Effect of Nanosized NbC Precipitates on Hydrogen Diffusion in X80 Pipeline Steel
Abstract
:1. Introduction
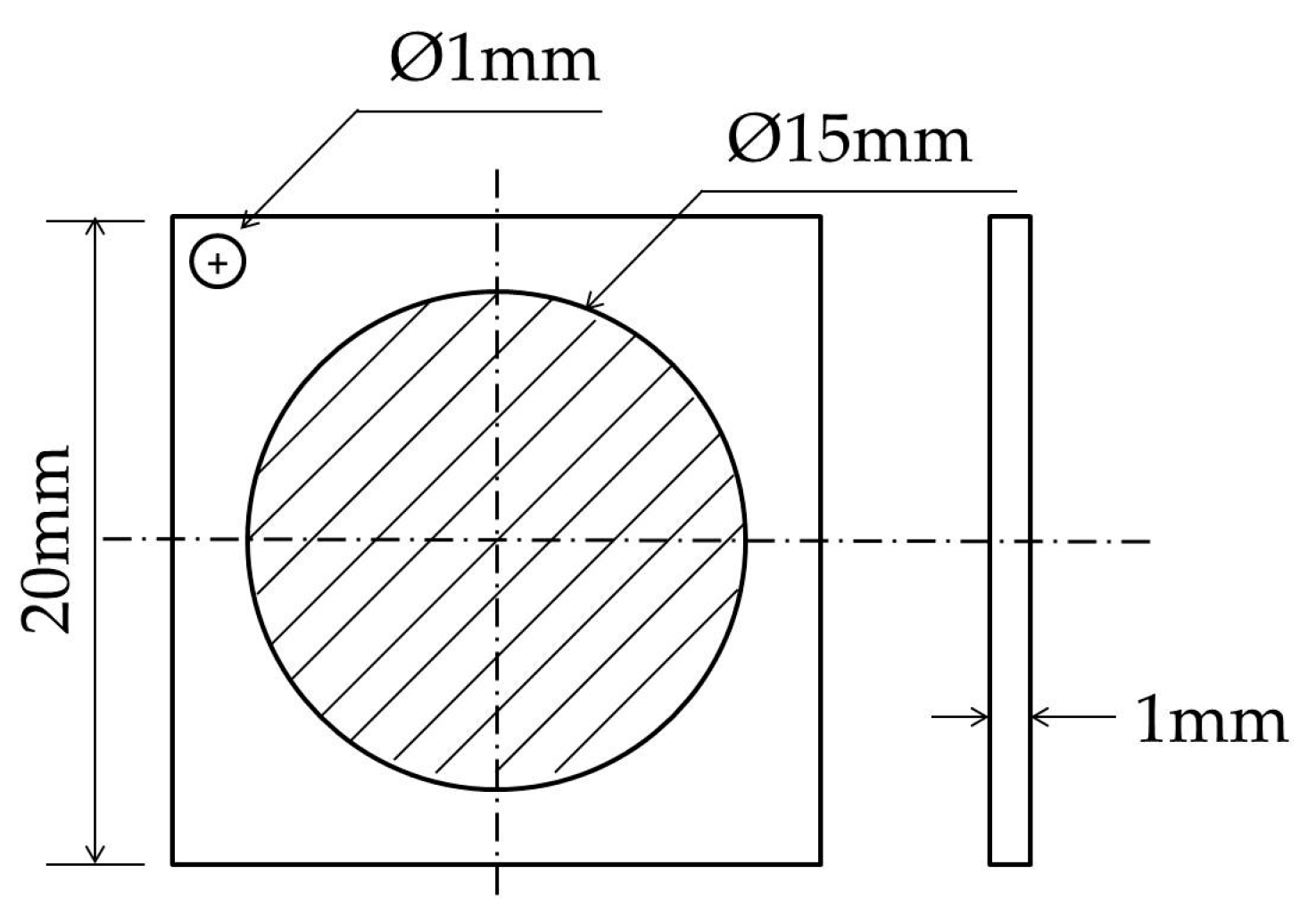
2. Test Materials and Methods
2.1. Test Materials
2.2. Test Methods
2.2.1. Microstructure Observation
2.2.2. Thermal Desorption Spectroscopy
2.2.3. Electrochemical Hydrogen Permeation
3. Results and Discussion
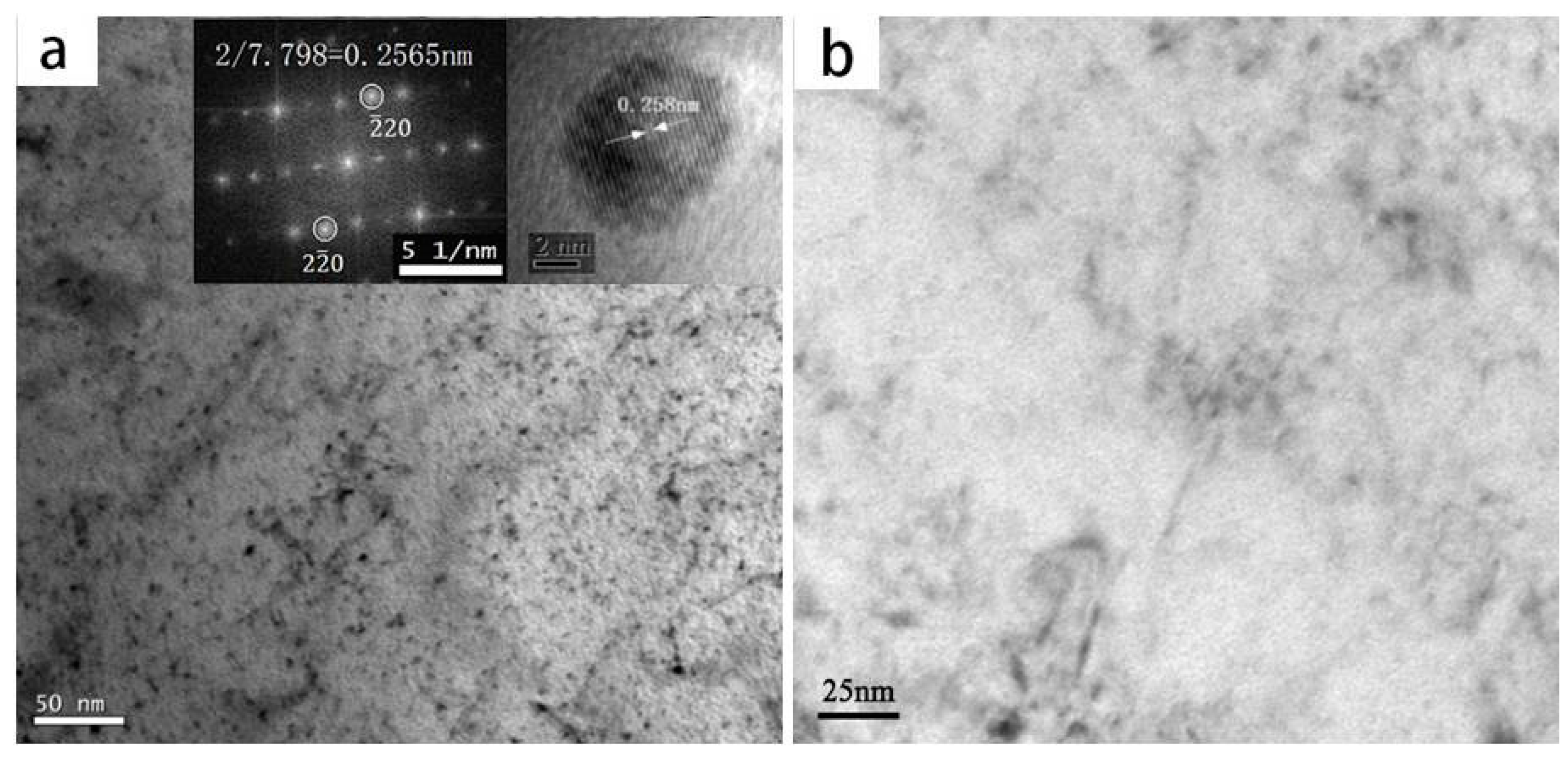
3.1. Microstructure and Precipitates
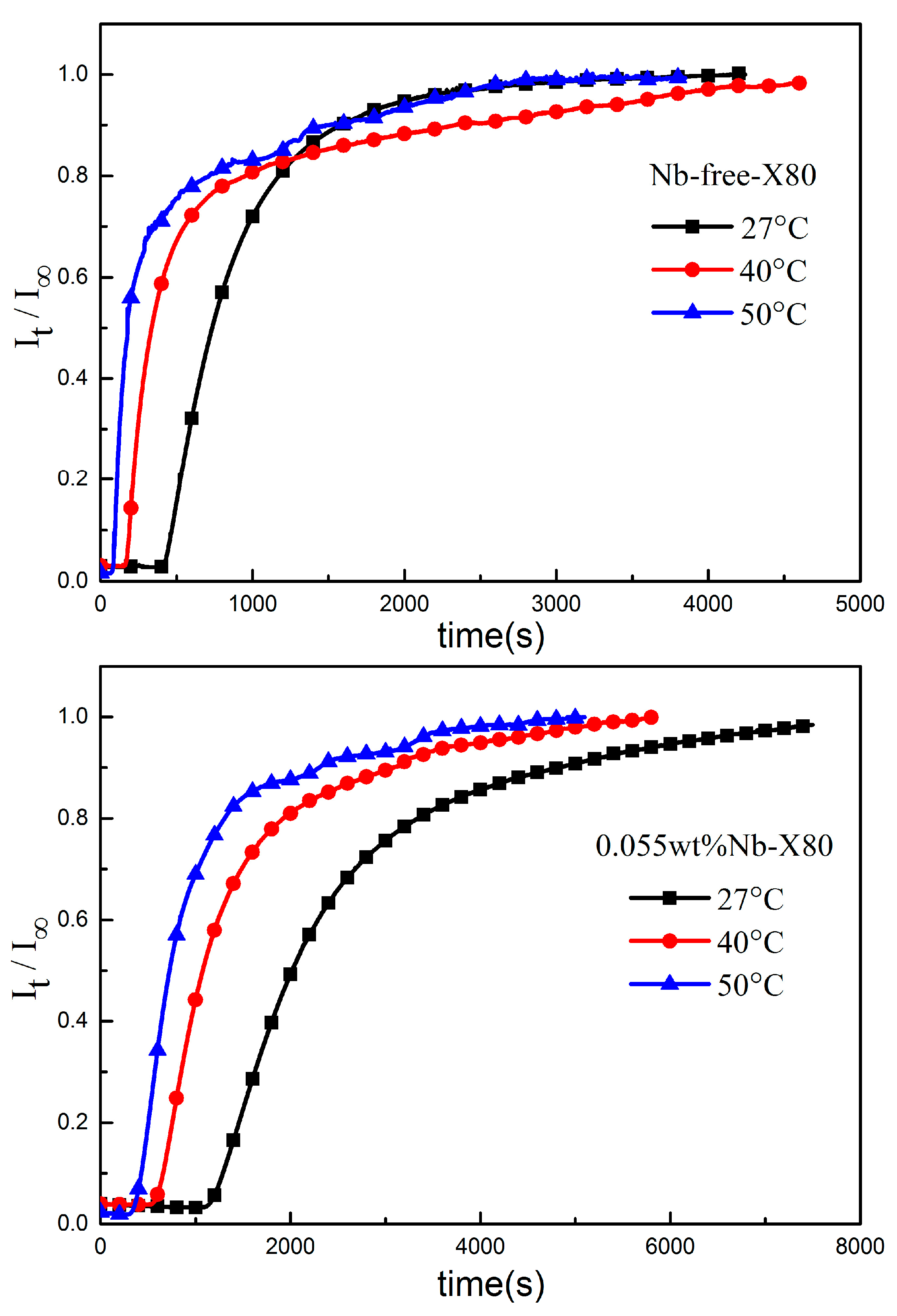
3.2. Hydrogen Diffusion in Steel with and without NbC
3.3. Effects of Traps on Hydrogen Diffusion
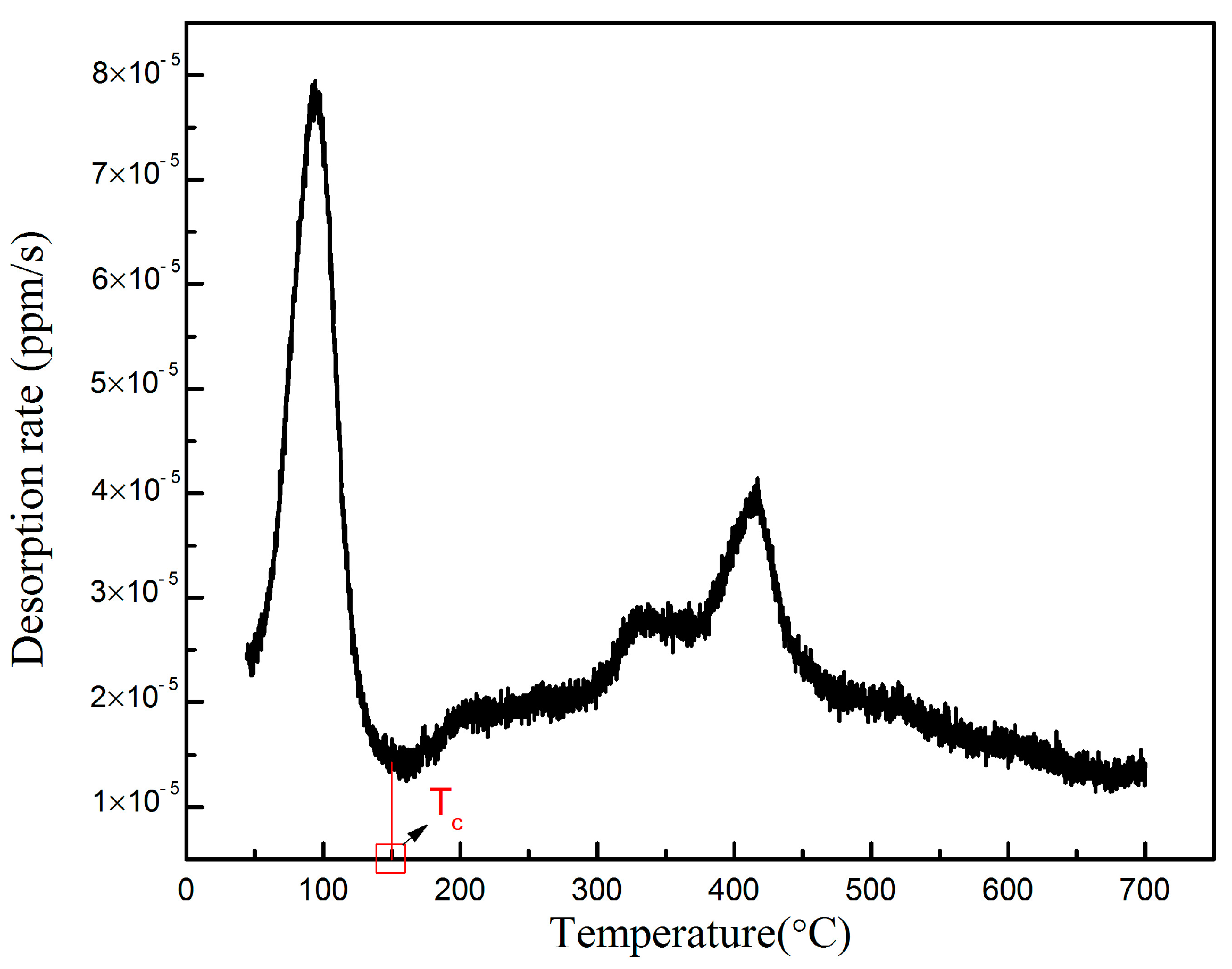
3.3.1. Types of Hydrogen Trap
3.3.2. Hindering Effects of Traps on Hydrogen Permeation
4. Conclusions
- The relationship between the diffusion coefficient of hydrogen and temperature in Nb-free X80 steel was established as lnD = −5024.89/T + 3.57 cm2/s, and the relationship in 0.055 wt% Nb X80 steel was found to be lnD = −4757.94/T + 1.73 cm2/s. The equation of Nb-bearing steel provides an alternative for the prediction of hydrogen diffusion in high-strength low-alloy steels with the Nb microalloying element.
- For 0.055 wt% Nb X80 steel, the critical temperature between the reversible and irreversible traps was measured as 150 °C, and the ratio of hydrogen captured by the reversible traps, relative to that of the irreversible traps, was determined to be 4:9.
- The irreversible hydrogen traps in Nb-bearing X80 steel were formed by NbC precipitates with sizes of 3~10 nm, which were misfit and were coherent with the bcc-Fe matrix. The irreversible traps markedly hindered hydrogen diffusion in steel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bueno, A.H.S.; Moreira, E.D.; Gomes, J.A.C.P. Evaluation of stress corrosion cracking and hydrogen embrittlement in an API grade steel. Eng. Fail. Anal. 2014, 36, 423–431. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Razavi-Tousi, S.S. A comparative study of hydrogen induced cracking behavior in API 5L X60 and X70 pipeline steels. Eng. Fail. Anal. 2013, 33, 163–175. [Google Scholar] [CrossRef]
- Addach, H.; Bercot, P.; Rezrazi, M.; Takadoum, J. Study of the electrochemical permeation of hydrogen in iron. Corros. Sci. 2009, 51, 263–267. [Google Scholar] [CrossRef]
- Zhao, W.M.; Zhang, T.M.; Zhao, Y.J.; Sun, J.B.; Wang, Y. Hydrogen permeation and embrittlement susceptibility of X80 welded joint under high-pressure coal gas environment. Corros. Sci. 2016, 111, 84–97. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The adsorption and diffusion of electrolytic hydrogen in palladium. Proc. R. Soc. Lond. Ser. A 1962, 270, 90–102. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates. J. Electrochem. Soc. 1964, 111, 619–623. [Google Scholar] [CrossRef]
- Padhy, G.K.; Komizo, Y.I. Diffusible hydrogen in steel weldments: A status review. Trans. JWRI 2013, 42, 39–62. [Google Scholar]
- Hong, S.G.; Kang, K.B.; Park, C.G. Strain-induced precipitation of NbC in Nb and Nb-Ti microalloy HSLA steels. Scr. Mater. 2002, 46, 163–168. [Google Scholar] [CrossRef]
- Spencer, G.L.; Duquette, D.J. The Role of Vanadium Carbide Traps in Reducing the Hydrogen Embrittlement Susceptibility of High Strength Alloy Steels; Technical Report: Arccb-TR-98016; Benet Laboratories, Army Armament Research, Development, and Engineering Center: Watervliet, NY, USA, 1998. [Google Scholar]
- Nagao, A.; Martin, M.L.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The effect of nanosized (Ti,Mo) C precipitates on hydrogen embrittlement of tempered lath martensitic steel. Acta Mater. 2014, 74, 244–254. [Google Scholar] [CrossRef]
- Xian, A.P.; Li, P.J.; Wang, Y.K.; Li, X. The capture of hydrogen in precipitated TiC in low carbon steel. Acta Metall. Sin. 1986, 22, A181–A188. [Google Scholar]
- Grabke, H.J.; Riecke, E. Absorption and diffusion of hydrogen in steels. Mater. Tehnol. 2000, 34, 331–342. [Google Scholar]
- Wei, F.G.; Tsuzaki, K. Quantitative analysis on hydrogen trapping of TiC particles in steel. Metall. Mater. Trans. A 2006, 37, 331–353. [Google Scholar] [CrossRef]
- Wallaert, E.; Depover, T.; Arafin, M.; Verbeken, K. Thermal desorption spectroscopy evaluation of the hydrogen-trapping capacity of NbC and NbN precipitates. Metall. Mater. Trans. A 2014, 45, 2412–2420. [Google Scholar] [CrossRef]
- Wei, F.G.; Hara, T.; Tsuzaki, K. Nano-precipitates design with hydrogen trapping character in high strength steels. In Proceedings of the 2008 International Conference on Effects of Hydrogen on Materials, Jackson Lake Lodge, Moran, WY, USA, 7–10 September 2008; Somerday, B., Sofronis, P., Jones, R., Eds.; pp. 448–455. [Google Scholar]
- Huang, F.; Liu, J.; Deng, Z.J.; Cheng, J.H.; Lu, Z.H.; Li, X.G. Effect of microstructure and inclusions on hydrogen induced cracking susceptibility and hydrogen trapping efficiency of X120 pipeline steel. Mater. Sci. Eng. A 2010, 527, 6997–7001. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Huang, Y.H.; Sun, B.T.; Liao, Q.L.; Lu, H.Z.; Jian, B.; Mohrbacher, H.; Zhang, W.; Guo, A.M.; Zhang, Y. Effect of Nb on hydrogen-induced delayed fracture in high strength hot stamping steels. Mater. Sci. Eng. A 2015, 626, 136–143. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Huang, Y.H.; Liao, Q.L.; Zhao, L.C.; Hong, J.Y.; Zhang, Y. Hydrogen diffusion and hydrogen-induced delayed cracking of ultra-high strength steels for hot stamping. J. Iron Steel Res. 2014, 26, 47–52. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zhang, T.Y. Effects of absorption and desorption on hydrogen permeation-II. Experimental measurements of activation energies. Acta Mater. 1998, 46, 5035–5043. [Google Scholar] [CrossRef]
- Cheng, Y.F. Analysis of electrochemical hydrogen permeation through X-65 pipeline steel and its implications on pipeline stress corrosion cracking. Int. J. Hydrogen Energy 2007, 32, 1269–1276. [Google Scholar] [CrossRef]
- Svoboda, J.; Mori, G.; Prethaler, A.; Fischer, F.D. Determination of trapping parameters and the chemical diffusion coefficient from hydrogen permeation experiments. Corros. Sci. 2014, 82, 93–100. [Google Scholar] [CrossRef]
- Chen, Y.X.; Chang, Q.G. Effect of hydrogen trap on hydrogen diffusion coefficient in 20 g pure steel. Acta Metall. Sin. 2011, 47, 548–552. [Google Scholar]



| Material | C | Si | Mn | P | S | Nb | Ti | Mo | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| Nb-free | 0.058 | 0.28 | 1.85 | 0.004 | 0.006 | 0 | 0.016 | 0.26 | 0.26 | 0.26 |
| 0.055 wt% Nb | 0.060 | 0.27 | 1.84 | 0.004 | 0.005 | 0.055 | 0.015 | 0.25 | 0.26 | 0.26 |
| Parameters | T/°C | |||
|---|---|---|---|---|
| 27 | 40 | 50 | ||
| Nb-free-X80 | Penetration time tb/s | 403 | 156 | 80 |
| Lag time t0.63/s | 869 | 444 | 263 | |
| Apparent diffusion coefficient Dapp/(cm2·s−1) | 1.92 × 10−6 | 3.75 × 10−6 | 6.34 × 10−6 | |
| Stable current density I∞/(µA·cm−2) | 4.88 | 6.39 | 8.32 | |
| Surface hydrogen concentration C0/(µmol·cm−3) | 2.63 | 1.77 | 1.36 | |
| 0.055 wt% Nb-X80 | Penetration time tb/s | 1135 | 565 | 370 |
| Lag time t0.63/s | 2390 | 1301 | 883 | |
| Apparent diffusion coefficient Dapp/(cm2·s−1) | 6.97 × 10−7 | 1.28 × 10−6 | 1.89 × 10−6 | |
| Stable current density I∞/(µA·cm−2) | 4.99 | 7.31 | 7.96 | |
| Surface hydrogen concentration C0/(µmol·cm−3) | 7.42 | 5.92 | 4.37 | |
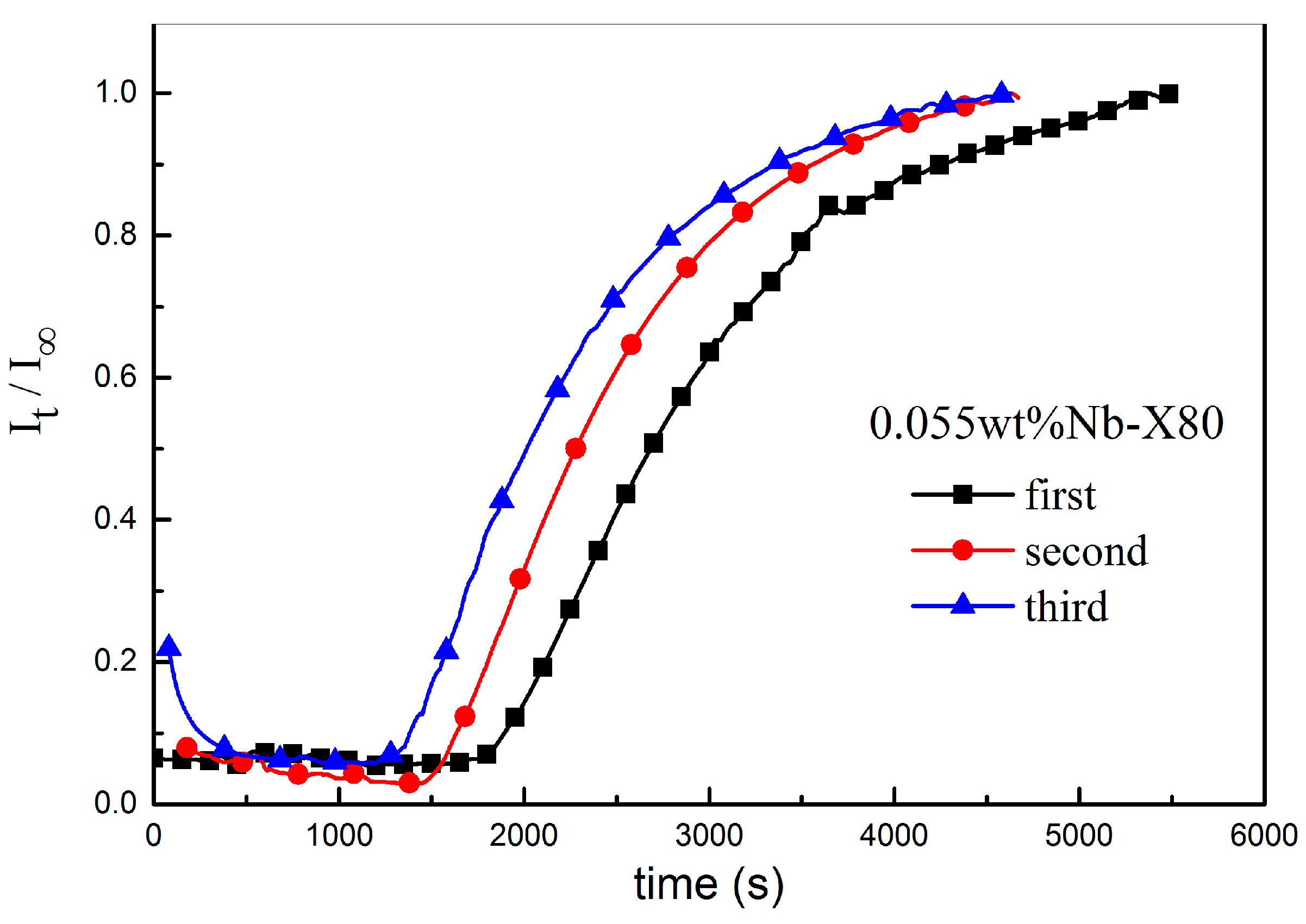
| Parameters | First | Second | Third |
|---|---|---|---|
| penetration time tb/s | 1698 | 1468 | 1236 |
| lag time t0.63/s | 2990 | 2542 | 2282 |
| Dapp/(cm2 s−1) | 5.57 × 10−7 | 6.56 × 10−7 | 7.30 × 10−7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Q.; Wu, J.; Xie, D.; Wu, X.; Huang, Y.; Li, X. Effect of Nanosized NbC Precipitates on Hydrogen Diffusion in X80 Pipeline Steel. Materials 2017, 10, 721. https://doi.org/10.3390/ma10070721
Cui Q, Wu J, Xie D, Wu X, Huang Y, Li X. Effect of Nanosized NbC Precipitates on Hydrogen Diffusion in X80 Pipeline Steel. Materials. 2017; 10(7):721. https://doi.org/10.3390/ma10070721
Chicago/Turabian StyleCui, Qiaoqi, Junsheng Wu, Donghan Xie, Xiaoguang Wu, Yunhua Huang, and Xiaogang Li. 2017. "Effect of Nanosized NbC Precipitates on Hydrogen Diffusion in X80 Pipeline Steel" Materials 10, no. 7: 721. https://doi.org/10.3390/ma10070721






