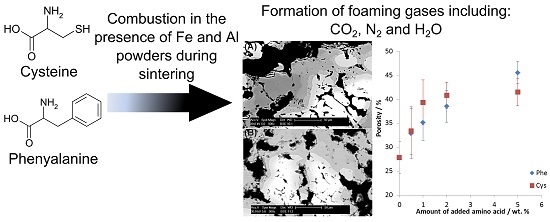
Amino Acids Aided Sintering for the Formation of Highly Porous FeAl Intermetallic Alloys
Abstract
:1. Introduction
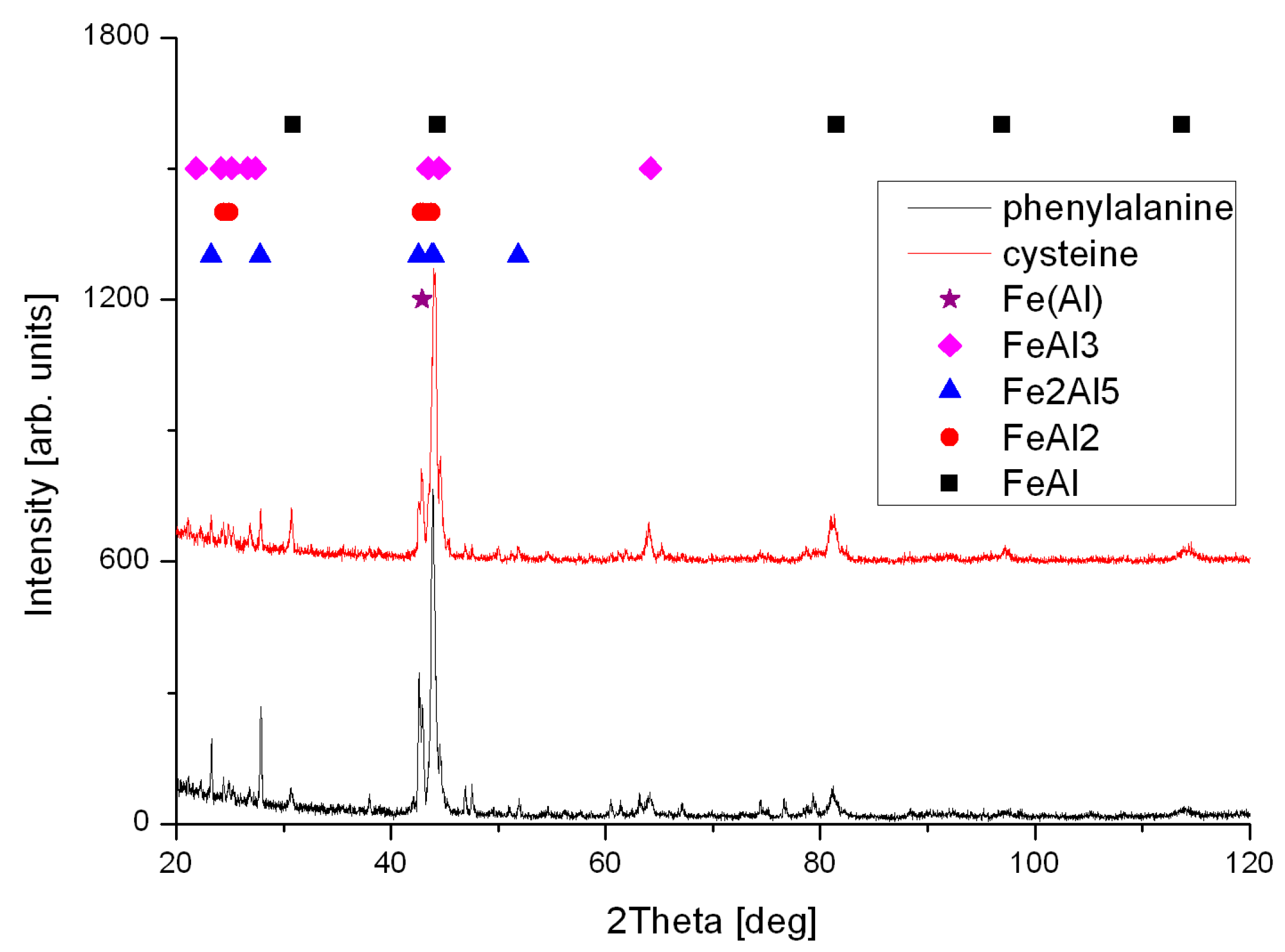
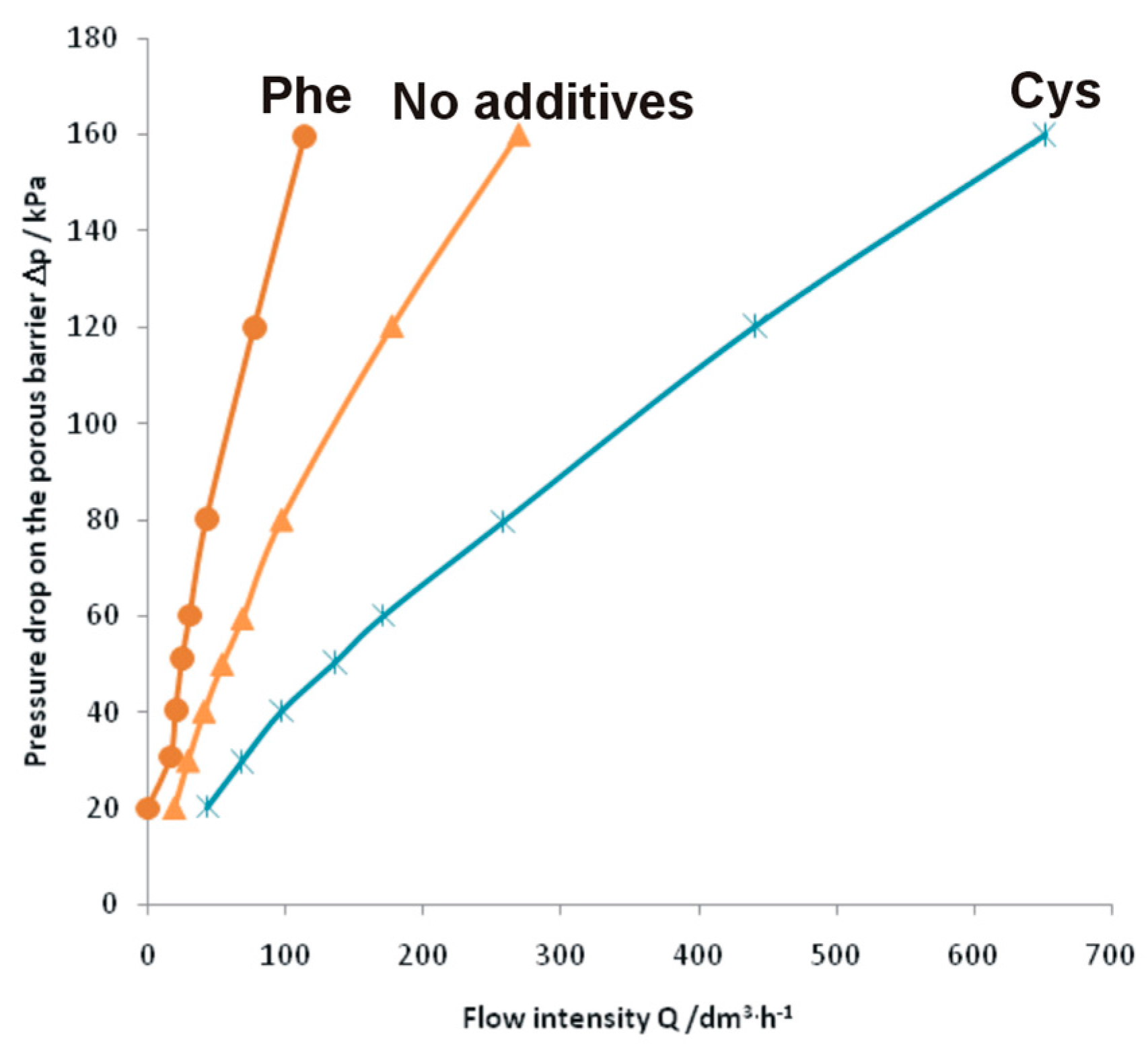
2. Results and Discussion
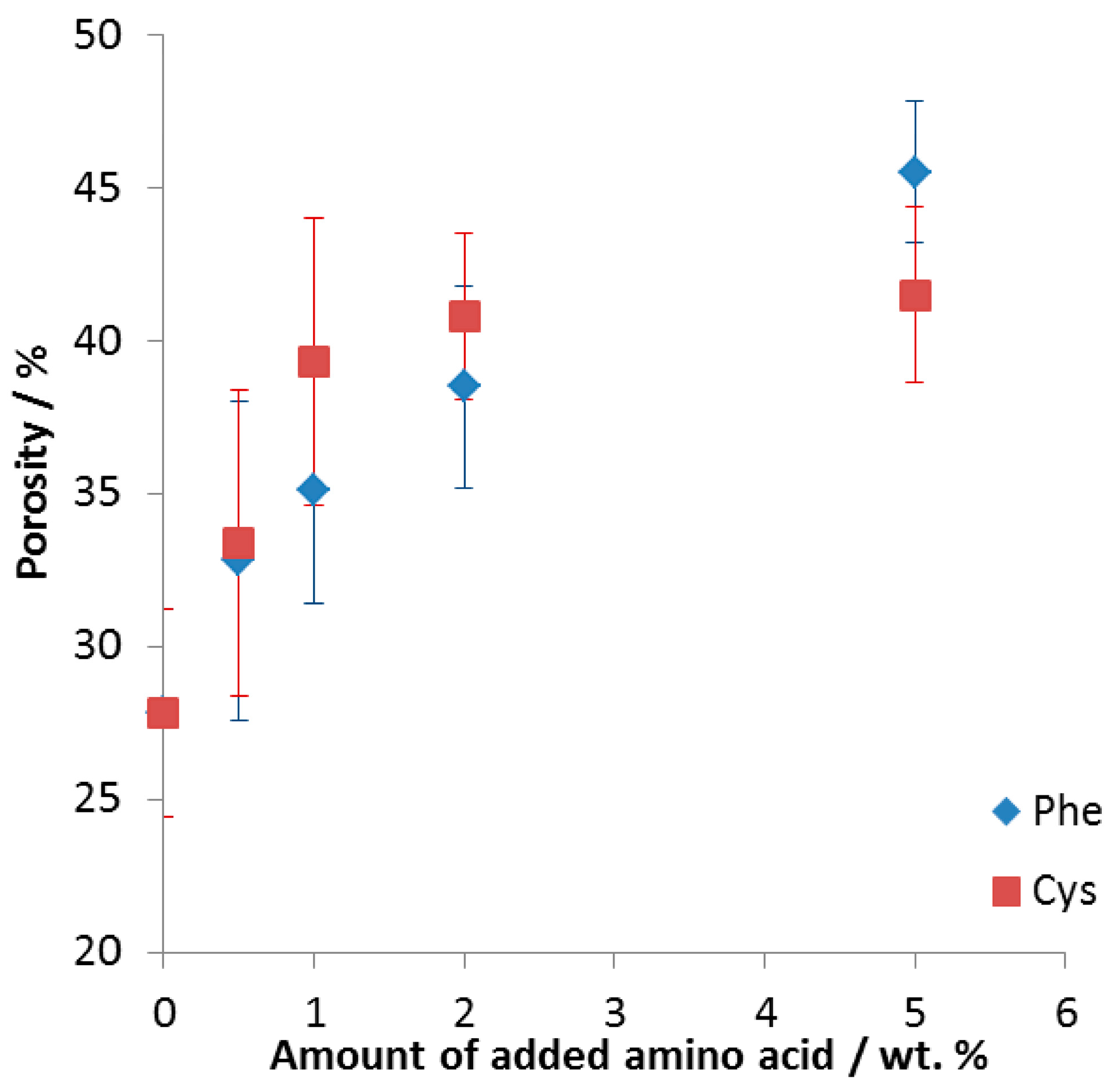
- Intermetallic foams were formed with porosity up to 42 ± 3% and 46 ± 2% for 5 wt % addition of cysteine and phenylalanine, respectively.
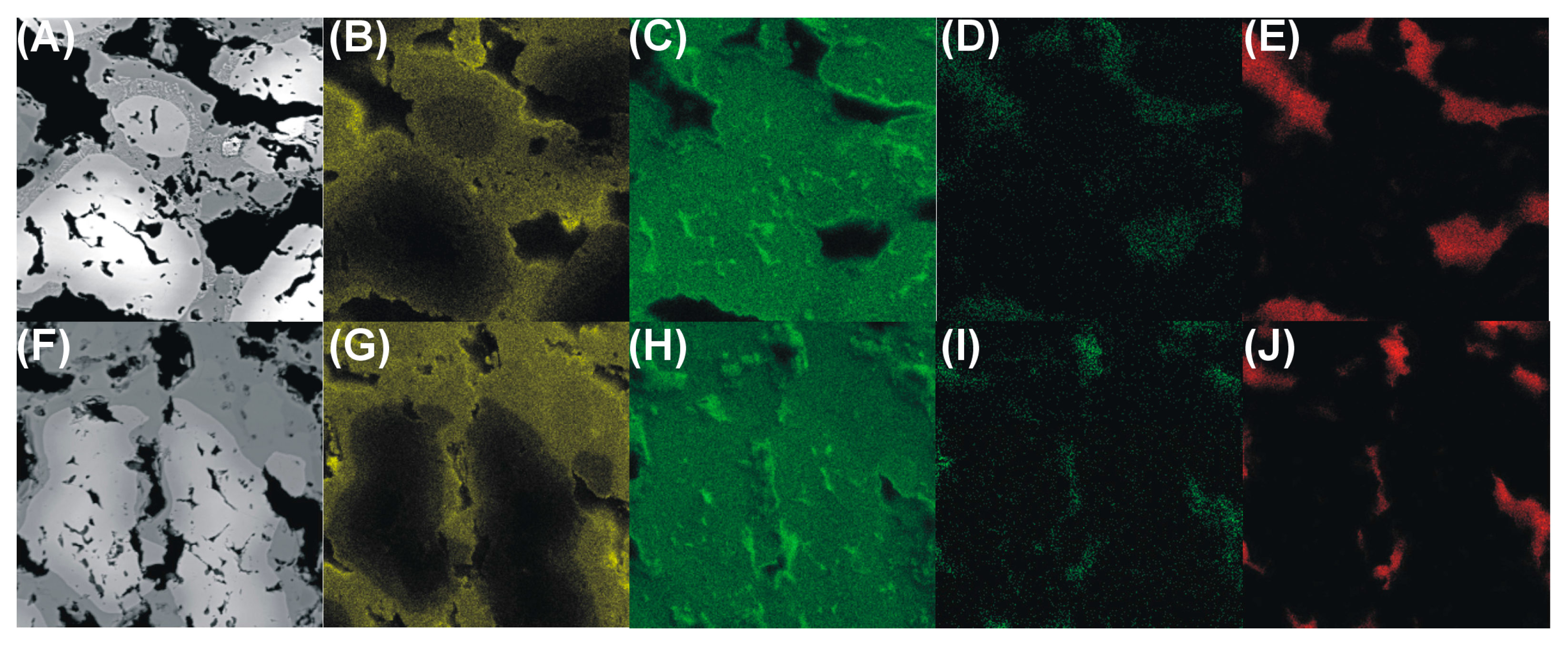
- Only traces of carbon and oxygen were noticed after the sintering, which allows us to conclude that gaseous nitrogen formed during the process made the leaching out of the combustion products easier.
- Only the intermetallic foams formed with the use of cysteine showed improved gas permeability, likely provided by numerous pore interconnections and thus enlarged path for the flowing gases, making them possible candidate materials for use as porous gas filters.
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Łyszkowski, R.; Bystrzycki, J. Hot deformation and processing maps of a Fe–Al intermetallic alloy. Mater. Charact. 2014, 96, 196–205. [Google Scholar] [CrossRef]
- Baker, I.; Gaydosh, D.J. Flow and fracture of Fe-AI. Mater. Sci. Eng. 1987, 96, 147–158. [Google Scholar] [CrossRef]
- Baker, I.; Yang, Y. On the yield stress anomaly in stoichiometric FeAl. Mater. Sci. Eng. 1997, 240, 109–117. [Google Scholar] [CrossRef]
- Nagpal, P.; Baker, I. Room temperature fracture of FeAI and NiAI. Mater. Charact. 1991, 1, 167–173. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Cinca, N.; Dosta, S.; Lima, C.R.C. High-temperature oxidation of Fe40Al coatings obtained by HVOF thermal spray. Intermetallics 2007, 15, 1384–1394. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Lima, C.R.C.; Cinca, N.; Miguel, J.R. Studies of Fe–40Al coatings obtained by high velocity oxy-fuel. Surf. Coat. Technol. 2006, 201, 2072–2079. [Google Scholar] [CrossRef]
- Verdian, M.M. Fabrication of FeAl (Cu) intermetallic coatings by plasma spraying of vacuum annealed powders. Vacuum 2016, 132, 5–9. [Google Scholar] [CrossRef]
- Cinca, N.; Roberto, C.; Lima, C.; Maria, J. An overview of intermetallics research and application: Status of thermal spray coatings. Integr. Med. Res. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Chawake, N.; Koundinya, N.T.B.N.; Kashyap, S.; Srivastav, A.K.; Yadav, D.; Mondal, R.A.; Sankar, R. Materials characterization formation of amorphous alumina during sintering of nanocrystalline B2 aluminides. Mater. Charact. 2016, 119, 186–194. [Google Scholar] [CrossRef]
- Senderowski, C.; Chodala, M.; Bojar, Z. Corrosion behavior of detonation gun sprayed Fe-Al type intermetallic coating. Materials 2015, 4, 1108–1123. [Google Scholar] [CrossRef]
- Józwiak, S.; Karczewski, K.; Bojar, Z. The effect of loading mode changes during the sintering process on the mechanical properties of FeAl intermetallic sinters. Intermetallics 2013, 33, 99–104. [Google Scholar] [CrossRef]
- Curfs, C.; Turrillas, X.; Vaughan, G.B.M.; Terry, A.E.; Kvick, A.; Rodríguez, M.A. Al–Ni intermetallics obtained by SHS; A time-resolved X-ray diffraction study. Intermetallics 2007, 15, 1163–1171. [Google Scholar] [CrossRef]
- Pochec, E.; Józwiak, S.; Karczewski, K.; Bojar, Z. Fe–Al phase formation around SHS reactions under isothermal conditions. J. Alloys Compd. 2011, 509, 1124–1128. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Casagrande, A.; Leonelli, C. Microwave ignition of the combustion synthesis of aluminides and field-related effects. J. Alloys Compd. 2016, 657, 59–67. [Google Scholar] [CrossRef]
- Hassani, A.; Habibolahzadeh, A.; Bafti, H. Production of graded aluminum foams via powder space holder technique. Mater. Des. 2012, 40, 510–515. [Google Scholar] [CrossRef]
- Goodall, R.; Despois, J.; Mortensen, A. The plasticity size effect in replicated microcellular aluminium. Scr. Mater. 2013, 69, 469–472. [Google Scholar] [CrossRef]
- Abdulla, T.; Yerokhin, A.; Goodall, R. Science Direct Enhancement in specific strength of open cell aluminium foams through plasma electrolytic oxidation treatment. Scr. Mater. 2014, 75, 38–41. [Google Scholar] [CrossRef]
- Ran, H.; Liu, Z.; Feng, P.; Xu, C.; Wang, J.; Akhtar, F. Processing, microstructure and properties of hierarchically porous Cu. Mater. Express 2016, 6, 271–276. [Google Scholar] [CrossRef]
- Łazinska, M.; Durejko, T.; Lipinski, S.; Polkowski, W.; Czujko, T.; Varin, R.A. Porous graded FeAl intermetallic foams fabricated by sintering process using NaCl space holders. Mater. Sci. Eng. A 2015, 636, 407–414. [Google Scholar] [CrossRef]
- Osorio-hernández, J.O.; Suarez, M.A.; Goodall, R.; Lara-rodriguez, G.A.; Alfonso, I.; Figueroa, I.A. Manufacturing of open-cell Mg foams by replication process and mechanical properties. Mater. Des. 2014, 64, 136–141. [Google Scholar] [CrossRef]
- Trinidad, J.; Marco, I.; Arruebarrena, G.; Wendt, J.; Letzig, D.; Saenz de Argandona, E.; Goodall, R. Processing of magnesium porous structures by in filtration casting for biomedical applications. Adv. Eng. Mater. 2014, 16, 241–247. [Google Scholar] [CrossRef]
- Shbeh, M.M.; Goodall, R. Open pore titanium foams via metal injection molding of metal powder with a space holder. Met. Powder Rep. 2016, 71, 450–455. [Google Scholar] [CrossRef]
- Ran, H.; Niu, J.; Song, B.; Wang, X.; Feng, P.; Wang, J. Microstructure and properties of Ti5Si3-based porous intermetallic compounds fabricated via combustion synthesis. J. Alloys Compd. 2014, 612, 337–342. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, X.; Feng, P.; Wang, X.; Liu, Z. Intermetallics highly porous open cellular TiAl-based intermetallics fabricated by thermal explosion with space holder process. Intermetallics 2016, 68, 95–100. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, X.; Kang, X.; Feng, P.; Zhang, L.; Wang, J.; Akhtar, F. Hierarchical porous TiAl3 intermetallics synthesized by thermal explosion with a leachable space-holder material. Mater. Lett. 2016, 181, 261–264. [Google Scholar] [CrossRef]
- Ismail, M.H.; Goodall, R.; Davies, H.A.; Todd, I. Porous NiTi alloy by metal injection moulding/sintering of elemental powders: Effect of sintering temperature. Mater. Lett. 2012, 70, 142–145. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Feng, P.; Ran, H.; Song, B.; Wang, J.; Ge, Y. Synthesis, microstructure and properties of Ti–Al thermal explosion reaction. RSC Adv. 2015, 5, 46339–46347. [Google Scholar] [CrossRef]
- Shahzeydi, M.H.; Parvanian, A.M.; Panjepour, M. Materials characterization the distribution and mechanism of pore formation in copper foams fabricated by Lost Carbonate Sintering method. Mater. Charact. 2016, 111, 21–30. [Google Scholar] [CrossRef]
- Diao, K.K.; Xiao, Z.; Zhao, Y.Y. Specific surface areas of porous Cu manufactured by Lost Carbonate Sintering: Measurements by quantitative stereology and cyclic voltammetry. Mater. Chem. Phys. 2015, 162, 571–579. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fung, T.; Zhang, L.P.; Zhang, F.L. Lost carbonate sintering process for manufacturing metal foams. Scr. Mater. 2005, 52, 295–298. [Google Scholar] [CrossRef]
- Laptev, A.; Bram, M.; Buchkremer, H.P.; Sto, D. Study of production route for titanium parts combining very high porosity and complex shape. Powder Metall. 2004, 47, 85–92. [Google Scholar] [CrossRef]
- Karczewski, K.; Stępniowski, W.J.; Chojnacki, M.; Jóźwiak, S. Crystalline oxalic acid aided FeAl intermetallic alloy sintering. Fabrication of intermetallic foam with porosity above 45%. Mater. Lett. 2016, 164, 32–34. [Google Scholar] [CrossRef]
- Karczewski, K.; Stępniowski, W.J.; Jóźwiak, S. Highly-porous FeAl intermetallic foams formed via sintering with Eosin Y as a gas releasing agent. Mater. Lett. 2016, 178, 268–271. [Google Scholar] [CrossRef]
- Karczewski, K.; Stępniowski, W.J.; Kaczor, P.; Jóźwiak, S. Fabrication of Fe-Al Intermetallic Foams via Organic Compounds Assisted Sintering. Materials 2015, 8, 2217–2226. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Durejko, T.; Michalska-Domanska, M.; Łazinska, M.; Aniszewska, J. Characterization of nanoporous anodic aluminum oxide formed on laser pre-treated aluminum. Mater. Charact. 2016, 122, 130–136. [Google Scholar] [CrossRef]

| Name, Formula, and Molar Mass | Structural Formula | Produced Gases | Produced Gas Volume 1/dm3 | Reference |
|---|---|---|---|---|
| Cysteine, C3H7NO2S, 121.16 g/mol |  | CO2, H2O, N2, SO2 | 1.48 | This work |
| Phenylalanine, C9H11NO2, 165.19 g/mol | 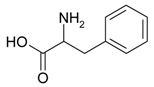 | CO2, H2O, N2 | 2.03 | This work |
| Eosin Y, C20H6Br4O52−2Na+, 647.89 g/mol | 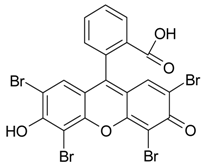 | CO2, H2O, Br2 | 0.9 | [33] |
| Crystalline oxalic acid, H2C2O4·2H2O, 126.07 g/mol | 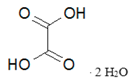 | CO2, H2O | 0.9 | [32] |
| Palmitic acid, C15H31COOH, 256.42 g/mol |  | CO2, H2O | 2.98 | [34] |
| Foaming Agent | Carbon Content | Oxygen Content | ||
|---|---|---|---|---|
| wt % | at % | wt % | at % | |
| Cysteine | 0.2 ± 0.1 | 0.9 ± 0.1 | 0.3 ± 0.1 | 1.1 ± 0.6 |
| Phenylalanine | 0.11 ± 0.04 | 0.3 ± 0.1 | 0.14 ± 0.06 | 0.4 ± 0.2 |
| Foaming Agent | ECD/µm | |
|---|---|---|
| In the Sample Core | At the sample surface | |
| Without additive | 28 ± 20 | 30 ± 17 |
| Cysteine | 29 ± 15 | 28 ± 16 |
| Phenylalanine | 32 ± 15 | 25 ± 11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczewski, K.; Stepniowski, W.J.; Salerno, M. Amino Acids Aided Sintering for the Formation of Highly Porous FeAl Intermetallic Alloys. Materials 2017, 10, 746. https://doi.org/10.3390/ma10070746
Karczewski K, Stepniowski WJ, Salerno M. Amino Acids Aided Sintering for the Formation of Highly Porous FeAl Intermetallic Alloys. Materials. 2017; 10(7):746. https://doi.org/10.3390/ma10070746
Chicago/Turabian StyleKarczewski, Krzysztof, Wojciech J. Stepniowski, and Marco Salerno. 2017. "Amino Acids Aided Sintering for the Formation of Highly Porous FeAl Intermetallic Alloys" Materials 10, no. 7: 746. https://doi.org/10.3390/ma10070746