Lateral Tension-Induced Penetration of Particles into a Liposome
Abstract
:1. Introduction
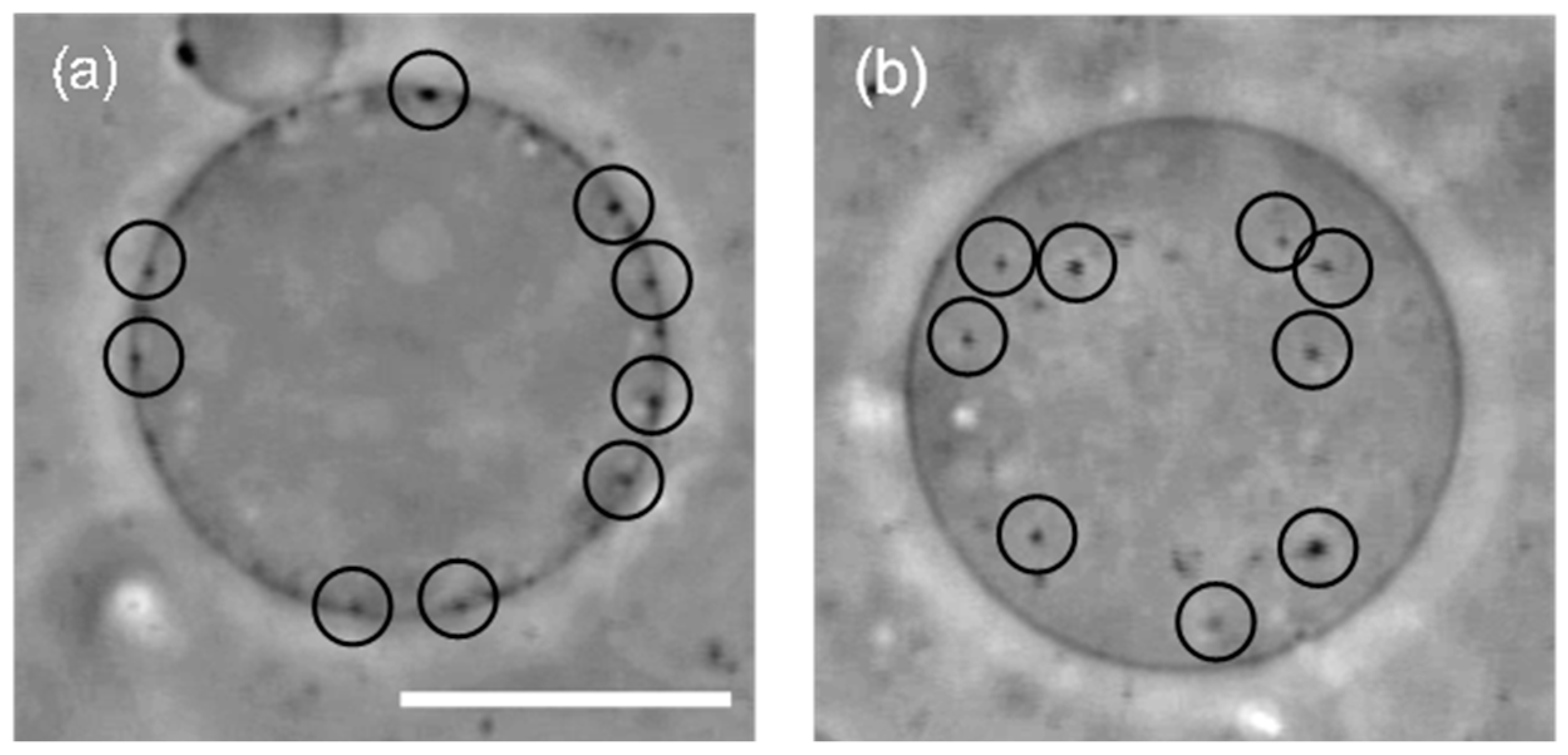
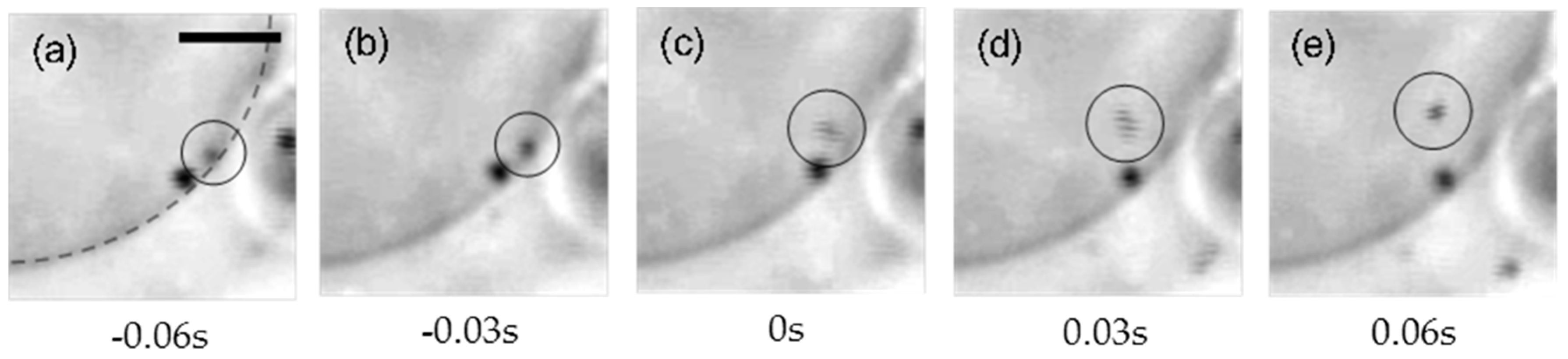
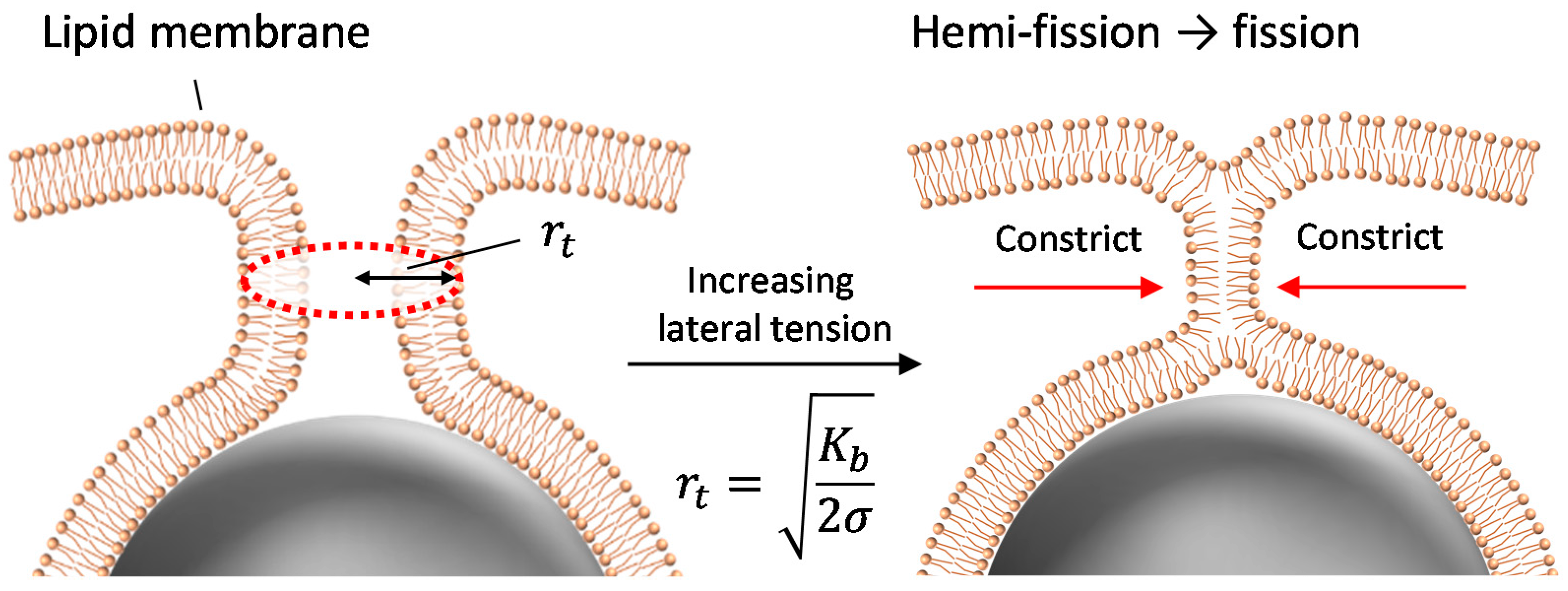
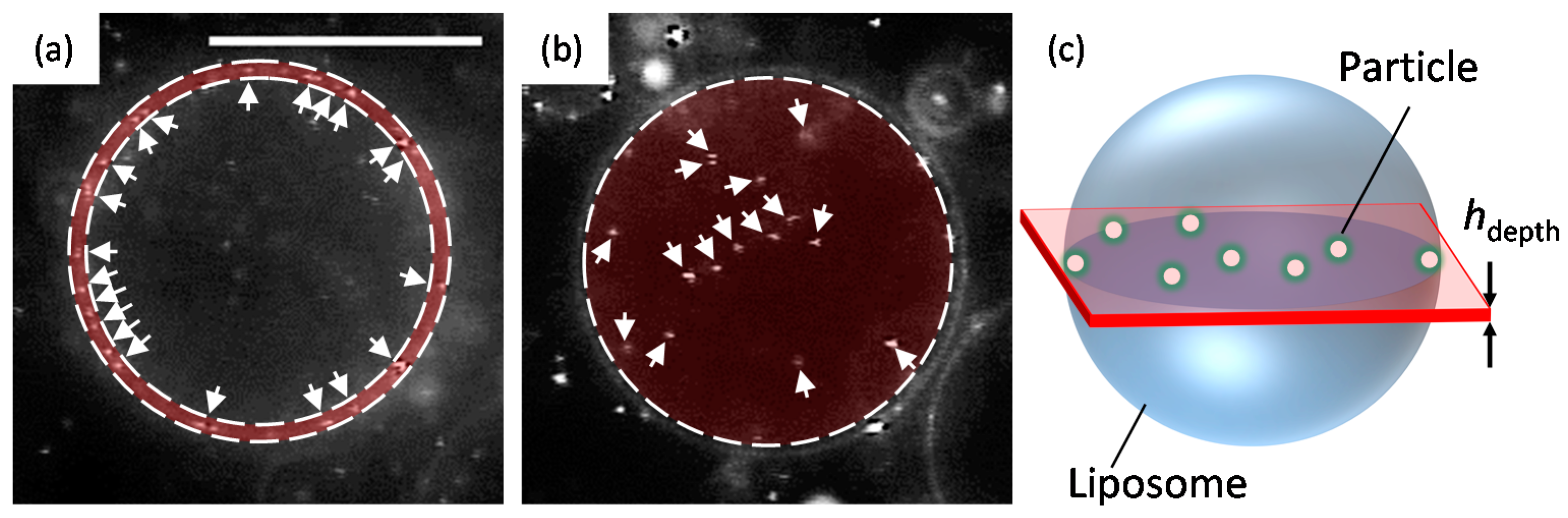
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ting, C.; Wang, Z.-G. Interactions of a charged nanoparticle with a lipid membrane: Implications for gene delivery. Biophys. J. 2011, 100, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Sehrig, F.; Samiei, M.; Milani, M.; Abbasi, E.; Dadashzadeh, K.; Akbarzadeh, A. Magnetic nanoparticles: Applications in gene delivery and gene therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Li, K.; Sun, C.; Du, W.; Cui, J.; Zhao, X.; Chen, W. A Magnetic Nanoparticle-Based Multiple-Gene Delivery System for Transfection of Porcine Kidney Cells. PLoS ONE 2014, 9, e102886. [Google Scholar] [CrossRef] [PubMed]
- Zauner, W.; Farrow, N.; Haines, A. In vitro uptake of polystyrene microspheres: Effect of particle size, cell line and cell density. J. Control. Release 2001, 71, 39–51. [Google Scholar] [CrossRef]
- Seemork, J.; Sansureerungsikul, T.; Sathornsantikun, K.; Sinthusake, T.; Shigyou, K.; Tree-Udom, T.; Jiangchareon, B.; Chiablaem, K.; Lirdprapamongkol, K.; Svasti, J.; et al. Penetration of Oxidized Carbon Nanospheres through Lipid Bilayer Membrane: Comparison to Graphene Oxide and Oxidized Carbon Nanotubes, and Effects of pH and Membrane Composition. ACS Appl. Mater. Interf. 2016, 8, 23549–23557. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, H.; Numata, T.; Morone, N.; Kaneko, S.; Mori, Y.; Imahori, H.; Murakami, T. Thermosensitive Ion Channel Activation in Single Neuronal Cells by Using Surface-Engineered Plasmonic Nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 11725–11729. [Google Scholar] [CrossRef] [PubMed]
- Spindler, S.; Ehrig, J.; König, K.; Nowak, T.; Piliarik, M.; Stein, H.; Taylor, R.; Garanger, E.; Lecommandoux, S.; Alves, I.; et al. Visualization of lipids and proteins at high spatial and temporal resolution via interferometric scattering (iSCAT) microscopy. J. Phys. D 2016, 49, 274002. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Morlot, S.; Roux, A. Mechanics of Dynamin-Mediated Membrane Fission. Annu. Rev. Biophys. 2013, 42, 629–649. [Google Scholar] [CrossRef] [PubMed]
- Morlot, S.; Galli, V.; Klein, M.; Chiaruttini, N.; Manzi, J.; Humbert, F.; Dinis, L.; Lenz, M.; Cappello, G.; Roux, A. Membrane Shape at the Edge of the Dynamin Helix Sets Location and Duration of the Fission Reaction. Cell 2012, 151, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Pucadyil, T.; Schmid, S. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 2008, 135, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Uyhazi, K.; Frost, A.; Camilli, P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 2006, 441, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Arayachukiat, S.; Seemork, J.; Pan-In, P.; Amornwachirabodee, K.; Sangphech, N.; Sansureerungsikul, T.; Sathornsantikun, K.; Vilaivan, C.; Shigyou, K.; Pienpinijtham, P.; et al. Bringing Macromolecules into Cells and Evading Endosomes by Oxidized Carbon Nanoparticles. Nano Lett. 2015, 15, 3370–3376. [Google Scholar] [CrossRef] [PubMed]
- Shigyou, K.; Nagai, K.; Hamada, T. Lateral Diffusion of a Submicrometer Particle on a Lipid Bilayer Membrane. Langmuir 2016, 32, 13771–13777. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y.; Idiart, M. Pore dynamics of osmotically stressed vesicles. Phys. A 2004, 331, 571–578. [Google Scholar] [CrossRef]
- Koster, G.; VanDuijn, M.; Hofs, B.; Dogterom, M. Membrane tube formation from giant vesicles by dynamic association of motor proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 15583–15588. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Dimitrov, D. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.C.; McIntosh, T.; Needham, D.; Evans, E. Effect of Chain Length and Unsaturation on Elasticity of Lipid Bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef]

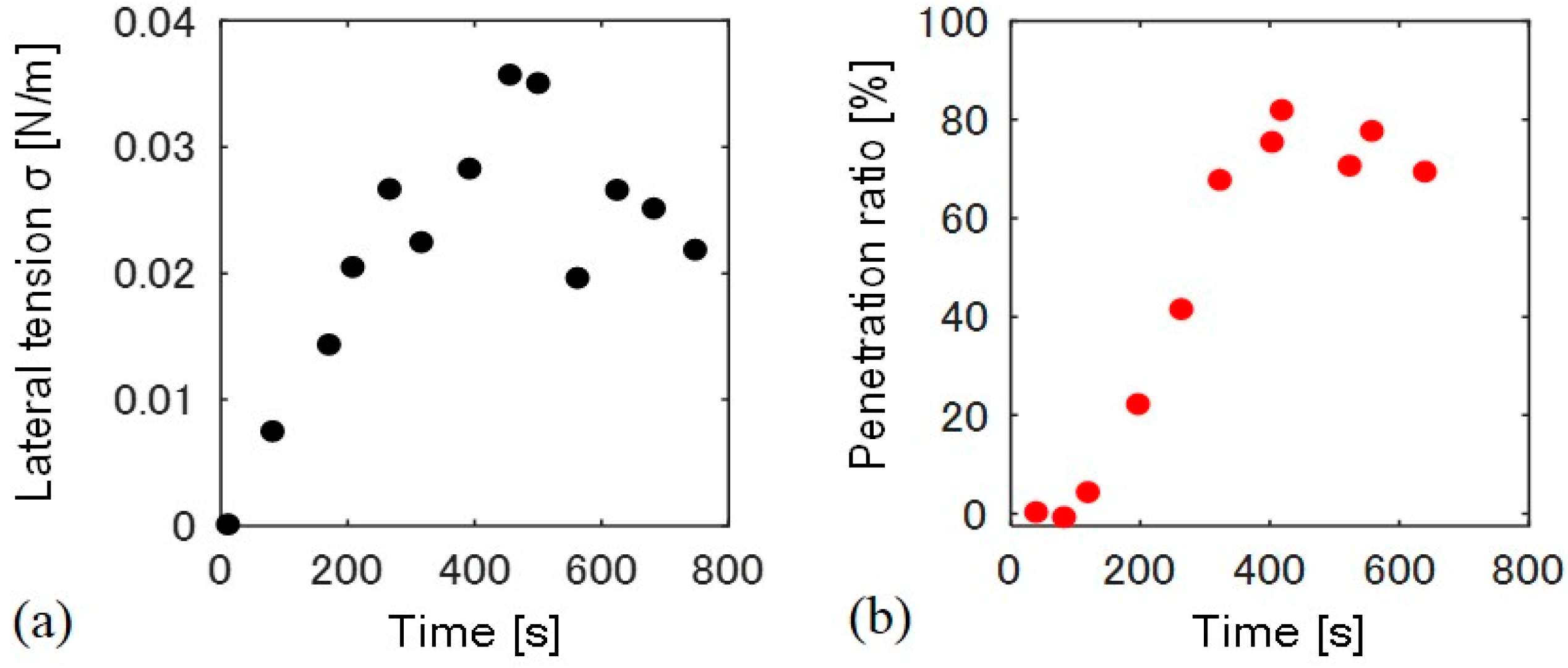

| Concentration Difference ΔC (Cin − Cout) (mM) | 2.5 | 10 | 10 | 12.5 | 25 | 50 |
|---|---|---|---|---|---|---|
| Lateral tension σf (N/m) | 0.003 | 0.017 | 0.027 | 0.032 | 0.027 | 0.026 |
| Penetration ratio (%) | −0.6 | 4.7 | 100 | 6.2 | 7.9 | 69 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigyou, K.; Nagai, K.H.; Hamada, T. Lateral Tension-Induced Penetration of Particles into a Liposome. Materials 2017, 10, 765. https://doi.org/10.3390/ma10070765
Shigyou K, Nagai KH, Hamada T. Lateral Tension-Induced Penetration of Particles into a Liposome. Materials. 2017; 10(7):765. https://doi.org/10.3390/ma10070765
Chicago/Turabian StyleShigyou, Kazuki, Ken H. Nagai, and Tsutomu Hamada. 2017. "Lateral Tension-Induced Penetration of Particles into a Liposome" Materials 10, no. 7: 765. https://doi.org/10.3390/ma10070765





