Corrosion Behavior of X80 Steel with Coupled Coating Defects under Alternating Current Interference in Alkaline Environment
Abstract
:1. Introduction
2. Experimental
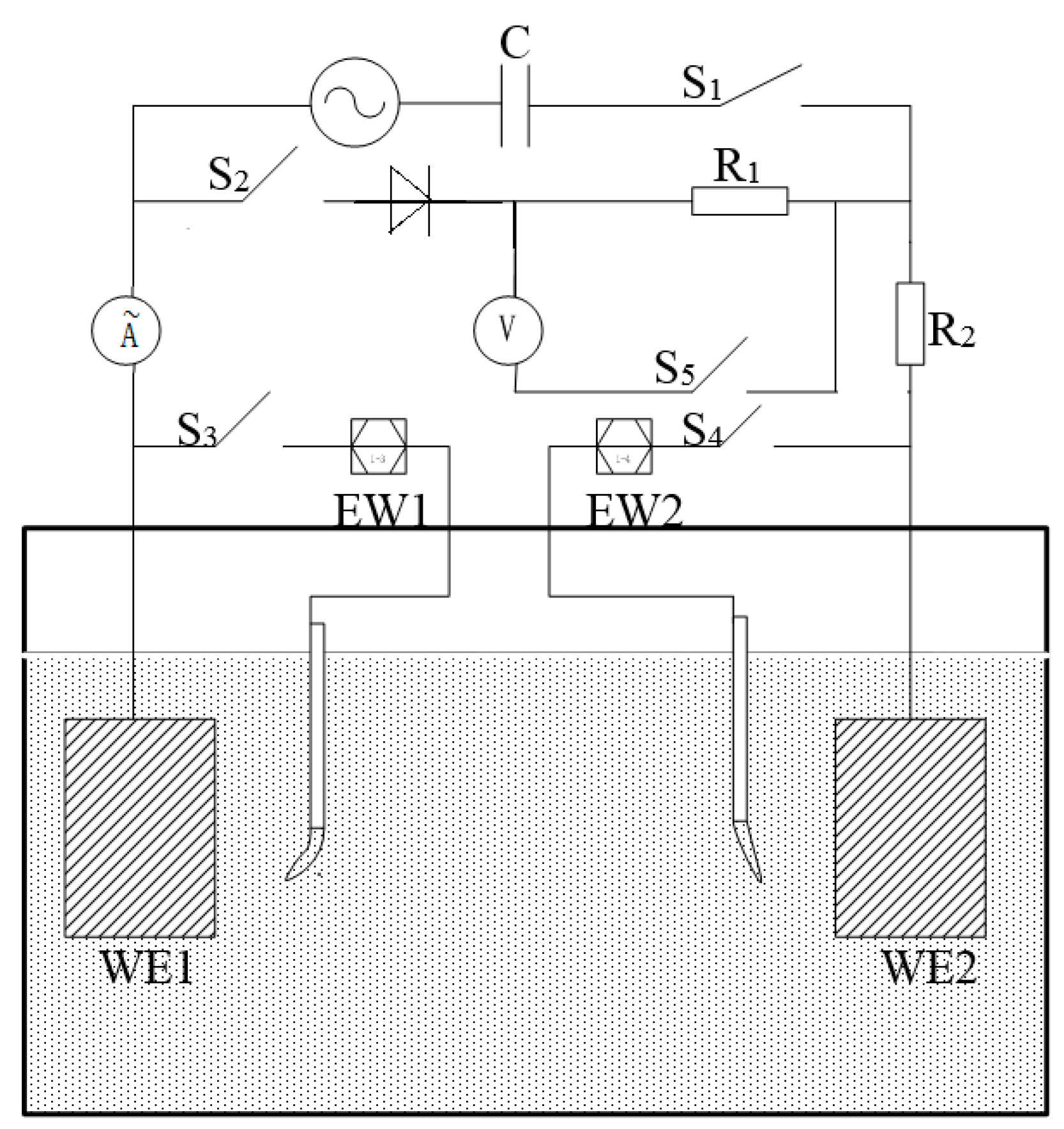
2.1. Electrode and Solution
2.2. Electrochemical Tests
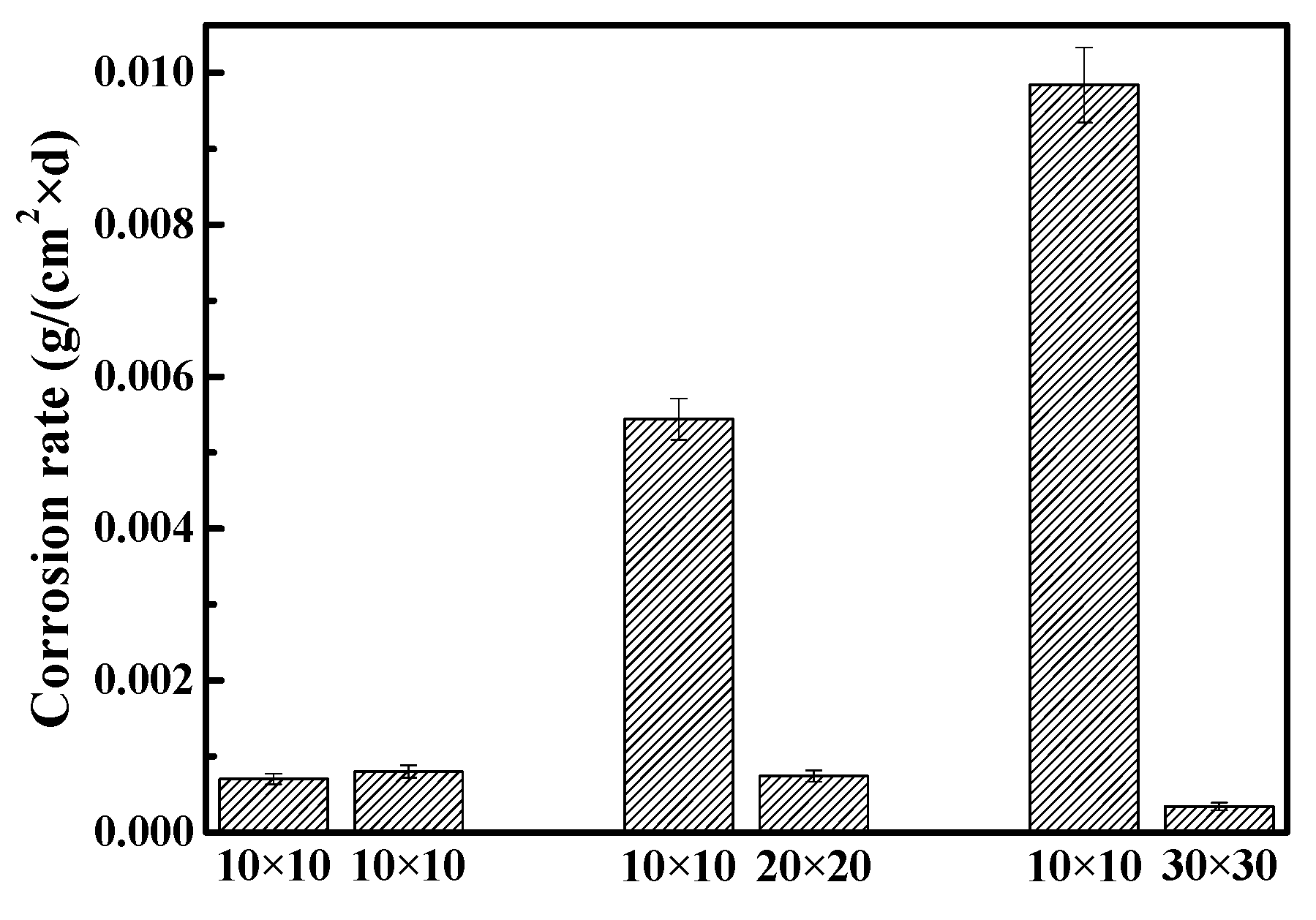
- Pair 1, 10 × 10/10 × 10 (mm2);
- Pair 2, 10 × 10/20 × 20 (mm2);
- Pair 3, 10 × 10/30 × 30 (mm2).
2.3. Electrochemical Parameter Measurement
3. Results and Discussion
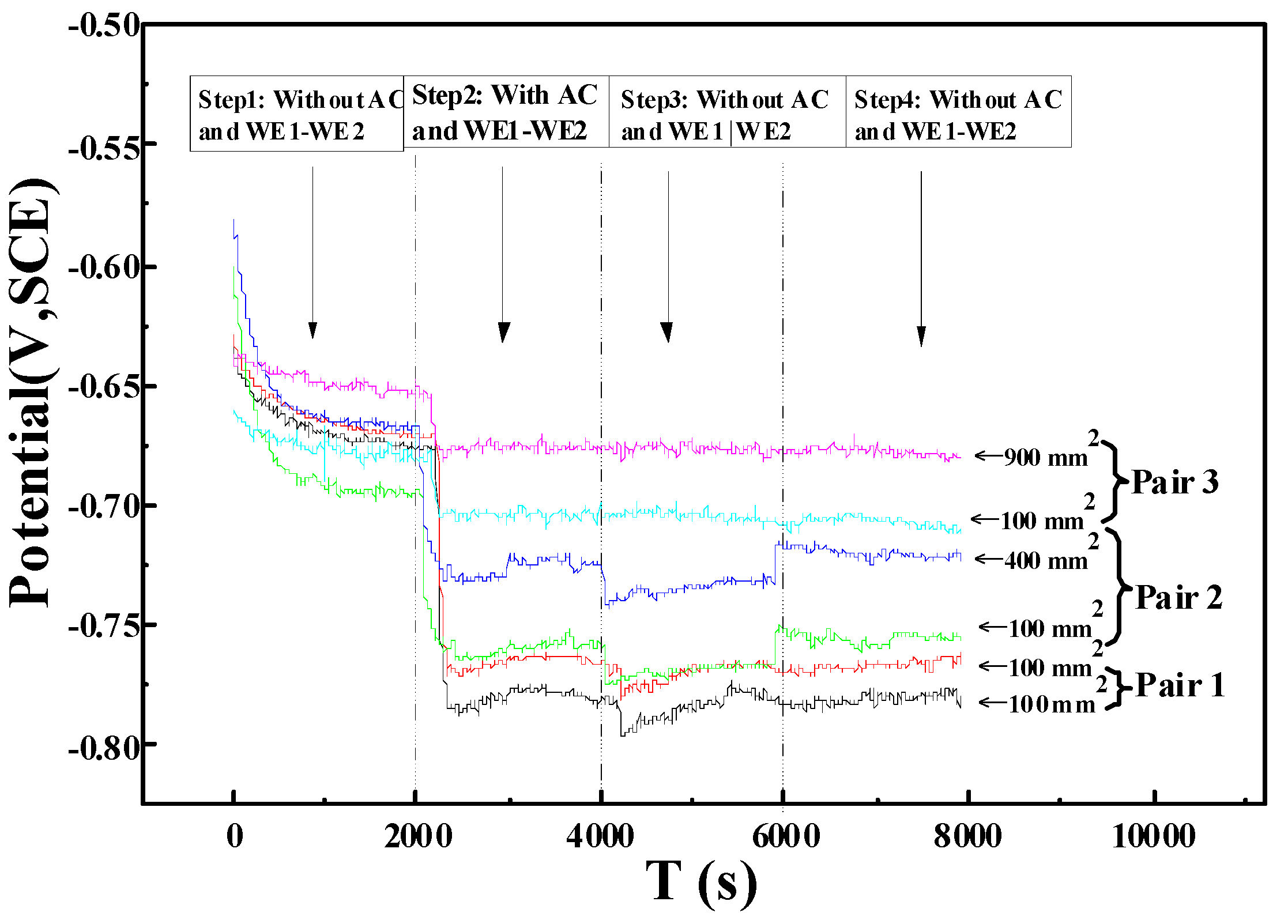
3.1. Electrochemical Analyses
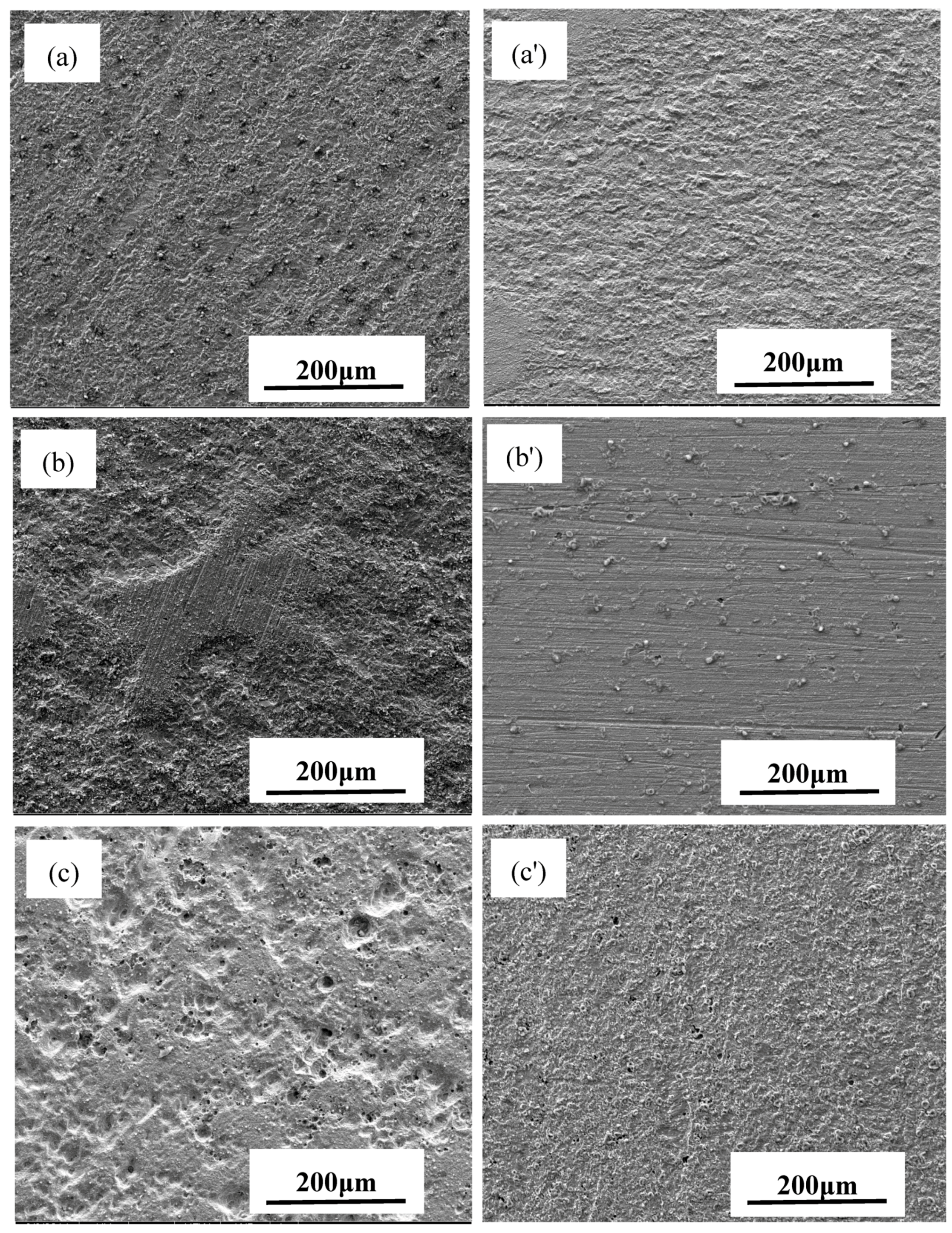
3.2. Corrosion Morphology
3.3. Corrosion Rates
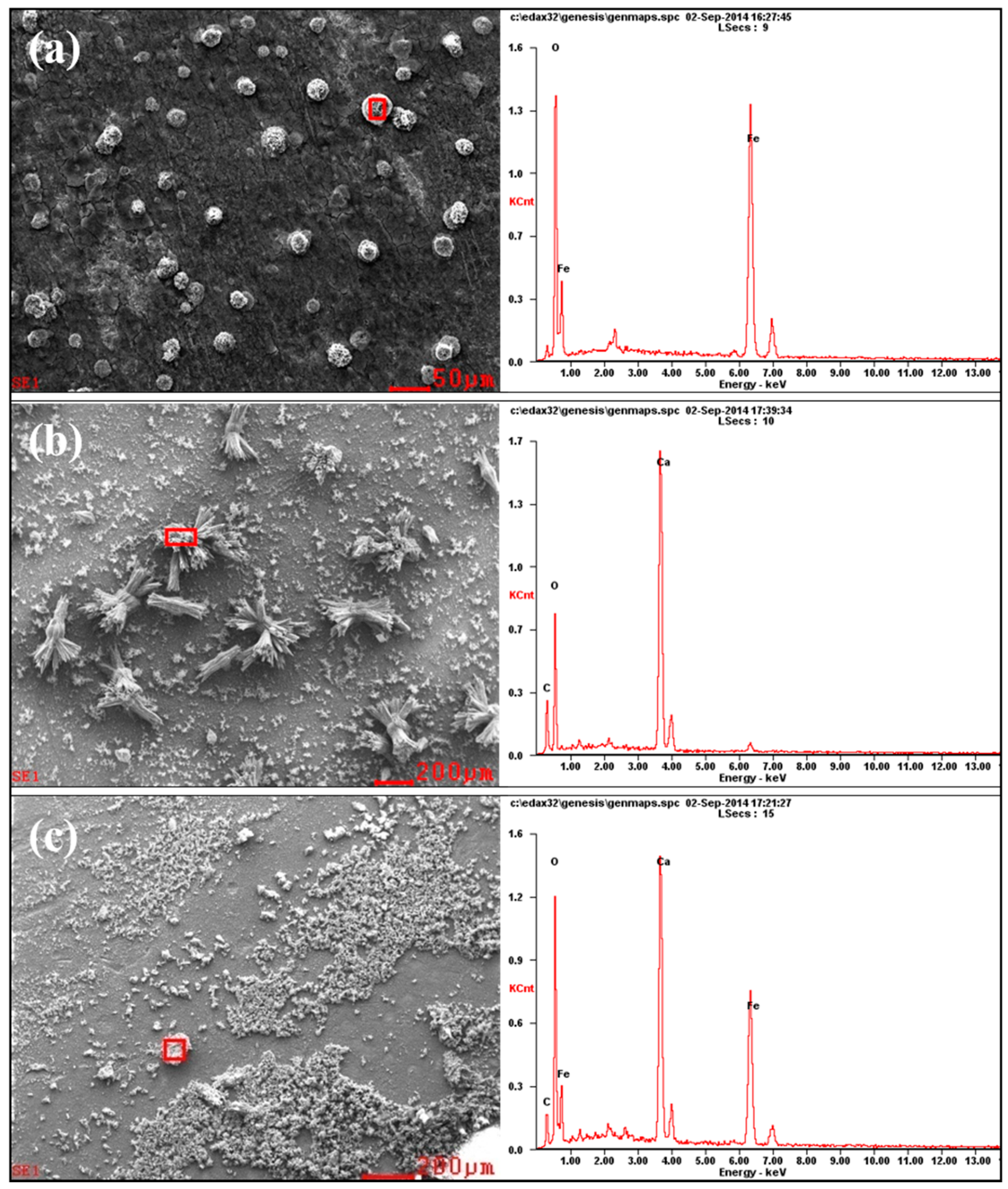
3.4. EDS Analysis of the Corrosion Products
4. Conclusions
- (1)
- The open circuit potential of the X80 steel specimen with a smaller coating defect area was lower than those with larger coating defects. This result showed that the specimen with the smaller coating defect was more prone to corrosion.
- (2)
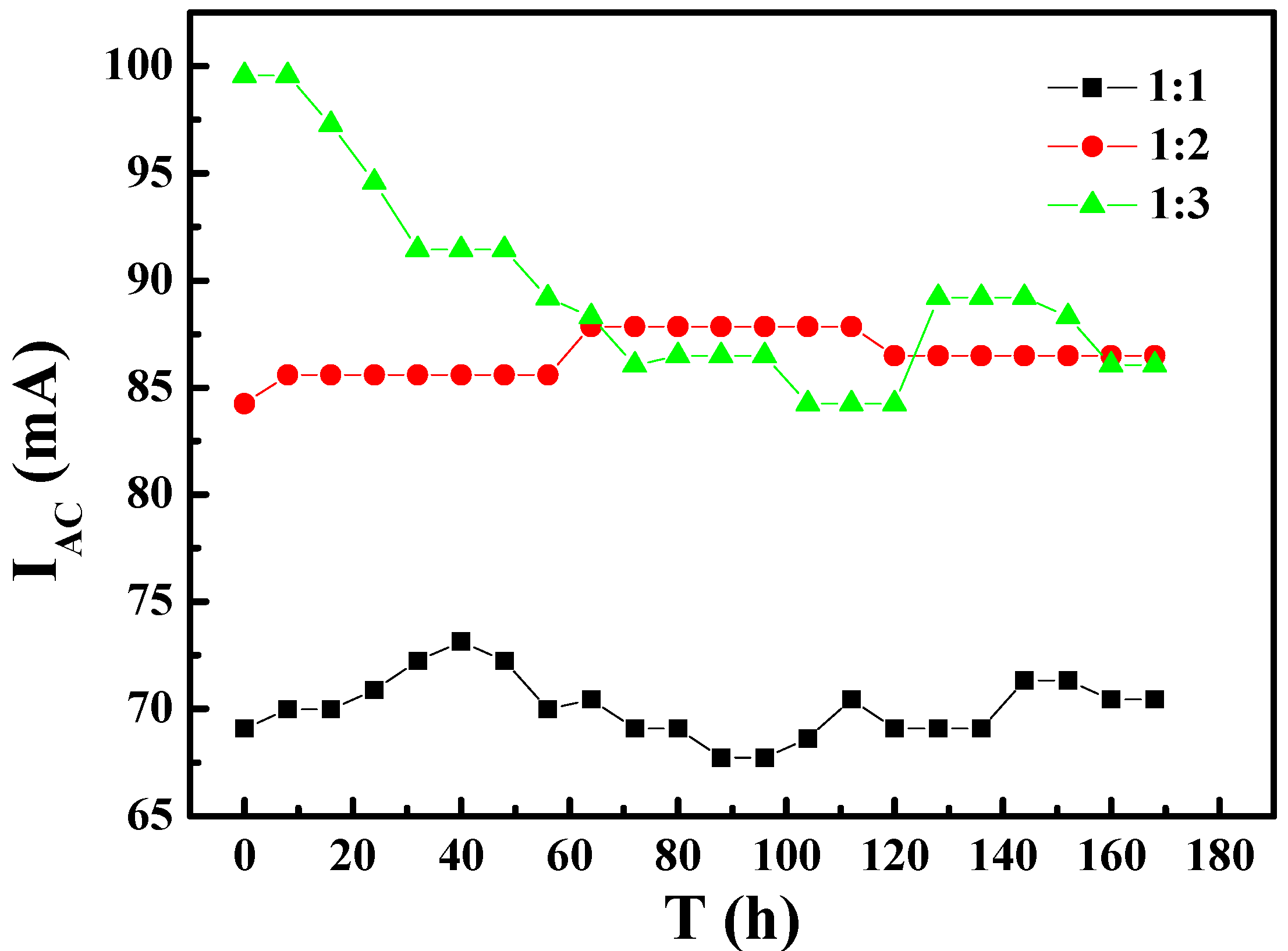
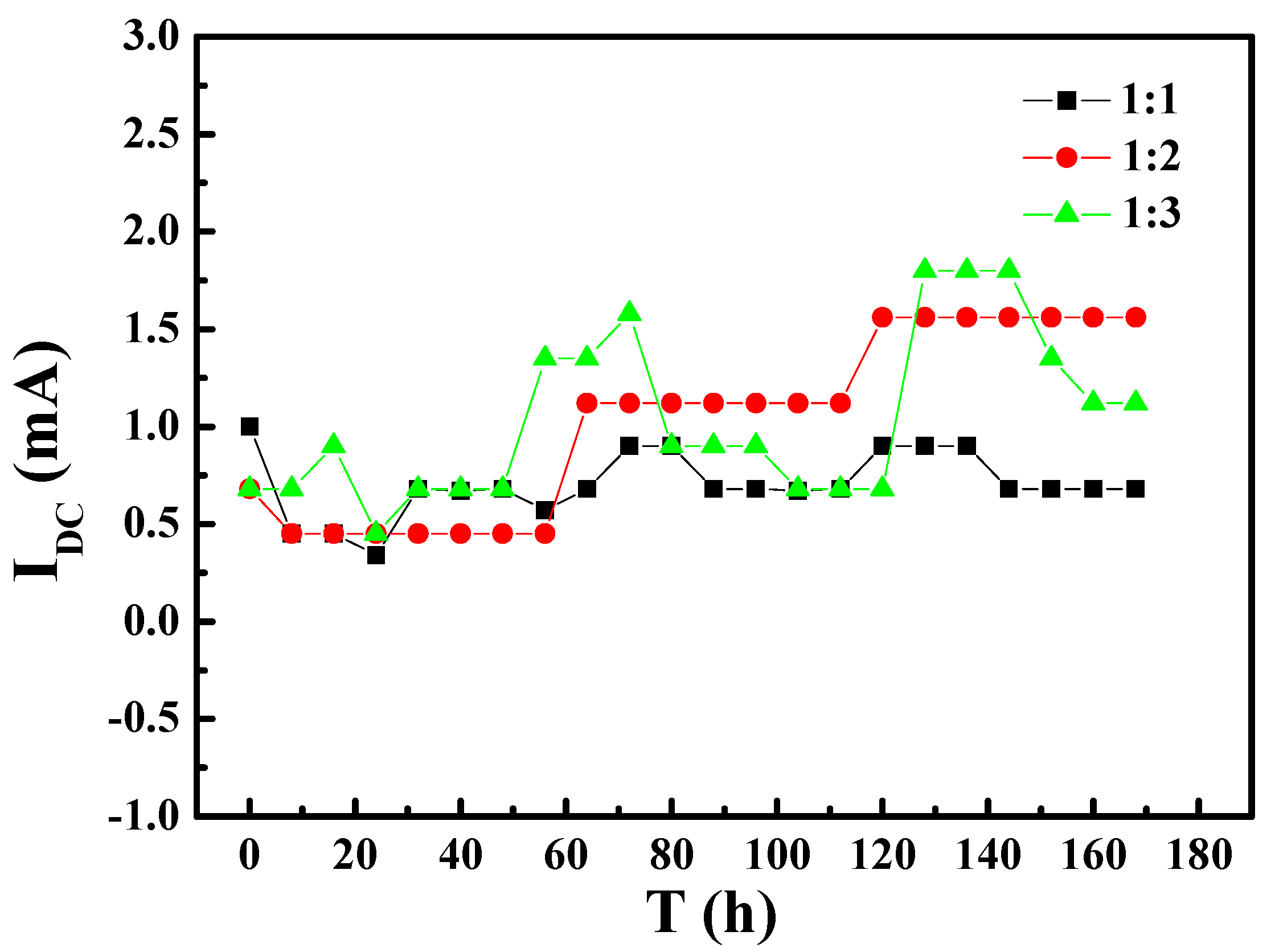
- The AC current induced by the AC interference on X80 steel with different coating defect areas increases with the same area of WE1 and increasing area of WE2. The DC signal was also induced between the two coating defects under AC interference.
- (3)
- Under the action of AC/DC currents, the corrosion rate of the X80 steel at the smaller coating defect increased significantly and that of X80 steel at the larger defect decreased gradually with the increase of the larger defect area at constant smaller defect area.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.G.; Zhang, D.W.; Liu, Z.Y.; Li, Z.; Du, C.W.; Dong, C.F. Share corrosion data. Nature 2015, 527, 441. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.; Kajiyama, F.; Fukuoka, T. Alternating current corrosion risk arising from alternating current-powered rail transit systems on cathodically protected buried steel pipelines and its measures. Corros. Eng. 2004, 60, 408–413. [Google Scholar] [CrossRef]
- Wang, S.; Du, C.W.; Li, X.G.; Liu, Z.Y.; Zhu, M.; Zhang, D.W. Field corrosion characterization of soil corrosion of X70 pipeline steel in a red clay soil. Prog. Nat. Sci. 2015, 25, 242–250. [Google Scholar] [CrossRef]
- Kuang, D.; Cheng, Y.F. AC corrosion at coating defect on pipelines. Corrosion 2014, 71, 267–276. [Google Scholar] [CrossRef]
- Yan, M.C.; Sun, C.; Xu, J.; Wu, T.; Yang, S.; Ke, W. Stress corrosion of pipeline steel under occluded coating disbondment in a red soil environment. Corros. Sci. 2015, 93, 27–38. [Google Scholar] [CrossRef]
- Yan, M.C.; Sun, C.; Xu, J.; Dong, J.H.; Ke, W. Role of Fe oxides in corrosion of pipeline steel in a red clay soil. Corros. Sci. 2014, 80, 309–317. [Google Scholar] [CrossRef]
- Yang, Y.; Akid, R. Electrochemical investigation of the corrosion of different microstructural phases of X65 pipeline steel under saturated carbon dioxide conditions. Materials 2015, 8, 2635–2649. [Google Scholar] [CrossRef]
- Zhu, M.; Du, C.W.; Li, X.G.; Liu, Z.Y.; Wang, S.R.; Li, J.K.; Zhang, D.W. Effect of AC current density on stress corrosion cracking behavior of X80 pipeline steel in high pH carbonate/bicarbonate solution. Electrochem. Acta 2014, 117, 351–359. [Google Scholar] [CrossRef]
- Tribollet, B.; Meyer, M. AC-induced corrosion of underground pipelines. Undergr. Pipeline Corros. 2014, 35–61. [Google Scholar] [CrossRef]
- Du, C.W.; Zhao, T.L.; Liu, Z.Y.; Li, X.G.; Zhang, D.W. Corrosion behavior and characteristics of the product film of API X100 steel in acidic simulated soil solution. Int. J. Min. Met. Mater. 2016, 23, 176–183. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization. Corros. Sci. 2012, 55, 54–60. [Google Scholar] [CrossRef]
- Qian, H.C.; Wang, L.T.; Wang, H.R.; Zheng, W.R.; Zhang, D.W.; Du, C.W. Electrochemical behavior and stress corrosion sensitivity of X70 steel under disbonded coatings in Korla soil solution. J. Mater. Eng. Perform. 2016, 25, 4657–4665. [Google Scholar] [CrossRef]
- Kuang, D.; Cheng, Y.F. Understand the AC induced pitting corrosion on pipelines in both high pH and neutral pH carbonate/bicarbonate solutions. Corros. Sci. 2014, 85, 204–210. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, X.H.; Cui, Z.Y.; Liu, Z.Y.; Du, C.W.; Li, X.G. Effect of alternating voltage on corrosion of X80 and X100 steels in a chloride containing solution—Investigated by AC voltammetry technique. Corros. Sci. 2014, 86, 213–222. [Google Scholar] [CrossRef]
- Kim, D.K.; Muralidharan, S.; Ha, T.H.; Bae, J.H.; Ha, Y.C.; Lee, H.G.; Scantlebury, G.D. Electrochemical studies on the alternating current corrosion of mild steel under cathodic protection condition in marine environments. Electrochim. Acta 2006, 51, 5259–5267. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion—Part 1: Effects on overpotentials of anodic and cathodic processes. Corros. Sci. 2010, 52, 491–497. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion. Part 2: Parameters influencing corrosion rates. Corros. Sci. 2010, 52, 916–922. [Google Scholar] [CrossRef]
- Kuang, D.; Cheng, Y.F. Effect of alternating current interference on coating disbondment and cathodic protection shielding on pipelines. Corros. Eng. Sci. Techn. 2015, 50, 211–217. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Lin, X.A. A theoretical approach for predicting AC-induced corrosion. Corros. Sci. 1994, 36, 1039–1046. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Zhang, G. The corrosion of carbon steel in a chloride environment due to periodic voltage modulation: Part I. Corros. Sci. 1995, 37, 1567–1582. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Zhang, G. The corrosion of carbon steel in a chloride environment due to periodic voltage modulation: Part II. Corros. Sci. 1995, 37, 1583–1598. [Google Scholar] [CrossRef]
- Zhang, R.; Vairavanathan, P.R.; Lalvani, S.B. Perturbation method analysis of AC-induced corrosion. Corros. Sci. 2008, 50, 1664–1671. [Google Scholar] [CrossRef]
- Ono, S.; Habazaki, H. Role of cathodic half-cycle on AC etch process of aluminium. Corros. Sci. 2010, 52, 2164–2171. [Google Scholar] [CrossRef]
- Xu, L.Y.; Su, X.; Cheng, Y.F. Effect of alternating current on cathodic protection on pipelines. Corros. Sci. 2013, 66, 263–268. [Google Scholar] [CrossRef]
- Xu, L.Y.; Su, X.; Yin, Z.X.; Tang, Y.H.; Cheng, Y.F. Development of a real-time AC/DC data acquisition technique for studies of AC corrosion of pipelines. Corros. Sci. 2012, 61, 215–223. [Google Scholar] [CrossRef]
- Fu, A.Q.; Cheng, Y.F. Effects of alternating current on corrosion of a coated pipeline steel in a chloride-containing carbonate/bicarbonate solution. Corros. Sci. 2010, 52, 612–619. [Google Scholar] [CrossRef]

| pH | NaCl | Na2SO4 | NaHCO3 | KNO3 | MgCl2·6H2O | CaCl2 |
|---|---|---|---|---|---|---|
| 9.4 | 3.4945 | 1.2603 | 0.146 | 0.2152 | 0.3383 | 0.1221 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, C.; Qian, H.; Li, J.; Huang, L.; Du, C. Corrosion Behavior of X80 Steel with Coupled Coating Defects under Alternating Current Interference in Alkaline Environment. Materials 2017, 10, 720. https://doi.org/10.3390/ma10070720
Li Z, Li C, Qian H, Li J, Huang L, Du C. Corrosion Behavior of X80 Steel with Coupled Coating Defects under Alternating Current Interference in Alkaline Environment. Materials. 2017; 10(7):720. https://doi.org/10.3390/ma10070720
Chicago/Turabian StyleLi, Zhong, Caiyu Li, Hongchang Qian, Jun Li, Liang Huang, and Cuiwei Du. 2017. "Corrosion Behavior of X80 Steel with Coupled Coating Defects under Alternating Current Interference in Alkaline Environment" Materials 10, no. 7: 720. https://doi.org/10.3390/ma10070720




