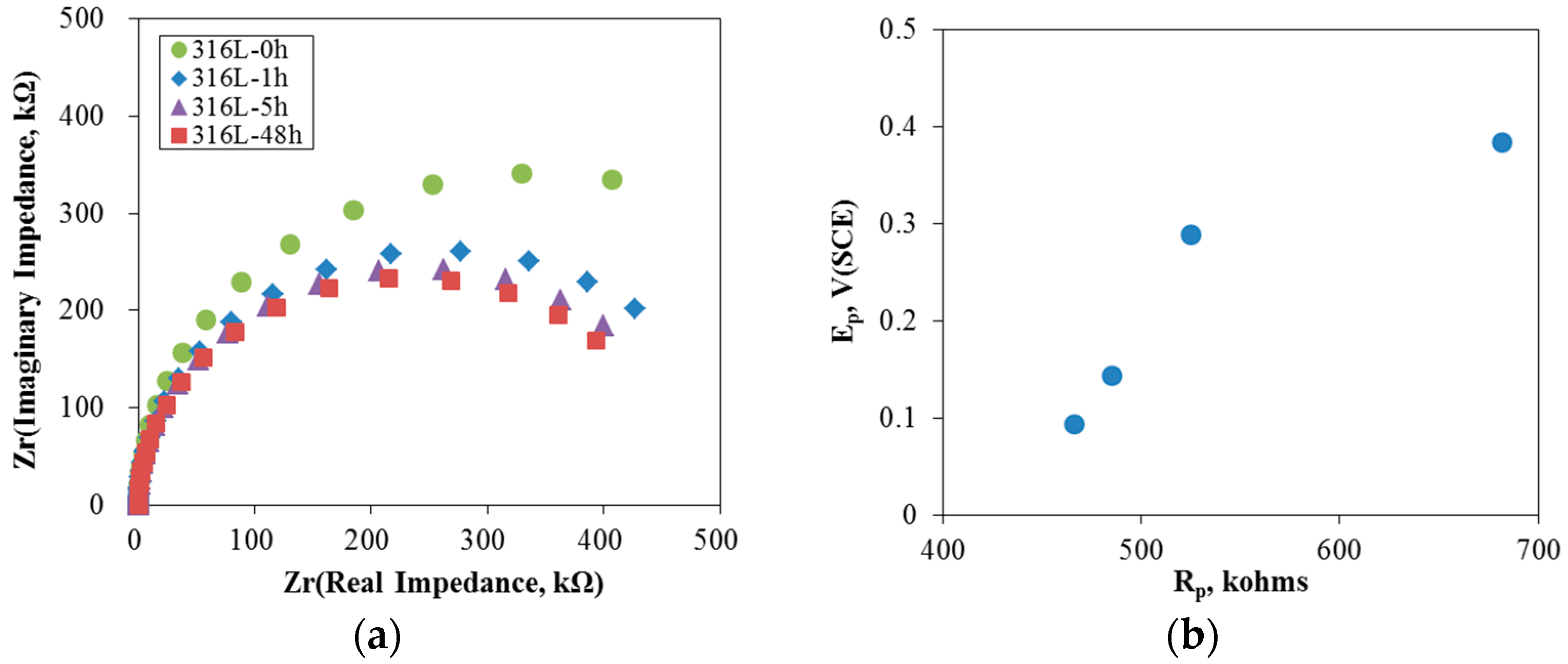
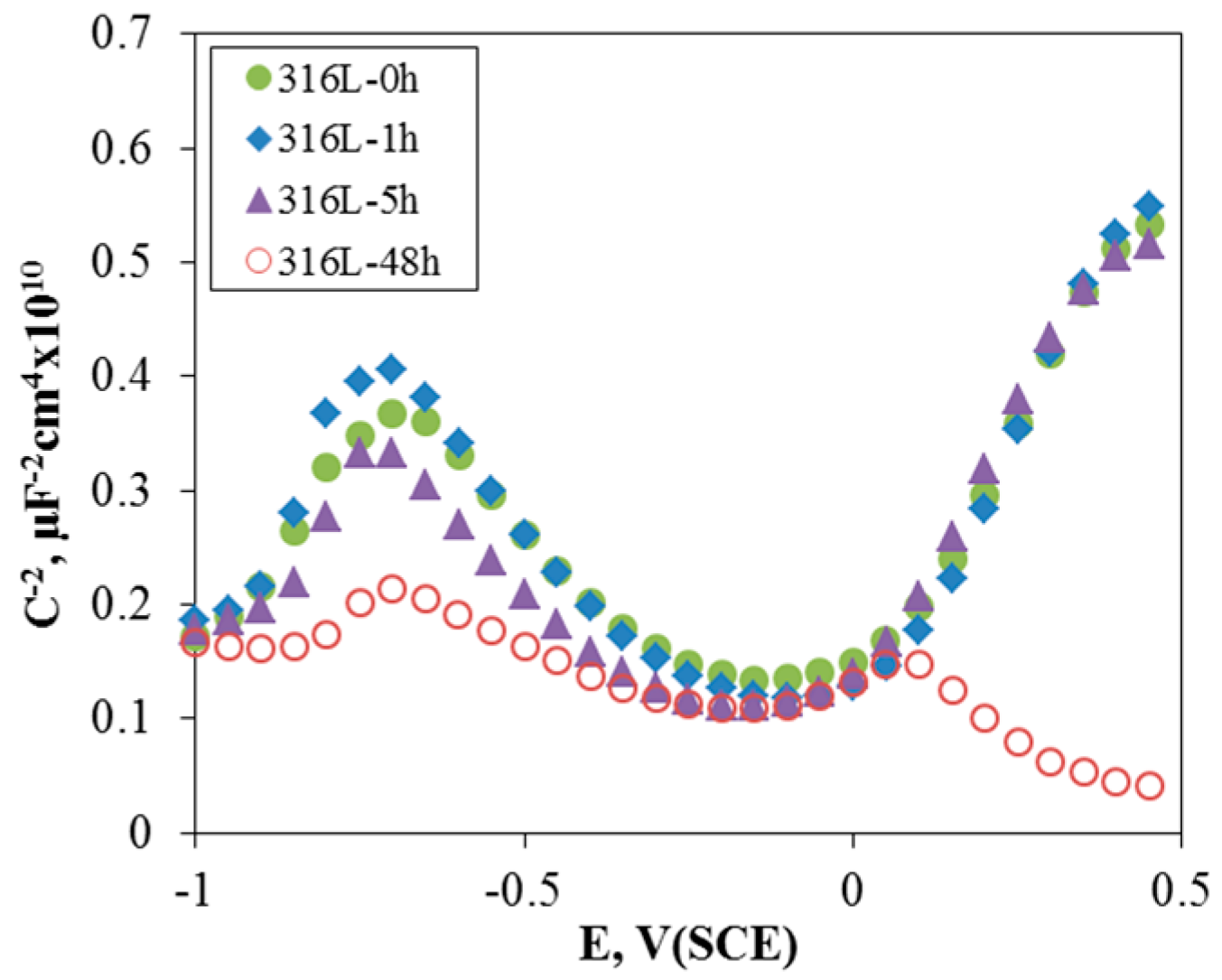
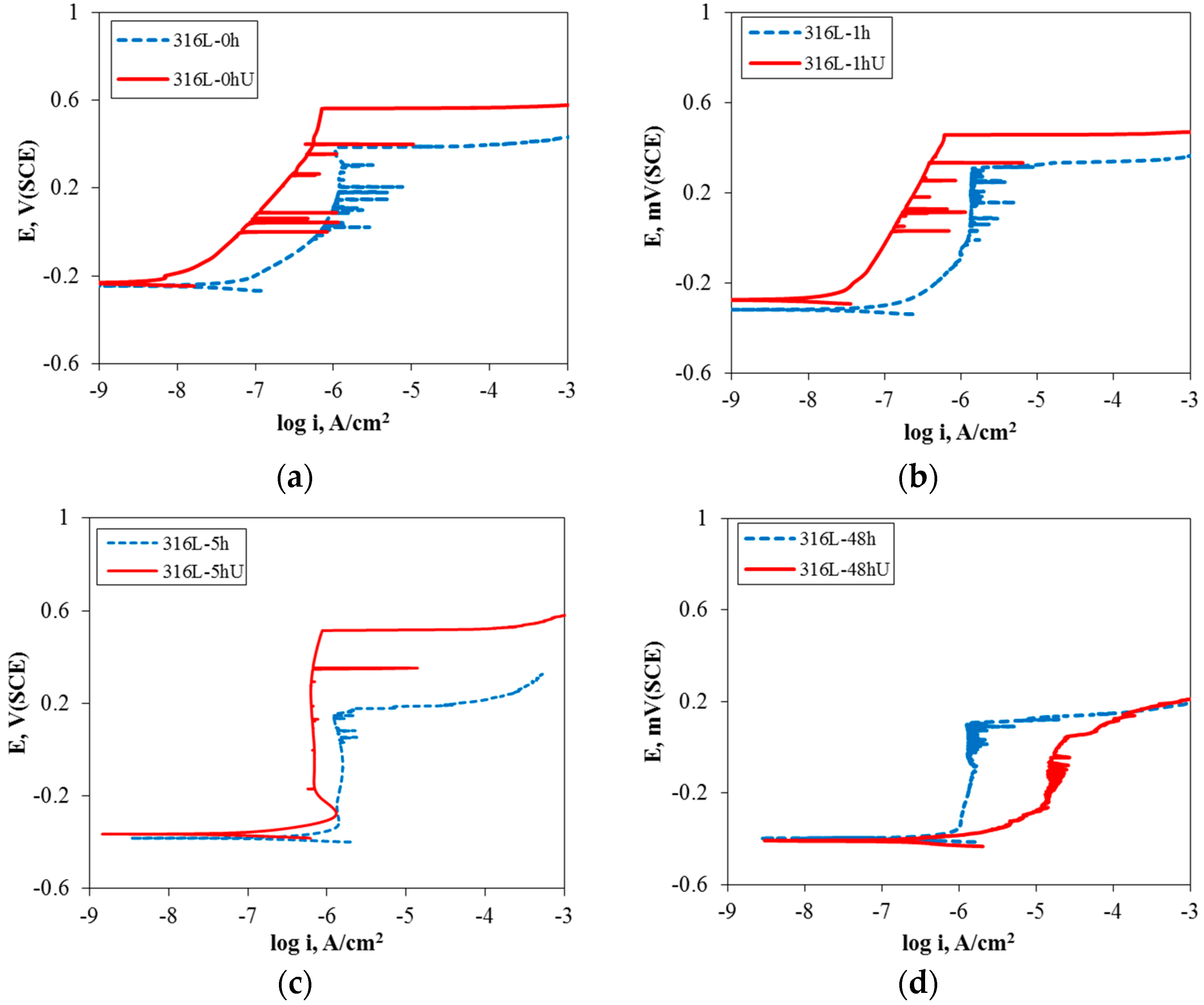
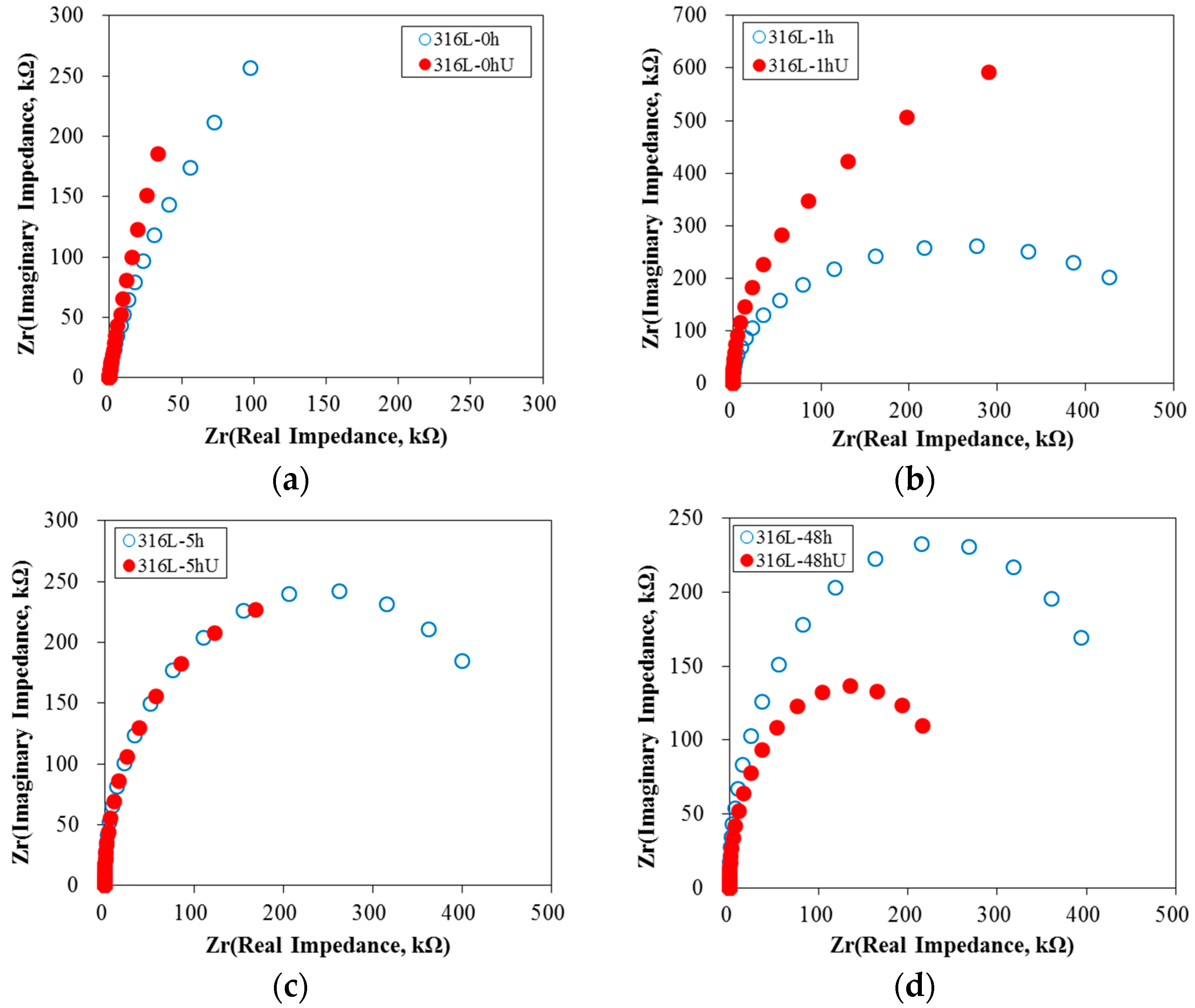
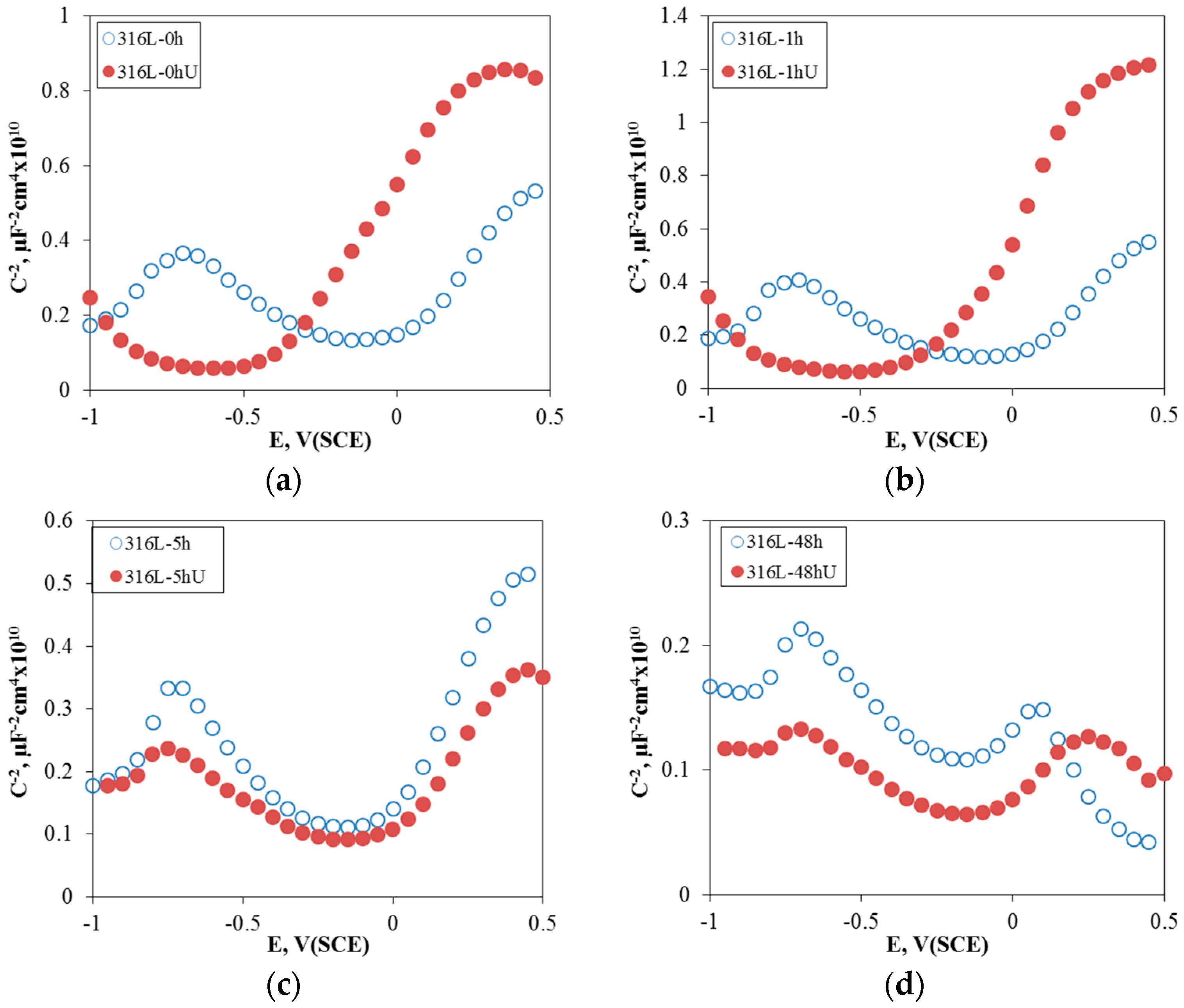
3.1. Effect of Aging Treatment on the Electrochemical Passivation Behavior
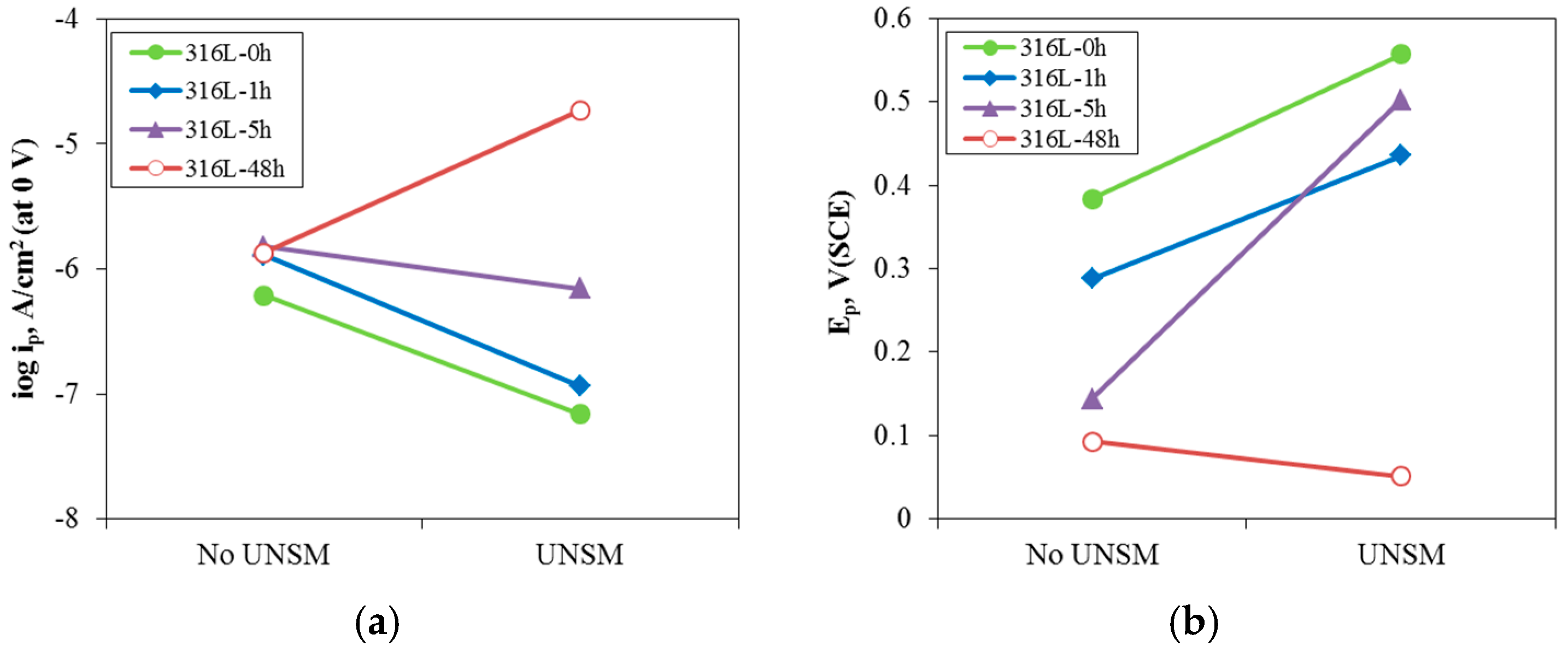
3.2. Effect of UNSM on the Electrochemical Passivation Behavior
4. Discussion
5. Conclusions
- (1)
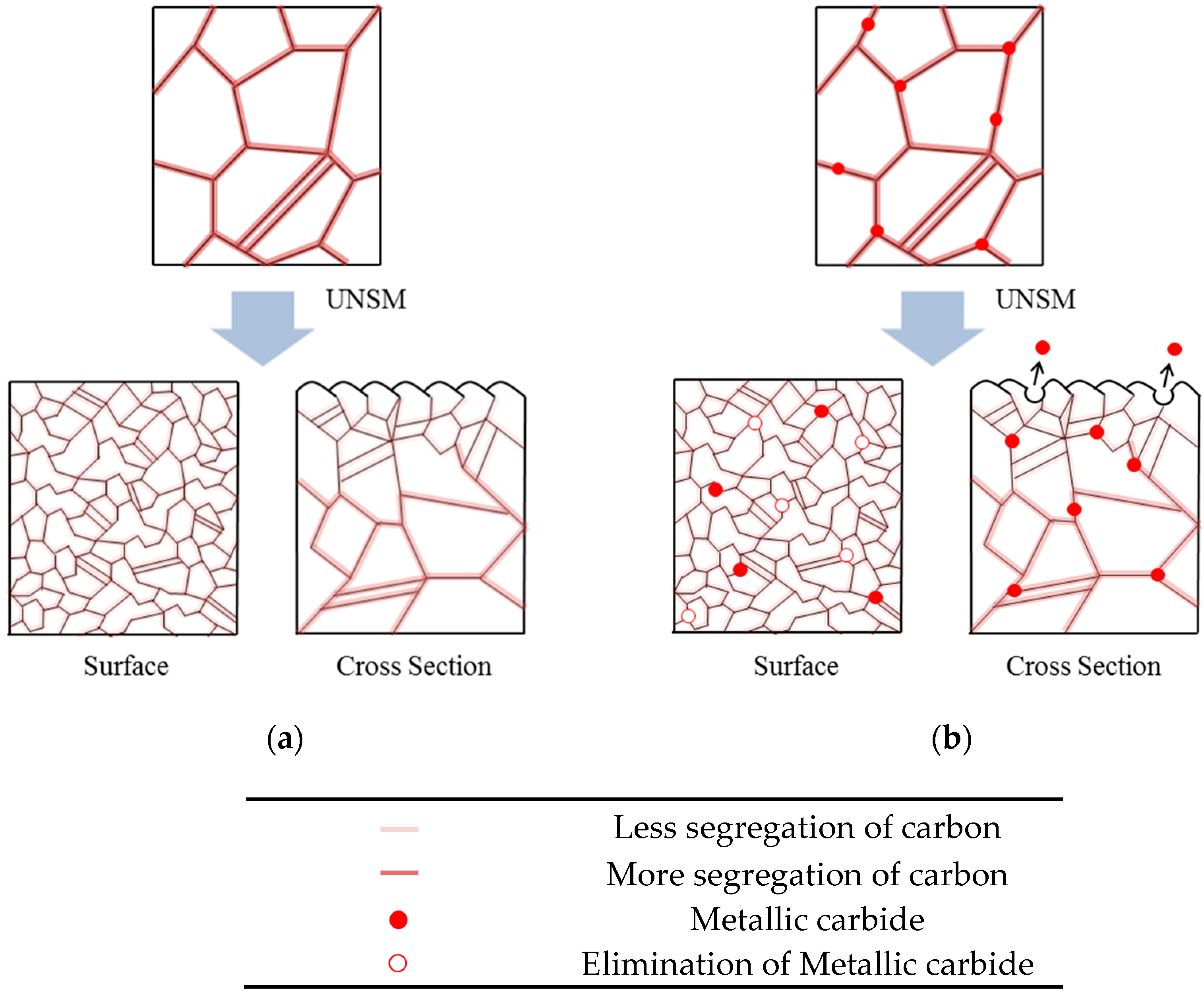
- In the case of slightly sensitized 316L stainless steel, the aging time drastically decreased the pitting potential, increased the passive current density, and lowered the resistance of the passive film, even though chromium carbide and the chromium depletion zone were not formed by aging. This behavior is due to the micro-galvanic corrosion between the matrix and the carbon segregated area, and shows the importance of carbon segregation in grain boundaries to the pitting corrosion resistance of stainless steel, in addition to the formation of the chromium depletion zone.
- (2)
- UNSM-treatment of the slightly sensitized 316L stainless steel increased the pitting potential, decreased the passive current density, and increased the resistance of the passive film. However, in the case of the heavily sensitized 316L stainless steel, the UNSM-treatment decreased the pitting potential, increased the passive current density, and decreased the resistance of the passive film. This behavior is due to the dual effects by UNSM-treatment. That is, UNSM-treatment reduced the carbon segregation regardless of whether the stainless steel 316L was slightly or heavily sensitized. However, since this treatment created mechanical flaws in the outer surface, in the case of the heavily sensitized stainless steel, the UNSM-treatment may eliminate chromium carbide, and this flaw can be a potential pitting initiation site, and therefore decrease the pitting corrosion resistance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dutta, R.S.; De, P.K.; Gadiyar, H.S. The sensitization and stress corrosion cracking of nitrogen-containing stainless steels. Corros. Sci. 1993, 34, 51–60. [Google Scholar] [CrossRef]
- Nishimoto, K.; Ogawa, K. Corrosion properties in weldments of stainless steels (1). Metallurgical factors affecting corrosion properties. Weld. Int. 1999, 13, 845–854. [Google Scholar] [CrossRef]
- Gooch, T.G. Corrosion behavior of welded stainless steel. Weld. J. Incl. Weld. Res. Suppl. 1996, 75, 135s–154s. [Google Scholar]
- Lee, J.H.; Kim, K.T.; Poun, Y.S.; Kim, Y.S. Intergranular corrosion mechanism of slightly-sensitized and UNSM-treated 316L stainless steel. Corros. Sci. Tech. 2016, 15, 226–236. [Google Scholar] [CrossRef]
- Kim, Y.S. Energy Materials and its Degradation; Hanti-media: Seoul, Korea, 2013; pp. 162–163. [Google Scholar]
- Fontana, M.G. Corrosion Engineering; McGraw-Hill Book Company: New York, NY, USA, 1987. [Google Scholar]
- Aydoğdu, G.H.; Aydinol, M.K. Determination of susceptibility to intergranular corrosion and electrochemical reactivation behaviour of AISI 316L type stainless steel. Corros. Sci. 2006, 48, 3565–3583. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Makhlouf, K.; Sidhom, H.; Philibert, J. Effects of ageing conditions on the precipitates evolution, chromium depletion and intergranular corrosion susceptibility of AISI 316L: Experimental and modeling results. Mater. Sci. Eng. A 2004, 372, 98–108. [Google Scholar] [CrossRef]
- Ogwu, A.A.; Davies, T.J. Improving the sensitisation resistance of ferritic stainless steels. Scr. Mater. 1997, 37, 259–263. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Carboneras, M.; Arrabal, R. Influence of Ti, C and N concentration on the intergranular corrosion behaviour of AISI 316Ti and 321 stainless steels. Acta Mater. 2007, 55, 2239–22551. [Google Scholar] [CrossRef]
- Hall, E.L.; Briant, C.L. Chromium depletion in the vicinity of carbides in sensitized austenitic stainless steels. Metall. Mater. Trans. A 1984, 15, 793–811. [Google Scholar] [CrossRef]
- Gates, J.D.; Jago, R.A. Effect of nitrogen contamination on intergranular corrosion of stabilized ferritic stainless steels. Mater. Sci. Technol. 1987, 3, 450–454. [Google Scholar] [CrossRef]
- Amunda, M.O.H.; Mridha, S. An overview of sensitization dynamics in ferritic stainless steel welds. Int. J. Corros. 2011, 2011, 305793. [Google Scholar]
- Rashid, M.W.A.; Gakim, M.; Rosli, Z.M.; Azam, M.A. Formation of Cr23C6 during the sensitization of AISI 304 stainless steel and its effect to pitting corrosion. Int. J. Electrochem. Sci. 2012, 7, 9465–9477. [Google Scholar]
- Kim, J.K.; Kim, Y.H.; Lee, B.H.; Kim, K.Y. New findings on intergranular corrosion mechanism of stabilized stainless steels. Electrochim. Acta 2011, 56, 1701–1710. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, Y.; Zhu, R. Stress corrosion cracking of type 304 stainless steel weldments in the active state. Corrosion 1994, 50, 171–175. [Google Scholar] [CrossRef]
- Krishnan, K.N.; Prasad, R.K. Effect of microstructure on stress corrosion cracking behavior of austenitic stainless steel weld metals. Mater. Sci. Eng. A 1991, 142, 79–85. [Google Scholar] [CrossRef]
- Lu, B.T.; Chen, Z.K.; Luo, J.L.; Patchett, B.M.; Xu, Z.H. Pitting and stress corrosion cracking behavior in welded austenitic stainless steel. Electrochim. Acta 2005, 50, 1391–1403. [Google Scholar] [CrossRef]
- Clauer, A.H. Shock Waves and High Strain Rate Phenomena in Metals; Plenum Press: New York, NY, USA, 1981; pp. 675–701. [Google Scholar]
- Sano, Y.; Mukai, N.; Okasaki, K.; Obata, M. Residual stress improvement in metal surface by underwater laser irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B 1997, 121, 432–436. [Google Scholar] [CrossRef]
- Peyre, P.; Fabbro, R.; Merrien, P.; Lieurade, H.P. Laser shock processing of aluminium alloys. Application to high cycle fatigue behavior. Mater. Sci. Eng. A 1996, 210, 102–113. [Google Scholar] [CrossRef]
- Peyre, P.; Scherpereel, X.; Berthe, L.; Carboni, C.; Fabbro, R.; Béranger, G.; Lemaitre, C. Surface modifications induced in 316L steel by laser peening and shot-peening. Influence on pitting corrosion resistance. Mater. Sci. Eng. A 2000, 280, 294–302. [Google Scholar] [CrossRef]
- Azar, V.; Hashemi, B.; Yazdi, M.R. The effect of shot peening on fatigue and corrosion behavior of 316L stainless steel in Ringer’s solution. Surf. Coat. Technol. 2010, 204, 3546–3551. [Google Scholar] [CrossRef]
- Liu, G.; Wang, S.C.; Lou, X.F.; Lu, J.; Lu, K. Low carbon steel with nanostructured surface layer induced by high-energy shot peening. Scr. Mater. 2001, 44, 1791–1795. [Google Scholar] [CrossRef]
- Sanjurjo, P.; Rodriguez, C.; Pariente, I.F.; Belzunce, F.J.; Canteli, A.F. The influence of shot peening on the fatigue behaviour of duplex stainless steels. Scri. Mater. 2010, 2, 1539–1546. [Google Scholar] [CrossRef]
- Peyre, P.; Braham, C.; Ledion, J.; Berthe, L.; Fabbro, R. Corrosion reactivity of laser-peened steel surfaces. J. Mater. Eng. Perform. 2000, 9, 656–662. [Google Scholar] [CrossRef]
- Trdan, U.; Grum, J. Evaluation of corrosion resistance of AA6082-T651 aluminium alloy after laser shock peening by means of cyclic polarization and ElS methods. Corros. Sci. 2012, 59, 324–333. [Google Scholar] [CrossRef]
- EPRI 1025839, Materials Reliability Program: Technical Basis for Primary Water Stress Corrosion Cracking Mitigation by Surface Stress Improvement (MRP-267, Revision 1); Electric Power Research Institute, Inc.: Palo Alto, CA, USA, 2012.
- Gujba, A.K.; Medraj, M. Laser peening process and its impact on materials properties in comparison with shot peening and ultrasonic impact peening. Materials 2014, 7, 7925–7974. [Google Scholar] [CrossRef]
- Telang, A.; Gill, A.S.; Tammana, D.; Wen, X.; Kumar, M.; Teysseyre, S.; Mannava, S. R.; Qian, D.; Vasudevan, V.K. Surface grain boundary engineering of Alloy 600 for improved resistance to stress corrosion cracking. Mater. Sci. Eng. A 2015, 648, 280–288. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, D.S.; Jung, J.S.; Pyun, Y.S.; Shin, K. Influence of peening on the corrosion properties of AISI 304 stainless steel. Corros. Sci. 2009, 51, 2826–2830. [Google Scholar] [CrossRef]
- Wang, T.; Yu, J.; Dong, B. Surface nanocrystallization induced by shot peening and its effect on corrosion resistance of 1Cr18Ni9Ti stainless steel. Surf. Coat. Technol. 2006, 200, 4777–4781. [Google Scholar] [CrossRef]
- Ye, W.; Li, Y.; Wang, F. Effects of nanocrystallization on the corrosion behavior of 309 stainless steel. Electrochim. Acta 2006, 51, 4426–4432. [Google Scholar]
- Takakuwa, O.; Soyama, H. Effect of residual stress on the corrosion behavior of austenitic stainless steel. Adv. Chem. Eng. Sci. 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Domankova, M.; Kocsisova, E.; Pinke, P.; Slatkovsky, I. The Microstructure Evolution and Its Effect on Corrosion Properties of 18Cr-12Ni-2,5Mo Steel Annealed at 500–900 °C. Acta Polytech. Hung. 2014, 11, 125–137. [Google Scholar]
- Wasnik, D.N.; Kain, V.; Samajdar, I.; Verlinden, B.; De, P.K. Resistance to sensitization and intergranular corrosion through extreme randomization of grain boundaries. Acta Mater. 2002, 50, 4587–4601. [Google Scholar] [CrossRef]
- Macdonald, D.D. Point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S. Intergranular corrosion of 316L stainless steel by aging and UNSM (ultrasonic nano-crystal surface modification) treatment. Corros. Sci. Technol. 2015, 14, 313–324. [Google Scholar] [CrossRef]
- Sakashita, M.; Sato, N. Bipolar fixed charged-induced passivity, in Passivity of Metals; The Electrochemical Society: Pennington, NJ, USA, 1978; pp. 483–497. [Google Scholar]
- Clayton, C.R.; Lu, Y.C. A bipolar model of the passivity of stainless steel: The role of Mo addition. J. Electrochem. Soc. 1986, 133, 2465–2473. [Google Scholar] [CrossRef]
- Clayton, C.R.; Halada, G.P.; Kearns, J.R. Passivity of high-nitrogen stainless alloys: The role of metal oxyanions and salt films. Mater. Sci. Eng. A 1995, 198, 135–144. [Google Scholar] [CrossRef]
- Hoar, T.P.; Mears, D.C.; Rothwell, G.P. The relationships between anodic passivity, brightening and pitting. Corros. Sci. 1965, 5, 279–289. [Google Scholar] [CrossRef]
- Macdonald, D.D. Passivity-the key to our metals-based civilization. Pure Appl. Chem. 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Popov, N.E. Corrosion Engineering Principles and Solved Problems; Elsevier: Waltham, MA, USA, 2015; pp. 290–303. [Google Scholar]
- Sedriks, A.J. Corrosion of Stainless Steels, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1996; pp. 231–262. [Google Scholar]

| Cr | Mo | Ni | C | Mn | Si | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| 16.69 | 1.99 | 10.19 | 0.01 | 1.19 | 0.59 | 0.04 | 0.01 | Bal |
| Alloys | 316L Stainless Steel |
|---|---|
| Amplitude | 30 μm |
| Static load | 10 N |
| Pitch | 0.07 mm |
| Speed | 1000 mm/min |
| Tip diameter | 2.38 mm (WC 1) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-T.; Lee, J.-H.; Kim, Y.-S. Effect of Ultrasonic Nano-Crystal Surface Modification (UNSM) on the Passivation Behavior of Aged 316L Stainless Steel. Materials 2017, 10, 713. https://doi.org/10.3390/ma10070713
Kim K-T, Lee J-H, Kim Y-S. Effect of Ultrasonic Nano-Crystal Surface Modification (UNSM) on the Passivation Behavior of Aged 316L Stainless Steel. Materials. 2017; 10(7):713. https://doi.org/10.3390/ma10070713
Chicago/Turabian StyleKim, Ki-Tae, Jung-Hee Lee, and Young-Sik Kim. 2017. "Effect of Ultrasonic Nano-Crystal Surface Modification (UNSM) on the Passivation Behavior of Aged 316L Stainless Steel" Materials 10, no. 7: 713. https://doi.org/10.3390/ma10070713




