Hydrothermal Fabrication of Highly Porous Titanium Bio-Scaffold with a Load-Bearable Property
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Porous Ti Samples
2.2. Surface Characterization
2.3. Compression and Nanoindentation Tests
2.4. In Vitro Tests
3. Results and Discussion
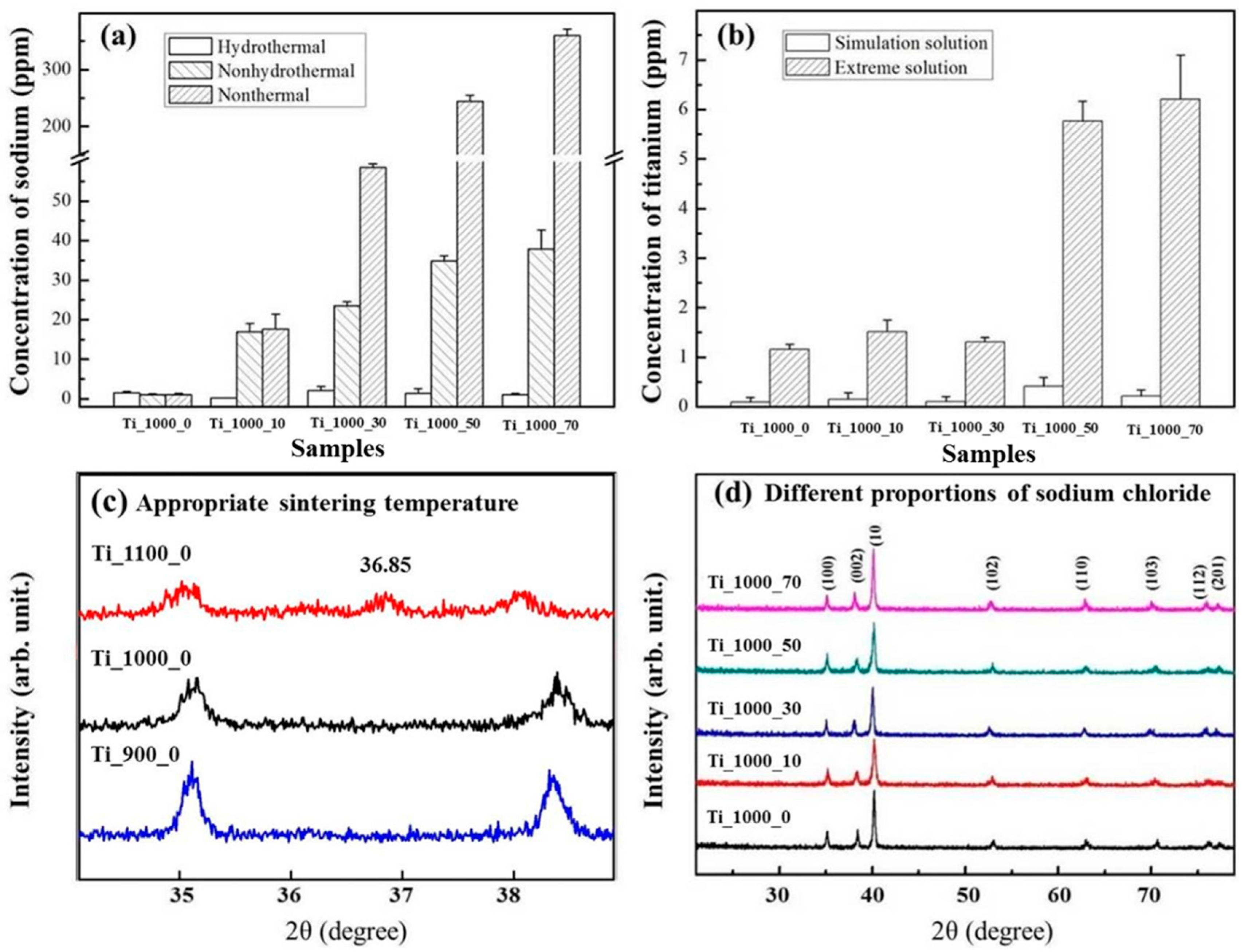
3.1. Composition of As-Prepared Porous Ti Samples
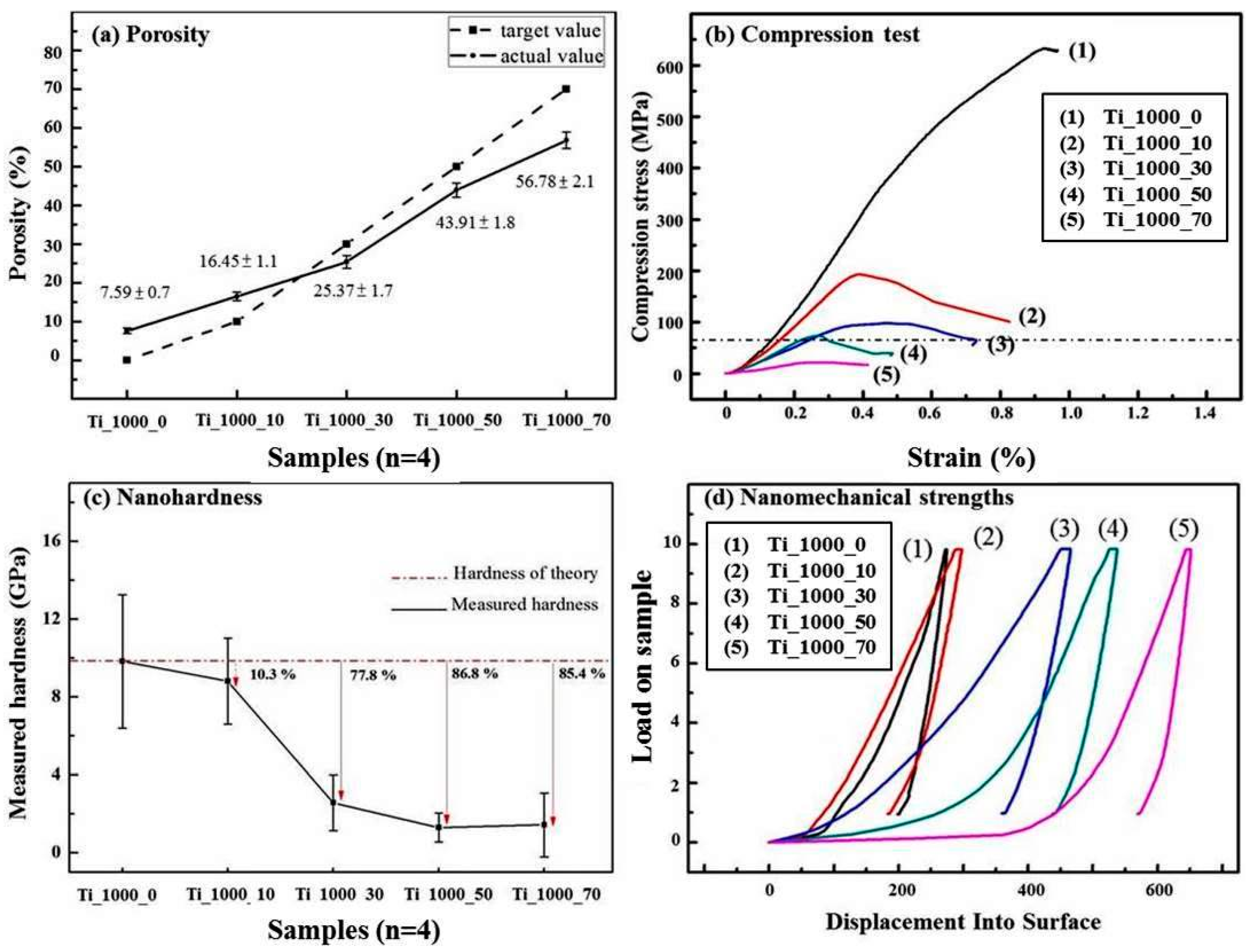
3.2. Load-Bearing Capacity of As-Prepared Porous Ti Samples
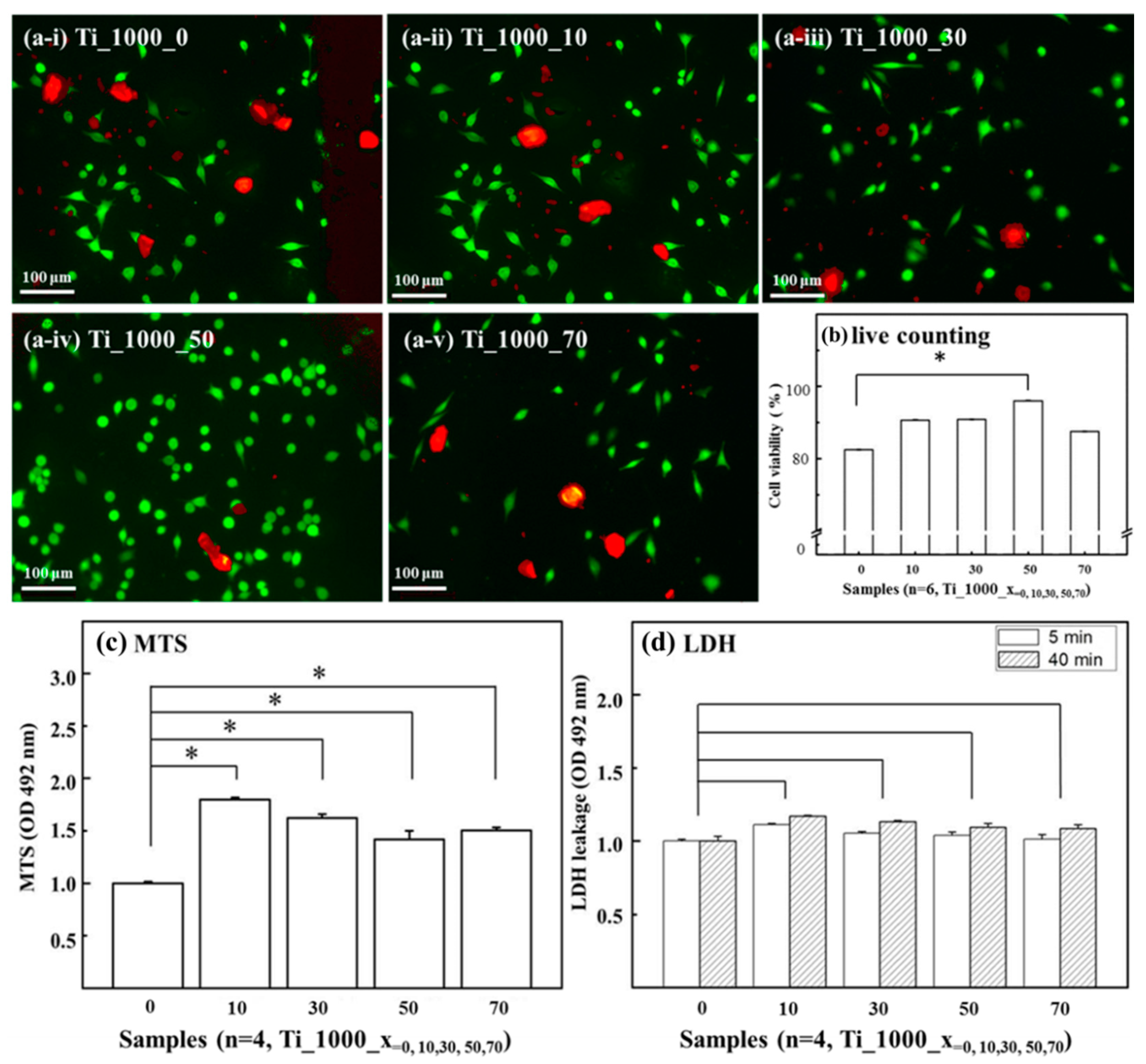
3.3. Cell Affinity of Porous Ti Samples
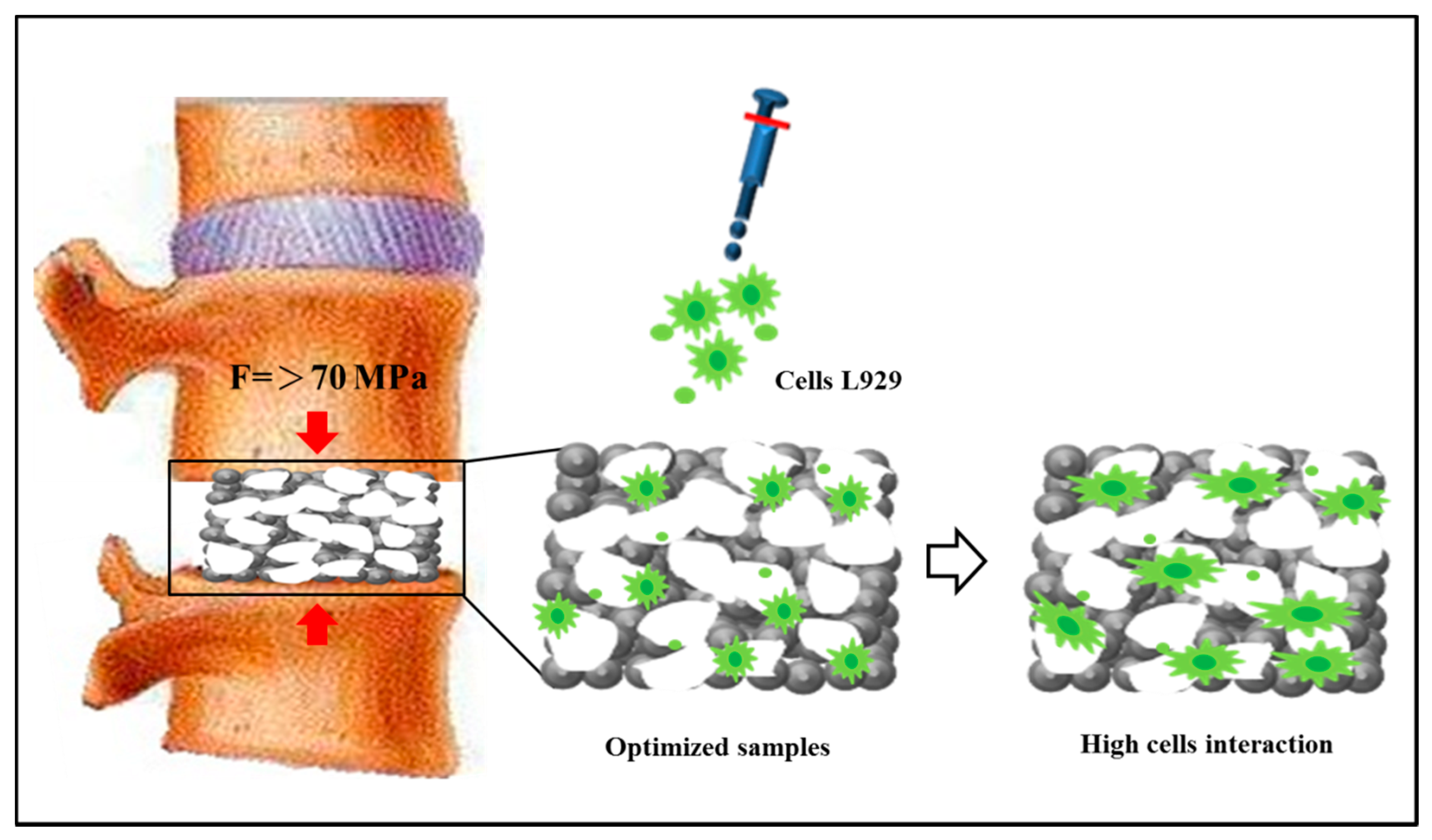
3.4. Comparison with Commercially Available Porous Ti-Based Scaffolds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nouri, A.; Hodgson, P.D.; Wen, C.E. Biomimetic Porous Titanium Scaffolds for Orthopaedic and Dental Applications; InTech: Hampshire, UK, 2010; pp. 415–450. [Google Scholar]
- Burr, D.B.; Martin, R.B. Errors in bone remodeling: Toward a unified theory of metabolic bone disease. Am. J. Anat. 1989, 186, 186–216. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Rajangam, T.; An, S.S.A. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int. J. Nanomed. 2013, 8, 3641–3662. [Google Scholar]
- Bansiddhi, A.; Sargeant, T.D.; Stupp, S.I.; Dunand, D.C. Porous NiTi for bone implants: A review. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Imwinkelried, T. Mechanical properties of open-pore titanium foam. J. Biomed. Mater. Res. Part A 2007, 81, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, G.; Alaqeeli, A.; Al Jawadi, A.; Foote, N.; Baron, E.; Albustanji, A. Reconstruction of residual mandibular defects by iliac crest bone graft in war-wounded Iraqi civilians, 2006–2011. Br. J. Oral Maxillofac. Surg. 2015, 53, e27–e31. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Coutinho, O.P.; Ducheyne, P.; Reis, R.L. Materials in particulate form for tissue engineering. J. Tissue Eng. Regen. Med. 2007, 1, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandal, S.; Barui, S.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basu, B. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater. Sci. Eng. R Rep. 2016, 103, 1–39. [Google Scholar] [CrossRef]
- Jaeggi, C.; Frauchiger, V.; Eitel, F.; Stiefel, M.; Schmotzer, H.; Siegmann, S. The effect of surface alloying of Ti powder for vacuum plasma spraying of open porous titanium coatings. Acta Mater. 2011, 59, 717–725. [Google Scholar] [CrossRef]
- Li, B.Q.; Wang, C.Y.; Lu, X. Effect of pore structure on the compressive property of porous Ti produced by powder metallurgy technique. Mater. Des. 2013, 50, 613–619. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, H.D.; Kang, M.H.; Kim, H.E.; Koh, Y.H.; Estrin, Y. Fabrication of porous titanium scaffold with controlled porous structure and net-shape using magnesium as spacer. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, E.; Li, M.; Zeng, S. Preparation, microstructure and mechanical properties of porous titanium sintered by Ti fibres. J. Mater. Sci. Mater. Med. 2008, 19, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.A.; Lam, C.X.; Hutmacher, D.W.; Goh, J.C.; Wong, H.K. Biological performance of a polycaprolactone-based scaffold used as fusion cage device in a large animal model of spinal reconstructive surgery. Biomaterials 2009, 30, 5086–5093. [Google Scholar] [CrossRef] [PubMed]
- Youm, I.; Youan, B.B.C. Uptake mechanism of furosemide-loaded pegylated nanoparticles by cochlear cell lines. Hear. Res. 2013, 304, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Monjo, M.; Rubert, M.; Verket, A.; Lyngstadaas, S.P.; Ellingsen, J.E.; Wohlfahrt, J.C. Porous ceramic titanium dioxide scaffolds promote bone formation in rabbit peri-implant cortical defect model. Acta Biomater. 2013, 9, 5390–5399. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T.T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.; Basirun, W.J.; Abas, W.A.B.W. Ceramic tantalum oxide thin film coating to enhance the corrosion and wear characteristics of Ti 6Al 4V alloy. J. Alloys Compd. 2016, 676, 369–376. [Google Scholar] [CrossRef]
- Ribeiro Filho, S.L.M.; Lauro, C.H.; Bueno, A.H.S.; Brandão, L.C. Influence cutting parameters on the surface quality and corrosion behavior of Ti-6Al-4V alloy in synthetic body environment (SBF) using Response Surface Method. Measurement 2016, 88, 223–237. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W.A.B.W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Ye, B.; Dunand, D.C. Titanium foams produced by solid-state replication of NaCl powders. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2010, 528, 691–697. [Google Scholar] [CrossRef]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.; Rodriguez, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Mondal, D.P.; Majumdar, J.D.; Badkul, A.; Jha, A.K.; Khare, A.K. Highly porous open cell Ti-foam using NaCl as temporary space holder through powder metallurgy route. Mater. Des. 2013, 47, 810–819. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 2011, 1, 3–56. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, J.S.; Hu, R.; Kou, H.C.; Zhou, L. Mechanical properties of porous titanium with different distributions of pore size. Trans. Nonferr. Met. Soc. China 2013, 23, 2317–2322. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Oxidation of Ti-6Al-4V alloy. J. Alloys Compd. 2009, 472, 241–246. [Google Scholar] [CrossRef]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Arifvianto, B.; Leeflang, M.A.; Zhou, J. The compression behaviors of titanium/carbamide powder mixtures in the preparation of biomedical titanium scaffolds with the space holder method. Powder Technol. 2015, 284, 112–121. [Google Scholar] [CrossRef]
- Aydoğmuş, T.; Bor, Ş. Processing of porous TiNi alloys using magnesium as space holder. J. Alloys Compd. 2009, 478, 705–710. [Google Scholar] [CrossRef]
- Mansourighasri, A.; Muhamad, N.; Sulong, A.B. Processing titanium foams using tapioca starch as a space holder. J. Mater. Process. Technol. 2012, 212, 83–89. [Google Scholar] [CrossRef]
- Torres, Y.; Pavón, J.J.; Rodríguez, J.A. Processing and characterization of porous titanium for implants by using NaCl as space holder. J. Mater. Process. Technol. 2012, 212, 1061–1069. [Google Scholar] [CrossRef]
- Esen, Z.; Bor, Ş. Processing of titanium foams using magnesium spacer particles. Scr. Mater. 2007, 56, 341–344. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.W.; Li, J.G.; Sun, X.D. Preparation and mechanical property of a novel 3D porous magnesium scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Caparrós, C.; Ortiz-Hernandez, M.; Molmeneu, M.; Punset, M.; Calero, J.A.; Aparicio, C.; Gil, F.J. Bioactive macroporous titanium implants highly interconnected. J. Mater. Sci. Mater. Med. 2016, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Ting, L.I.; Li, Y.M.; Hao, H.E.; Hu, Y.H. Porous titanium implants fabricated by metal injection molding. Trans. Nonferr. Met. Soc. China 2009, 19, 1174–1179. [Google Scholar] [CrossRef]
- Torres, Y.; Trueba, P.; Pavón, J.J.; Chicardi, E.; Kamm, P.; García-Moreno, F.; Rodríguez-Ortiz, J.A. Design, processing and characterization of titanium with radial graded porosity for bone implants. Mater. Des. 2016, 110, 179–187. [Google Scholar] [CrossRef]
- Jin, X.; Dong, L.; Xu, H.; Liu, L.; Li, N.; Zhang, X.; Han, J. Effects of porosity and pore size on mechanical and thermal properties as well as thermal shock fracture resistance of porous ZrB2–SiC ceramics. Ceram. Int. 2016, 42, 9051–9057. [Google Scholar] [CrossRef]
- Cimatti, B.; Engel, E.E.; Nogueira-Barbosa, M.H.; Frighetto, P.D.; Volpon, J.B. Physical and mechanical characterization of a porous cement for metaphyseal bone repair. Acta Ortop. Bras. 2015, 23, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Van de Graaf, G.M.M.; Zoppa, D.; do Valle, A.L.; Moreira, R.C.; Maestrelli, S.C.; Marques, R.F.C.; Campos, M.G.N. Morphological and mechanical characterization of chitosan-calcium phosphate composites for potential application as bone-graft substitutes. Res. Biomed. Eng. 2015, 31, 334–342. [Google Scholar] [CrossRef]
- Neacsu, P.; Gordin, D.M.; Mitran, V.; Gloriant, T.; Costache, M.; Cimpean, A. In vitro performance assessment of new beta Ti–Mo–Nb alloy compositions. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, S.; Hanawa, T.; Asami, K. Composition of surface oxide film of titanium with culturing murine fibroblasts L929. Biomaterials 2004, 25, 979–986. [Google Scholar] [CrossRef]
- Tianshi, W.; Renji, Z.; Yongnian, Y. Preparation of bioactive hydroxyapatite on pure titanium. J. Bioact. Compat. Polym. 2009, 24, 169–182. [Google Scholar] [CrossRef]
- Zhang, E.; Zou, C.; Yu, G. Surface microstructure and cell biocompatibility of silicon-substituted hydroxyapatite coating on titanium substrate prepared by a biomimetic process. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 298–305. [Google Scholar] [CrossRef]


| Sample | Pore Size (μm) | Porosity (%) | Mechanical Properties (MPa) | Biocompatibility |
|---|---|---|---|---|
| Ti_1000_50 | 340 ± 10 | 43.91 ± 1.8 | 73 | excellent |
| M-S | >300 | 80 | - | excellent |
| 848-05133 | <200 | - | - | excellent |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Liao, J.-D.; Sivashanmugan, K.; Liu, B.H.-C.; Su, Y.-H.; Yao, C.-K.; Juang, Y.-D. Hydrothermal Fabrication of Highly Porous Titanium Bio-Scaffold with a Load-Bearable Property. Materials 2017, 10, 726. https://doi.org/10.3390/ma10070726
Lee H, Liao J-D, Sivashanmugan K, Liu BH-C, Su Y-H, Yao C-K, Juang Y-D. Hydrothermal Fabrication of Highly Porous Titanium Bio-Scaffold with a Load-Bearable Property. Materials. 2017; 10(7):726. https://doi.org/10.3390/ma10070726
Chicago/Turabian StyleLee, Han, Jiunn-Der Liao, Kundan Sivashanmugan, Bernard Hao-Chih Liu, Yu-Han Su, Chih-Kai Yao, and Yung-Der Juang. 2017. "Hydrothermal Fabrication of Highly Porous Titanium Bio-Scaffold with a Load-Bearable Property" Materials 10, no. 7: 726. https://doi.org/10.3390/ma10070726





