Toxicity of Nine (Doped) Rare Earth Metal Oxides and Respective Individual Metals to Aquatic Microorganisms Vibrio fischeri and Tetrahymena thermophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Rare Earth Oxide (REO) Particles
2.2. Preparation and Characterization of REO Particle Suspensions
2.3. Soluble Metal Salts Analyzed for Toxicity
2.4. Quantification of Reactive Oxygen Species
2.5. Vibrio fischeri Kinetic Bioluminescence Inhibition Test (a Flash-Assay) in 2% NaCl and in PBS (Phosphate-Buffered Saline) Containing 2% NaCl
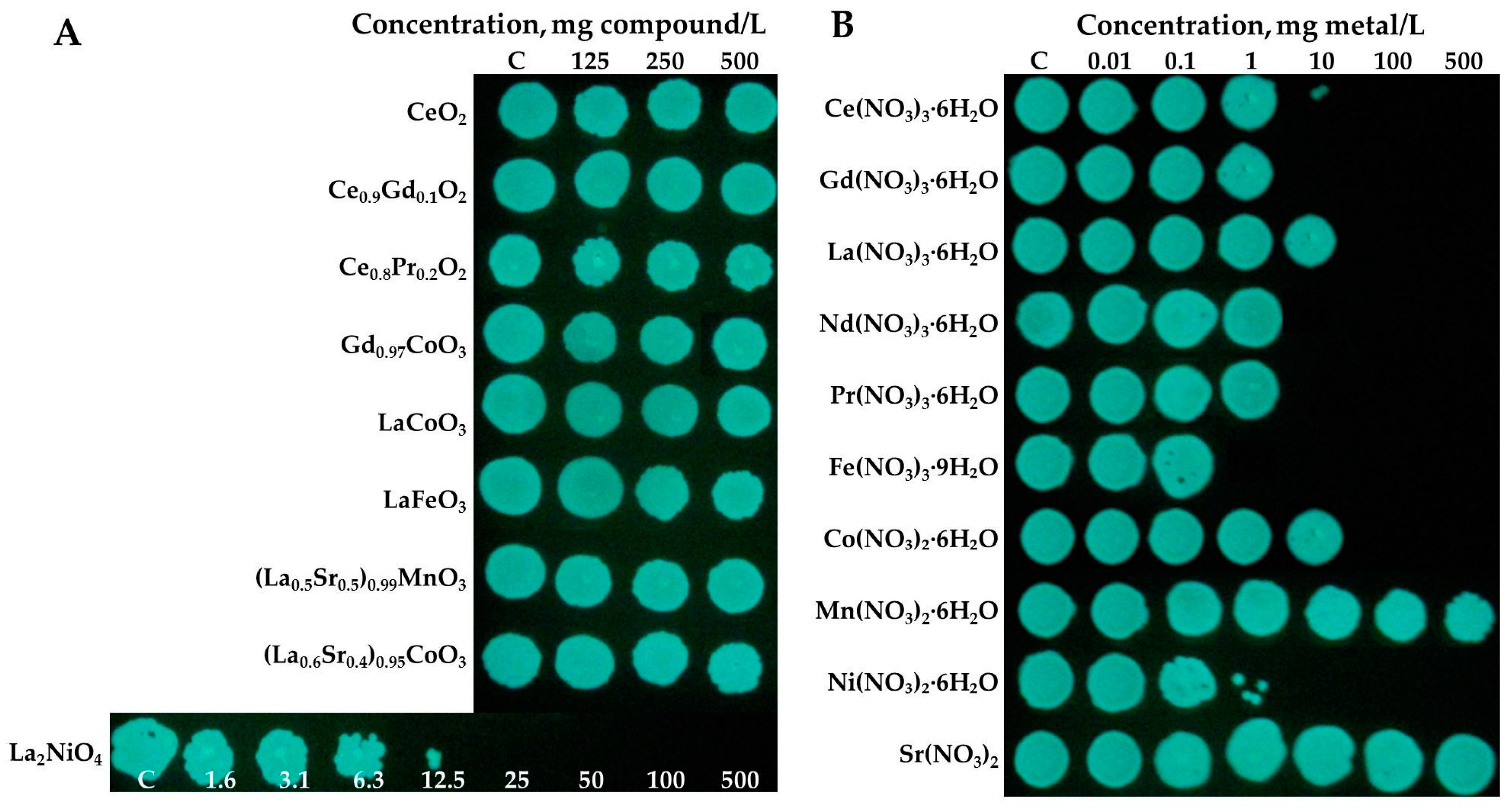
2.6. Vibrio fischeri Viability Assay (a ‘Spot Test’)
2.7. Tetrahymena thermophila Viability Assay
2.8. Data Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Studied Rare Earth Oxide (REO) Particles
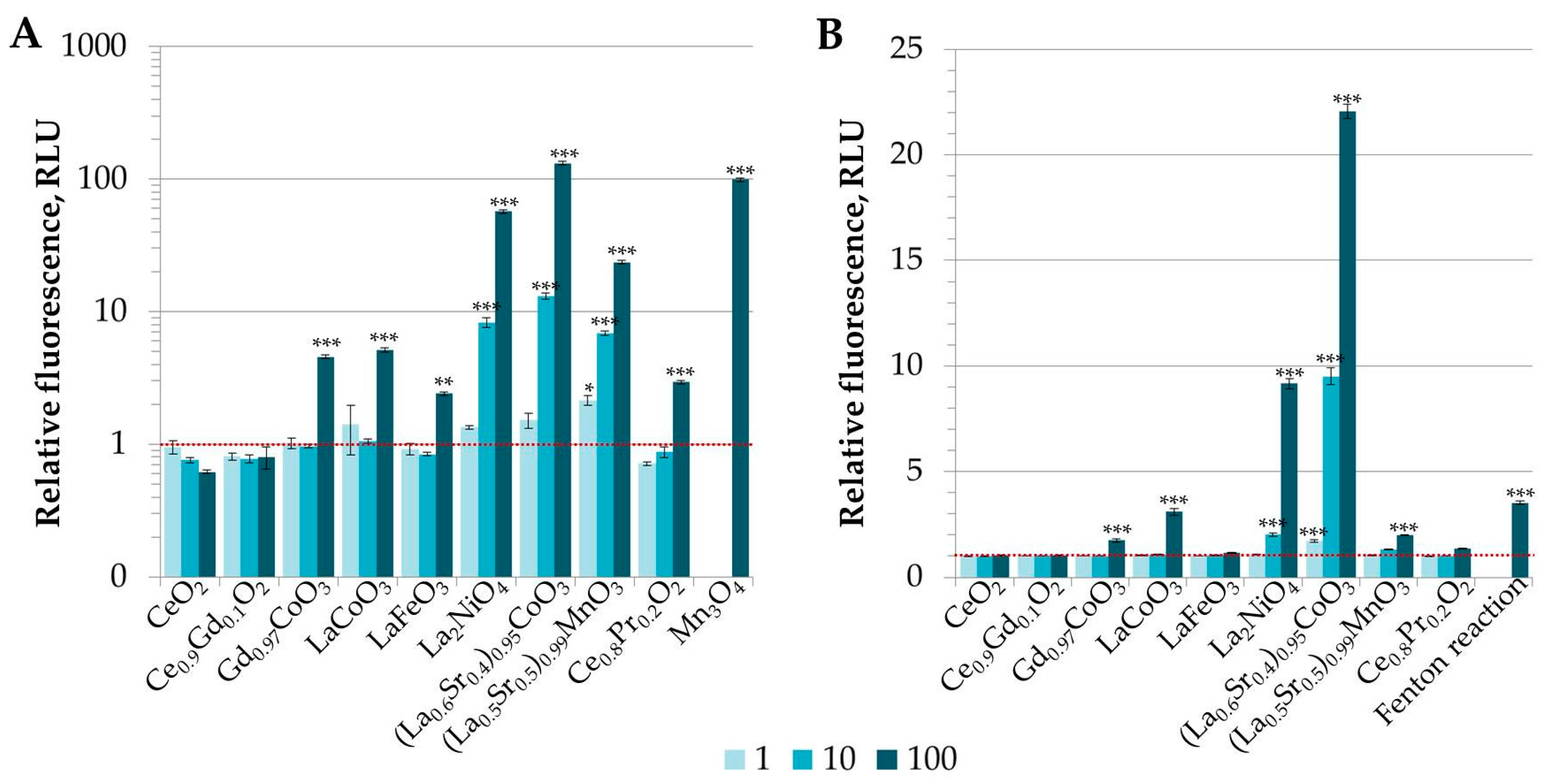
3.2. Analysis of Potential of REO Particles to Generate Reactive Oxygen Species (ROS) in Abiotic Conditions
3.3. Toxicity Evaluation of REEs and (Doped) REOs
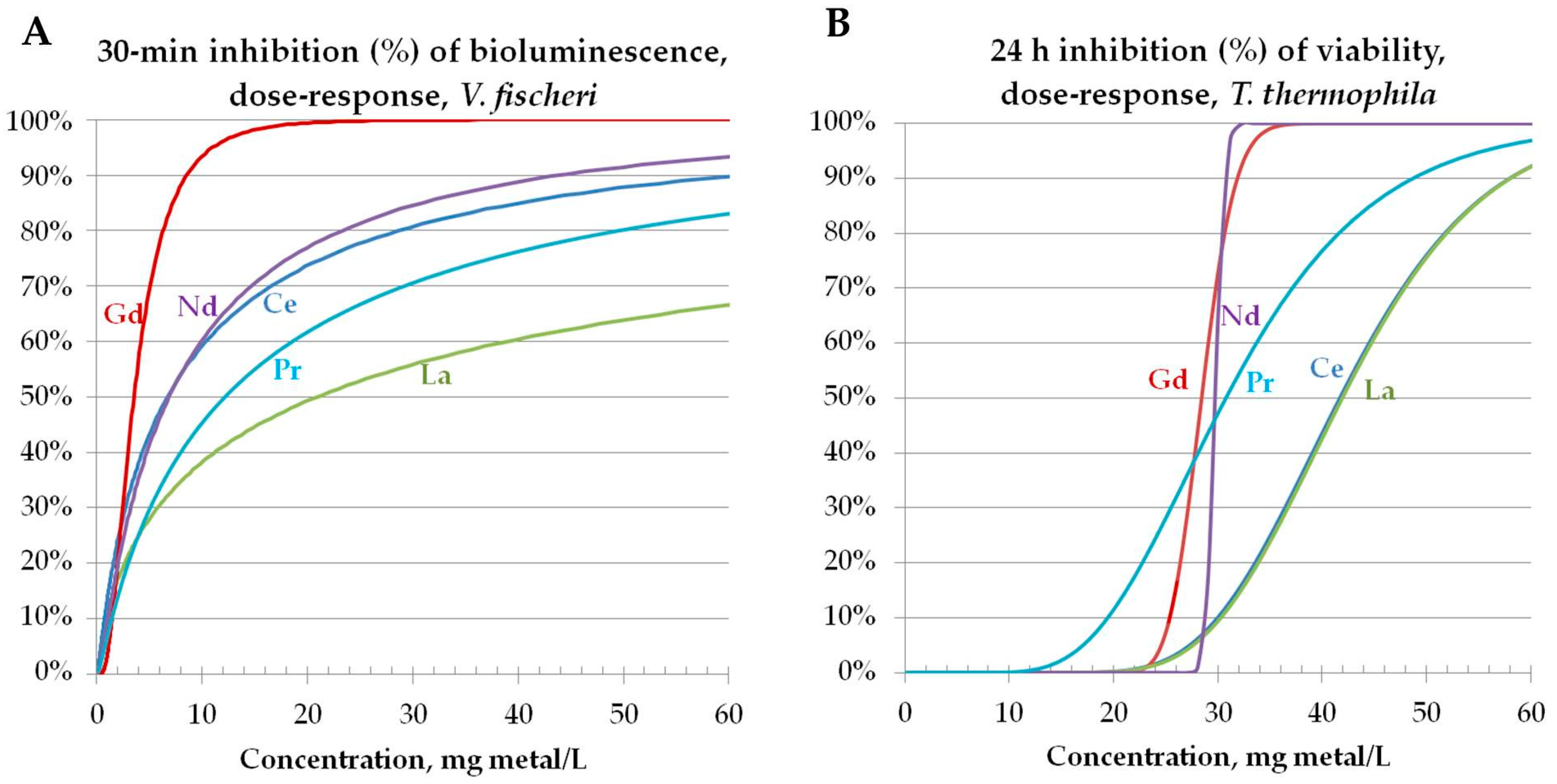
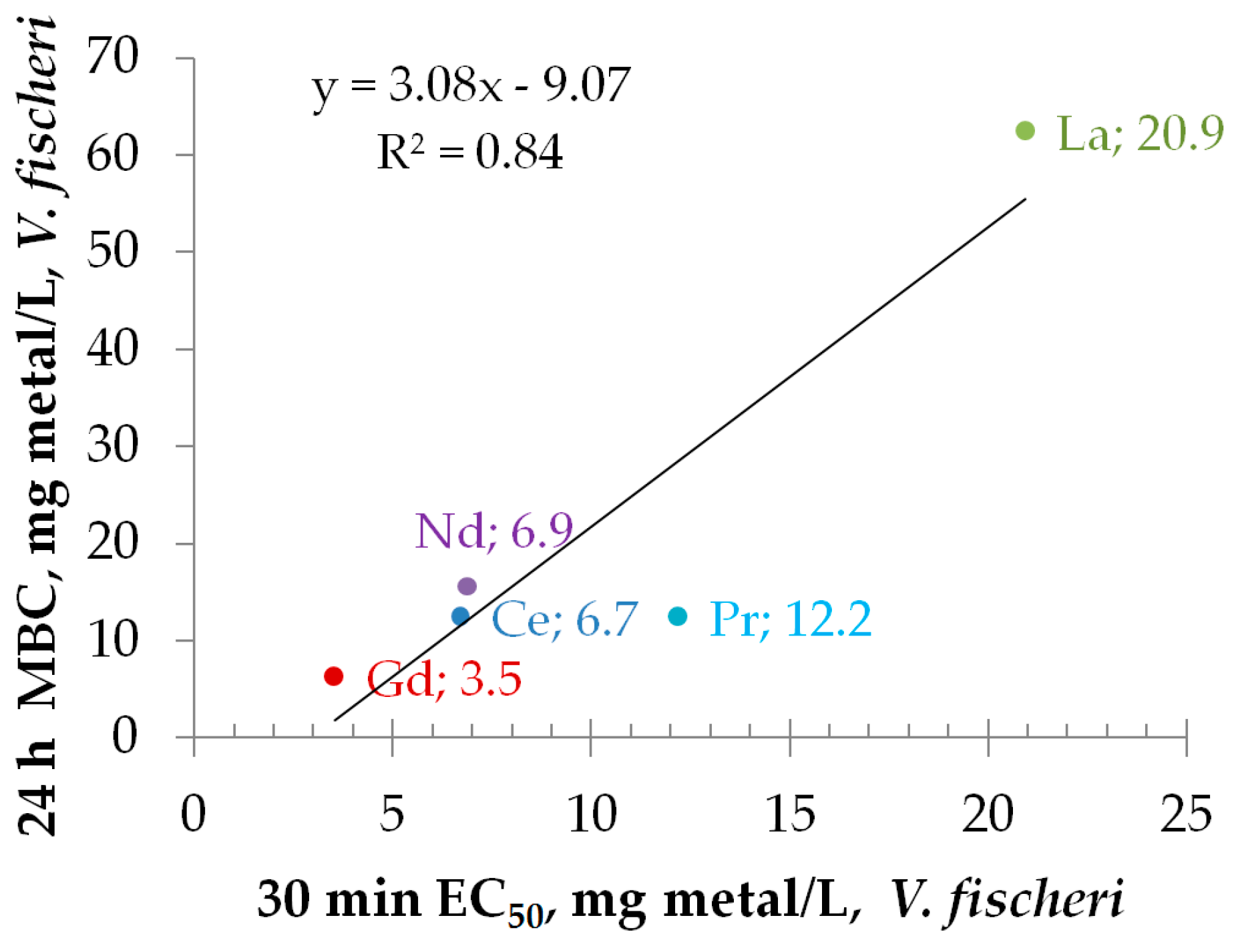
3.3.1. Toxicity to Bacteria Vibrio fischeri
3.3.2. Toxicity to Protozoa Tetrahymena thermophila
3.3.3. Toxicity to Algae Raphidocelis subcapitata (Data Taken from the Literature): Comparison of Toxicity Pattern of REEs and REOs to Three Aquatic Species (Bacteria, Protozoa, Algae)
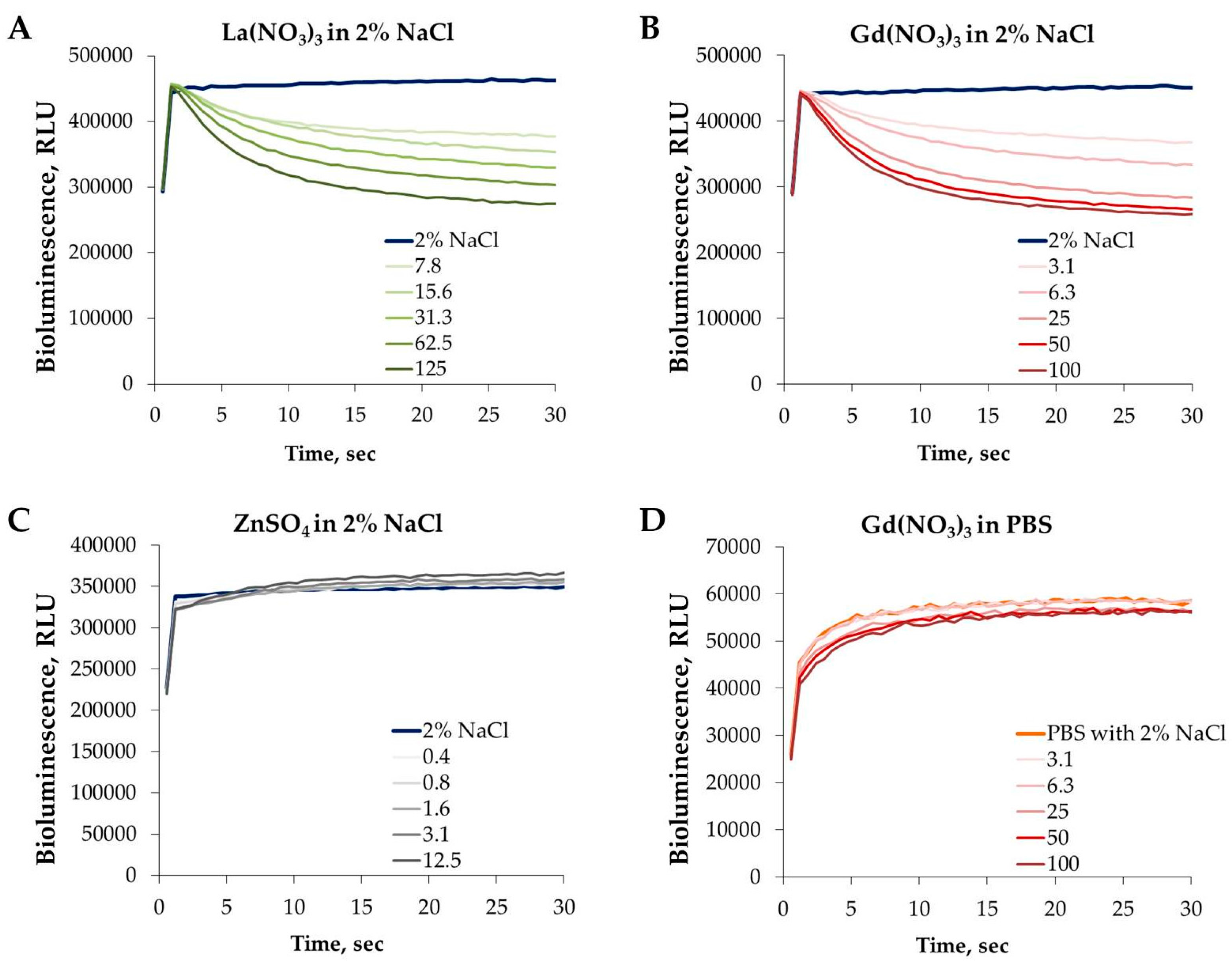
3.4. Effect of REEs on the Kinetics of Vibrio fischeri Bioluminescence
3.5. Toxicity of REEs to Aquatic Organisms Depends on Speciation
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gonzalez, V.; Vignati, D.A.L.; Leyval, C.; Giamberini, L. Environmental fate and ecotoxicity of lanthanides: Are they a uniform group beyond chemistry? Environ. Int. 2014, 71, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.A. Rare earth metals: A strategic concern. Miner. Econ. 2014, 27, 21–31. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614. [Google Scholar] [CrossRef]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Tuduri, J.; Charles, N.; Vaxelaire, S.; Dubois, D.; Fargier, H. Material flow analysis applied to rare earth elements in Europe. J. Clean. Prod. 2015, 107, 215–228. [Google Scholar] [CrossRef]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Volokh, A.A.; Gorbunov, A.V.; Gundorina, S.F.; Revich, B.A.; Frontasyeva, M.V.; Pal, C.S. Phosphorus-fertilizer production as a source of rare-earth elements pollution of the environment. Sci. Total Environ. 1990, 95, 141–148. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.C.; Peng, A. Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. Environ. Sci. Pollut. Res. 2002, 9, 143–148. [Google Scholar] [CrossRef]
- Liu, X.S.; Wang, J.C.; Yang, J.; Fan, Y.B.; Wu, Y.P.; Zhang, H. Application of rare earth phosphate fertilizer in western area of China. J. Rare Earths 2006, 24, 423–426. [Google Scholar]
- Telgmann, L.; Sperling, M.; Karst, U. Determination of gadolinium-based MRI contrast agents in biological and environmental samples: A review. Anal. Chim. Acta 2013, 764, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hatje, V.; Bruland, K.W.; Flegal, A.R. Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco bay over a 20 year record. Environ. Sci. Technol. 2016, 50, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, S.; Bau, M. Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine river and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet. Sci. Lett. 2013, 362, 43–50. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Leguay, S.; Campbell, P.G.C.; Fortin, C. Determination of the free-ion concentration of rare earth elements by an ion-exchange technique: Implementation, evaluation and limits. Environ. Chem. 2016, 13, 478–488. [Google Scholar] [CrossRef]
- Carpenter, D.; Boutin, C.; Allison, J.E.; Parsons, J.L.; Ellis, D.M. Uptake and effects of six rare earth elements (REEs) on selected native and crop species growing in contaminated soils. PLoS ONE 2015, 10, e0129936. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Nolde, J.; Berger, S.; Heise, S. Aquatic ecotoxicity of lanthanum—A review and an attempt to derive water and sediment quality criteria. Ecotoxicol. Environ. Saf. 2016, 124, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.K.; Zhu, W.Z.; Wang, Z.J.; Witkamp, G.J. Distributions of rare earths and heavy metals in field-grown maize after application of rare earth-containing fertilizer. Sci. Total Environ. 2002, 293, 97–105. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Shan, X.Q. Speciation of rare earth elements in soil and accumulation by wheat with rare earth fertilizer application. Environ. Pollut. 2001, 112, 395–405. [Google Scholar] [CrossRef]
- Gonzalez, V.; Vignati, D.A.L.; Pons, M.N.; Montarges-Pelletier, E.; Bojic, C.; Giamberini, L. Lanthanide ecotoxicity: First attempt to measure environmental risk for aquatic organisms. Environ. Pollut. 2015, 199, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Babula, P.; Adam, V.; Opatrilova, R.; Zehnalek, J.; Havel, L.; Kizek, R. Uncommon heavy metals, metalloids and their plant toxicity: A review. Environ. Chem. Lett. 2008, 6, 189–213. [Google Scholar] [CrossRef]
- Juganson, K.; Ivask, A.; Blinova, I.; Mortimer, M.; Kahru, A. NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 1788–1804. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.S.; Merrifield, R.; Williams, T.D.; Chipman, J.K.; Lead, J.R.; Viant, M.R. Molecular toxicity of cerium oxide nanoparticles to the freshwater alga Chlamydomonas reinhardtii is associated with supra-environmental exposure concentrations. Nanotoxicology 2016, 10, 32–41. [Google Scholar] [PubMed]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.T.; Arai, Y. Environmental geochemistry of cerium: Applications and toxicology of cerium oxide nanoparticles. Int. J. Environ. Res. Public Health 2015, 12, 1253–1278. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, S.; Nel, A.E.; Madler, L. Custom-designed nanomaterial libraries for testing metal oxide toxicity. Acc. Chem. Res. 2013, 46, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Messing, G.L.; Zhang, S.C.; Jayanthi, G.V. Ceramic powder synthesis by spray-pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Joonas, E.; Aruoja, V.; Olli, K.; Syvertsen-Wiig, G.; Vija, H.; Kahru, A. Potency of (doped) rare earth oxide particles and their constituent metals to inhibit algal growth and induce direct toxic effects. Sci. Total Environ. 2017, 593–594, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Maedler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2015, 2, 630–644. [Google Scholar] [CrossRef]
- ISO 21338:2010—Water quality—Kinetic Determination of the Inhibitory Effects of Sediment, Other Solids and Coloured Samples on the Light Emission of Vibrio fischeri (Kinetic Luminescent Bacteria Test); International Organization for Standardization: Geneva, Switzerland, 2010.
- Vindimian, E. MSExcel Macro REGTOX EV7.0.5.xls. 2005. Available online: http://www.normalesup.org/~vindimian/en_download.html (accessed on 5 July 2011).
- Suppi, S.; Kasemets, K.; Ivask, A.; Künnis-Beres, K.; Sihtmäe, M.; Kurvet, I.; Aruoja, V.; Kahru, A. A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae. J. Hazard. Mater. 2015, 286, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Jemec, A.; Kahru, A.; Potthoff, A.; Drobne, D.; Heinlaan, M.; Boehme, S.; Geppert, M.; Novak, S.; Schirmer, K.; Rekulapally, R.; et al. An interlaboratory comparison of nanosilver characterisation and hazard identification: Harmonising techniques for high quality data. Environ. Int. 2016, 87, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, M.; Kasemets, K.; Kahru, A. Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila. Toxicology 2010, 269, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Dubourguier, H.-C.; Blinova, I.; Ivask, A.; Kasemets, K. Biotests and biosensors for ecotoxicology of metal oxide nanoparticles: A mini review. Sensors 2008, 8, 5153–5170. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review. Nanotoxicology 2014, 8, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Gonzalez-Flecha, B.; Kobzik, L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic. Biol. Med. 2003, 35, 327–340. [Google Scholar] [CrossRef]
- Arbab, A.S.; Bashaw, L.A.; Miller, B.R.; Jordan, E.K.; Lewis, B.K.; Kalish, H.; Frank, J.A. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology 2003, 229, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, K.S.; Sunderman, F.W.; Salnikow, K. Nickel carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 533, 67–97. [Google Scholar] [CrossRef]
- Pagano, G.; Guida, M.; Siciliano, A.; Oral, R.; Kocbas, F.; Palumbo, A.; Castellano, I.; Migliaccio, O.; Thomas, P.J.; Trifuoggi, M. Comparative toxicities of selected rare earth elements: Sea urchin embryogenesis and fertilization damage with redox and cytogenetic effects. Environ. Res. 2016, 147, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Karakoti, A.; Graham, R.P.; Maguire, J.L.; Reilly, C.M.; Seal, S.; Rattan, R.; Shridhar, V. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS ONE 2013, 8, e54578. [Google Scholar] [CrossRef] [PubMed]
- Kurvet, I.; Ivask, A.; Bondarenko, O.; Sihtmäe, M.; Kahru, A. LuxCDABE-transformed constitutively bioluminescent Escherichia coli for toxicity screening: Comparison with naturally luminous Vibrio fischeri. Sensors 2011, 11, 7865–7878. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.W.; Makemson, J.; Dunlap, P.V. How are growth and luminescence regulated independently in light organ symbionts. Symbiosis 1987, 4, 3–24. [Google Scholar]
- Bulich, A.A. A practical and reliable method for monitoring the toxicity of aquatic samples. Process Biochem. 1982, 17, 45–47. [Google Scholar]
- Kaiser, K.L.E.; Devillers, J. Ecotoxicity of Chemicals to Photobacterium phosphoreum. Handbook of Ecotoxicological Data; Gordon and Breach Science Publishers: Yverdon, Switzerland, 1994. [Google Scholar]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, J.; Juvonen, R.; Vaajasaari, K.; Karp, M. A new flash method for measuring the toxicity of solid and colored samples. Chemosphere 1999, 38, 1069–1083. [Google Scholar] [CrossRef]
- Gellert, G. Sensitivity and significance of luminescent bacteria in chronic toxicity testing based on growth and bioluminescence. Ecotoxicol. Environ. Saf. 2000, 45, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Velzeboer, I.; Hendriks, A.J.; Ragas, A.M.J.; Van de Meent, D. Aquatic ecotoxicity tests of some nanomaterials. Environ. Toxicol. Chem. 2008, 27, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Storz, G.; Imlay, J.A. Oxidative stress. Curr. Opin. Microbiol. 1999, 2, 188–194. [Google Scholar] [CrossRef]
- Sorokina, E.V.; Yudina, T.P.; Bubnov, I.A.; Danilov, V.S. Assessment of iron toxicity using a luminescent bacterial test with an Escherichia coli recombinant strain. Microbiology 2013, 82, 439–444. [Google Scholar] [CrossRef]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel toxicity in microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Sherr, E.B.; Sherr, B.F. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2002, 81, 293–308. [Google Scholar] [CrossRef]
- Sauvant, M.P.; Pepin, D.; Piccinni, E. Tetrahymena pyriformis: A tool for toxicological studies. A review. Chemosphere 1999, 38, 1631–1669. [Google Scholar] [CrossRef]
- Frankel, J. Cell biology of Tetrahymena thermophila. In Tetrahymena thermophila, Methods in Cell Biology; Asai, D.J., Forney, J.D., Eds.; Academic Press: New York, NY, USA, 2000; Volume 62, pp. 27–125. [Google Scholar]
- Koppelhus, U.; Hellunglarsen, P.; Leick, V. Physiological-parameters affecting the chemosensory response of Tetrahymena. Biol. Bull. 1994, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Juganson, K.; Mortimer, M.; Ivask, A.; Pucciarelli, S.; Miceli, C.; Orupõld, K.; Kahru, A. Mechanisms of toxic action of silver nanoparticles in the protozoan Tetrahymena thermophila: From gene expression to phenotypic events. Environ. Pollut. 2017, 225, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Juganson, K.; Mortimer, M.; Ivask, A.; Kasemets, K.; Kahru, A. Extracellular conversion of silver ions into silver nanoparticles by protozoan Tetrahymena thermophila. Environ. Sci. Processes Impacts 2013, 15, 244–250. [Google Scholar] [CrossRef]
- Mortimer, M.; Kahru, A.; Slaveykova, V.I. Uptake, localization and clearance of quantum dots in ciliated protozoa Tetrahymena thermophila. Environ. Pollut. 2014, 190, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, M.; Petersen, E.J.; Buchholz, B.A.; Orias, E.; Holden, P.A. Bioaccumulation of multiwall carbon nanotubes in Tetrahymena thermophila by direct feeding or trophic transfer. Environ. Sci. Technol. 2016, 50, 8876–8885. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H. Biochemistry of the Lanthanides; Springer Science+Business Media: New York, NY, USA, 1990; pp. p. 22, p. 221 and p. 341. [Google Scholar]
- Ekwall, B. Correlation between cyto-toxicity in vitro and LD50-values. Acta Pharmacol. Toxicol. 1983, 52, 80–99. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A. Ecotoxicological tests in non-ecotoxicological research: Contribution to 3Rs. Use of luminescent photobacteria for evaluating the toxicity of 47 MEIC reference chemicals. ALTEX 2006, 23, 302–308. [Google Scholar]
- Mortimer, M.; Kasemets, K.; Heinlaan, M.; Kurvet, I.; Kahru, A. High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicol. In Vitro 2008, 22, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Chatellier, X.; Hattori, K.H.; Kato, K.; Fortin, D. Adsorption of rare earth elements onto bacterial cell walls and its implication for REE sorption onto natural microbial mats. Chem. Geol. 2005, 219, 53–67. [Google Scholar] [CrossRef]
- Markai, S.; Andres, Y.; Montavon, G.; Grambow, B. Study of the interaction between europium (III) and Bacillus subtilis: Fixation sites, biosorption modeling and reversibility. J. Colloid Interface Sci. 2003, 262, 351–361. [Google Scholar] [CrossRef]
- Texier, A.C.; Andres, Y.; Illemassene, M.; Le Cloirec, P. Characterization of lanthanide ions binding sites in the cell wall of Pseudomonas aeruginosa. Environ. Sci. Technol. 2000, 34, 610–615. [Google Scholar] [CrossRef]
- Martinez, R.E.; Pourret, O.; Takahashi, Y. Modeling of rare earth element sorption to the gram positive Bacillus subtilis bacteria surface. J. Colloid Interface Sci. 2014, 413, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, B.T.; Mosselmans, J.F.W.; Magennis, M.; Atkinson, K.D.; Tourney, J.; Olive, V.; Ellam, R.M. Macroscopic and spectroscopic analysis of lanthanide adsorption to bacterial cells. Geochim. Cosmochim. Acta 2009, 73, 3134–3147. [Google Scholar] [CrossRef]
- Dijkmans, A.C.; Wilms, E.B.; Kamerling, I.M.C.; Birkhoff, W.; Ortiz-Zacarias, N.V.; van Nieuwkoop, C.; Verbrugh, H.A.; Touw, D.J. Colistin: Revival of an old polymyxin antibiotic. Ther. Drug Monit. 2015, 37, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Beaudry, F.; Theriault, W.; Letellier, A. Colistin in pig production: Chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front. Microbiol. 2016, 7, 1789. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.D.; Chen, Y.; Wang, X.R.; Deng, X.H. Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Weltje, L.; Verhoof, L.; Verweij, W.; Hamers, T. Lutetium speciation and toxicity in a microbial bioassay: Testing the free-ion model for lanthanides. Environ. Sci. Technol. 2004, 38, 6597–6604. [Google Scholar] [CrossRef] [PubMed]
- Luli, G.W.; Strohl, W.R. Comparison of growth, acetate production, and acetate inhibition of Escherichia-coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 1990, 56, 1004–1011. [Google Scholar] [PubMed]
- Li, R.B.; Ji, Z.X.; Chang, C.H.; Dunphy, D.R.; Cai, X.M.; Meng, H.; Zhang, H.Y.; Sun, B.B.; Wang, X.; Dong, J.Y.; et al. Surface interactions with compartmentalized cellular phosphates explain rare earth oxide nanoparticle hazard and provide opportunities for safer design. ACS Nano 2014, 8, 1771–1783. [Google Scholar] [CrossRef] [PubMed]

| Compound | Specific Surface Area (SSA), m2/g a | Primary Size, nm (BET) a | Hydro-Dynamic Size (nm) in DI (DLS) b | ζ-Potential (mV) in DI b | pH in DI c | pH in 2% NaCl c |
|---|---|---|---|---|---|---|
| Ce0.9Gd0.1O2 | 31.1 | 27 | 280 | −16.6 | 6.3 | 6.3 |
| LaFeO3 | 72 | 126 | 177 | 16.2 | 6.5 | 6.4 |
| Gd0.97CoO3 | 34 | 230 | 166 | 18.8 | 6.3 | 6.3 |
| LaCoO3 | 14 | 590 | 285 | −17.5 | 6.7 | 6.4 |
| (La0.5Sr0.5)0.99MnO3 | 7 | 137 | 194 | −1.8 | 7.3 | 6.9 |
| CeO2 | 22 | 38 | 172 | 8.5 | 6.5 | 6.5 |
| Ce0.8Pr0.2O2 | 361 | 23 | 147 | 16.6 | 6.3 | 6.3 |
| (La0.6Sr0.4)0.95CoO3 | 15 | 65 | 160 | 22.7 | 7.4 | 9.5 |
| La2NiO4 | 3 | 284 | 266 | −6.6 | 8.6 | 8.6 |
| Compound | Toxicity Endpoint | ||
|---|---|---|---|
| 24-h Decrease of Viability, % | 30-min Inhibition of Luminescence, % | Ability to Yield Colonies on Agar Plates after 24-h Exposure to Chemical | |
| Protozoa T. thermophila | Bacteria V. fischeri | Bacteria V. fischeri | |
| (Doped) rare earth oxides | EC50 a, mg compound/L | MBC b, mg compound/L | |
| Ce0.9Gd0.1O2 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| LaFeO3 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| Gd0.97CoO3 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| LaCoO3 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| (La0.5Sr0.5)0.99MnO3 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| CeO2 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| Ce0.8Pr0.2O2 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| (La0.6Sr0.4)0.95CoO3 | >100; N = 2 | >500; N = 2 | >500; N = 2 |
| La2NiO4 | >100; N = 2 | >500; N = 2 | 25; N = 4 |
| Rare earth elements | EC50, mg metal/L | MBC, mg metal/L | |
| Ce(NO3)3·6H2O | 41.7 (38.3–46.5); N = 3 | 6.70 (5. 81–8.48); N = 5 | 12.5; N = 3 |
| Gd(NO3)3·6H2O | 28.4 (27.3–30.6); N = 3 | 3.53 (3.38–3.76); N = 5 | 6.25; N = 3 |
| La(NO3)3·6H2O | 41.9 (38.0–47.2); N = 3 | 20.93 (17.07–25.94), N = 5 | 62.5; N = 4 |
| Nd(NO3)3·6H2O | 29.8 (29.5–31.2); N = 2 | 6.87 (6.66–8.57); N = 3 | 15.6; N = 4 |
| Pr(NO3)3·6H2O | 30.8 (26.6–35.8); N = 2 | 12.17 (11.08–14.82); N = 3 | 12.5; N = 4 |
| Dopant metals | EC50, mg metal/L | MBC, mg metal/L | |
| Fe(NO3)3·9H2O | 4.9 (4.4–5.3); N = 3 | 0.44 (0.40–0.46); N = 6 | 1.25; N = 4 |
| Co(NO3)2·6H2O | >100; N = 4 | 462 (404–564); N = 5 | 62.5; N = 5 |
| Mn(NO3)2·6H2O | 82.0 (76.9–90.4); N = 4 | >500; N = 5 | >500; N = 3 |
| Ni(NO3)2·6H2O | 2.7 (2.6–2.9); N = 3 | >500; N = 5 | 1.25; N = 5 |
| Sr(NO3)2 | >100; N = 2 | >500; N = 5 | >500; N = 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurvet, I.; Juganson, K.; Vija, H.; Sihtmäe, M.; Blinova, I.; Syvertsen-Wiig, G.; Kahru, A. Toxicity of Nine (Doped) Rare Earth Metal Oxides and Respective Individual Metals to Aquatic Microorganisms Vibrio fischeri and Tetrahymena thermophila. Materials 2017, 10, 754. https://doi.org/10.3390/ma10070754
Kurvet I, Juganson K, Vija H, Sihtmäe M, Blinova I, Syvertsen-Wiig G, Kahru A. Toxicity of Nine (Doped) Rare Earth Metal Oxides and Respective Individual Metals to Aquatic Microorganisms Vibrio fischeri and Tetrahymena thermophila. Materials. 2017; 10(7):754. https://doi.org/10.3390/ma10070754
Chicago/Turabian StyleKurvet, Imbi, Katre Juganson, Heiki Vija, Mariliis Sihtmäe, Irina Blinova, Guttorm Syvertsen-Wiig, and Anne Kahru. 2017. "Toxicity of Nine (Doped) Rare Earth Metal Oxides and Respective Individual Metals to Aquatic Microorganisms Vibrio fischeri and Tetrahymena thermophila" Materials 10, no. 7: 754. https://doi.org/10.3390/ma10070754





