Wear Performance of Calcium Carbonate-Containing Knee Spacers
Abstract
:1. Introduction
2. Material and Methods
2.1. Test Specimens
2.2. Wear Simulation
2.3. Wear Analysis
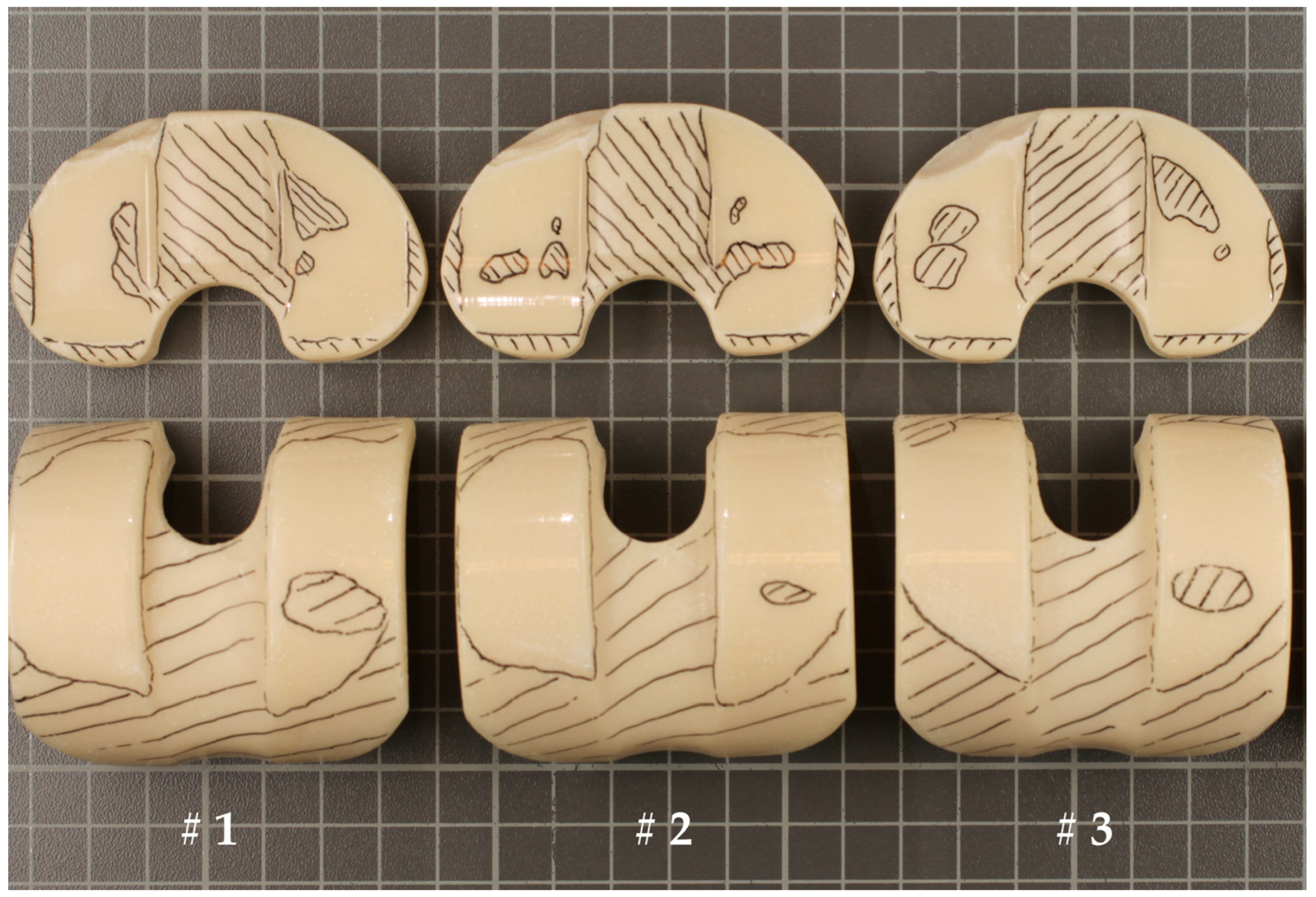
2.3.1. Photographic Documentation
2.3.2. Gravimetric Wear Measurements
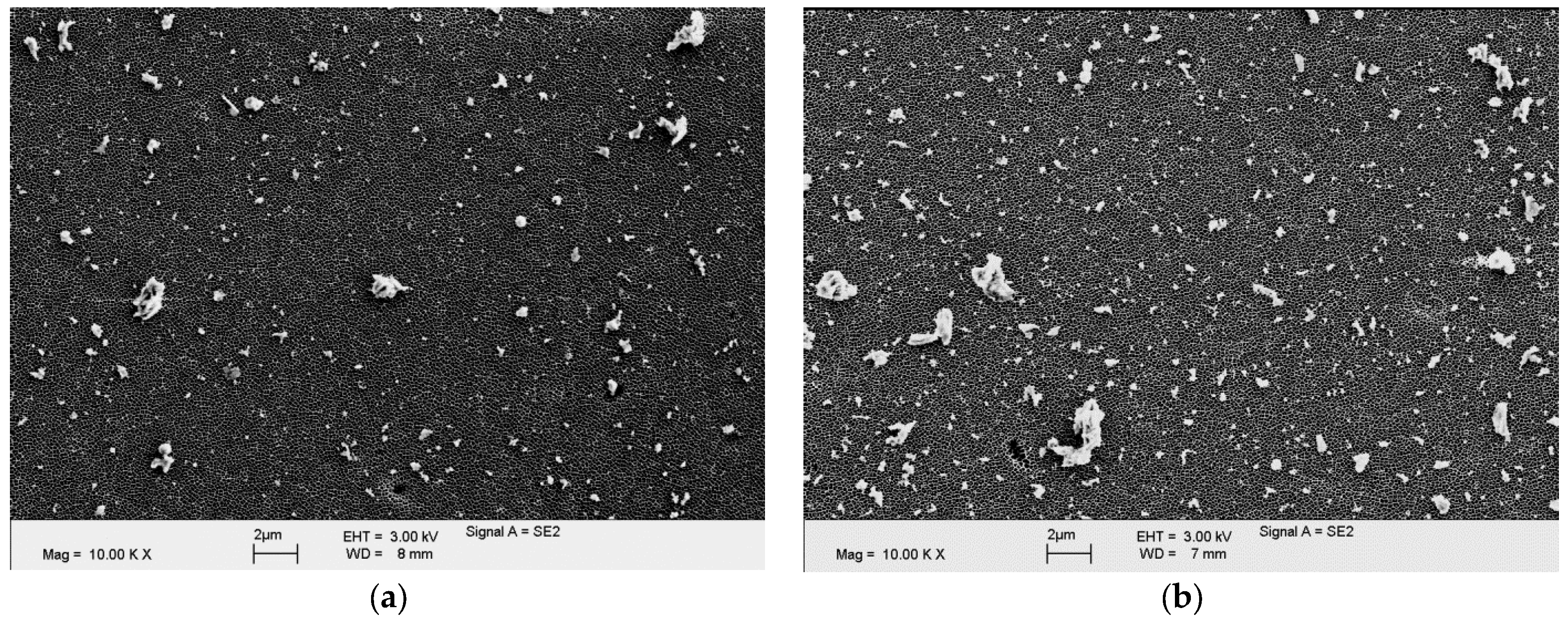
2.3.3. Particle Analysis
2.4. Statisitical Analysis
3. Results
3.1. Photographic Documentation
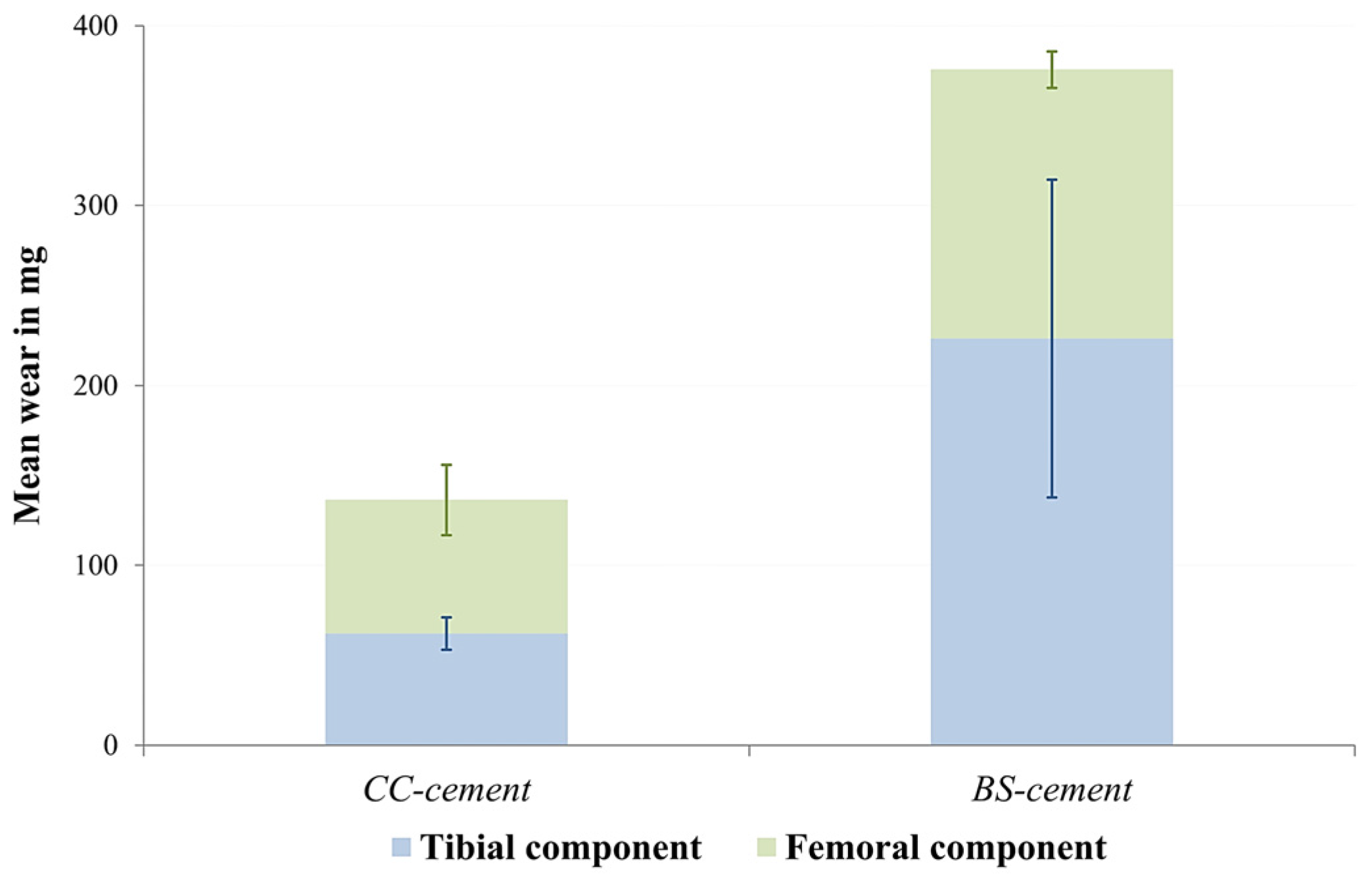
3.2. Gravimetric Measurements
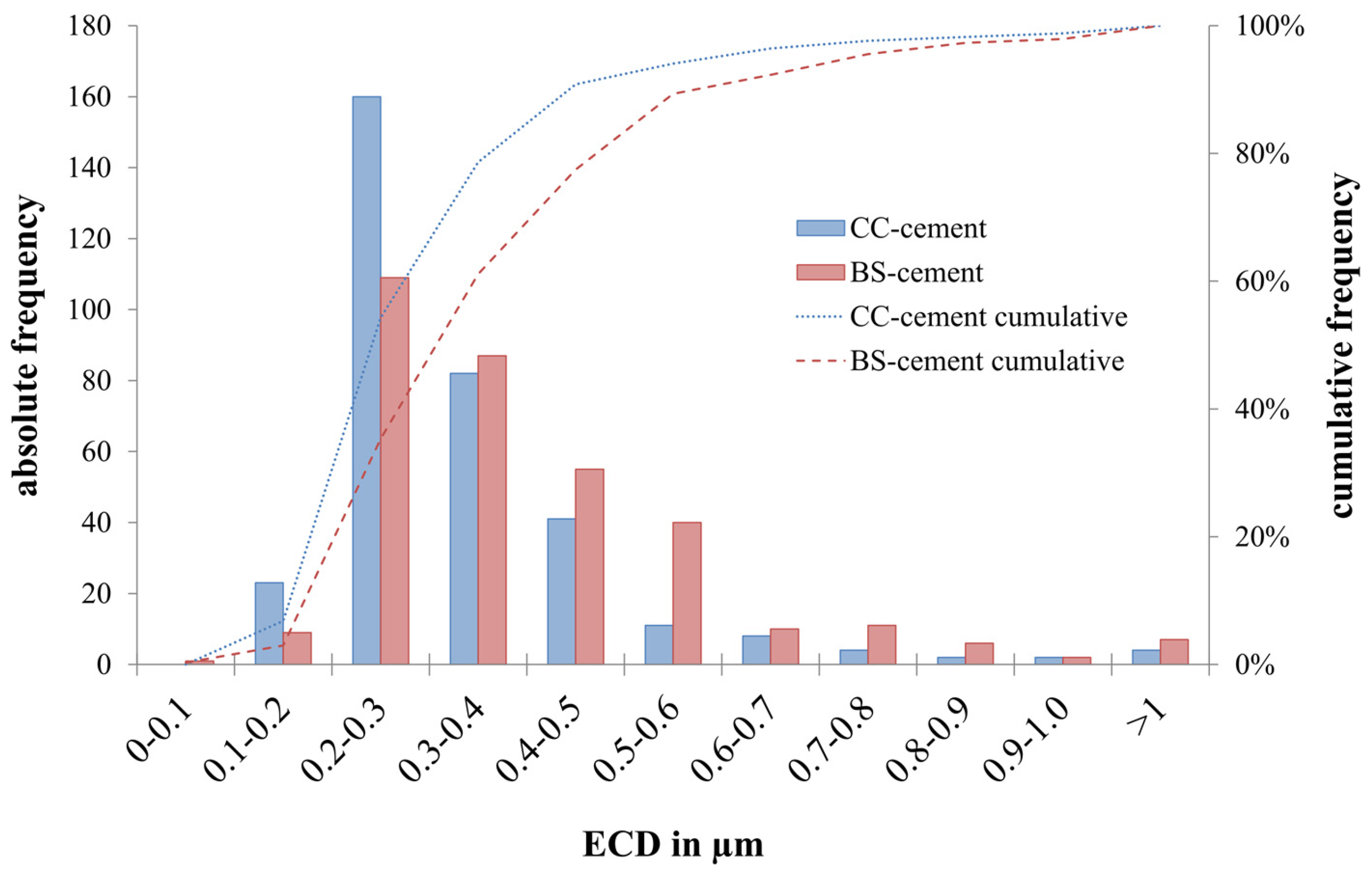
3.3. Particle Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMTI | Advanced Mechanical Technology Inc. (Watertown, MA) |
| ASTM | American Society for Testing and Materials |
| BS | Barium Sulphate |
| CC | Calcium Carbonate |
| EDX | Energy Dispersive X-ray analysis |
| ISO | International Standard Organization |
| PMMA | Polymethylmethacrylate |
| SEM | Scanning Electron Microscope |
References
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Sharkey, P.F.; Lichstein, P.M.; Shen, C.; Tokarski, A.T.; Parvizi, J. Why are total knee arthroplasties failing today—Has anything changed after 10 years? J. Arthroplast. 2014, 29, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Jamsen, E.; Stogiannidis, I.; Malmivaara, A.; Pajamaki, J.; Puolakka, T.; Konttinen, Y.T. Outcome of prosthesis exchange for infected knee arthroplasty: The effect of treatment approach. Acta. Orthop. 2009, 80, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Chen, L.H.; Chen, C.H.; Lee, M.S.; Yang, W.E.; Shih, C.H. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J. Trauma 2004, 56, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, P.R.; Dhotar, H.S.; Sternheim, A.; Gross, A.E.; Safir, O.; Backstein, D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: Techniques, controversies, and outcomes. J. Am. Acad. Orthop. Surg. 2014, 22, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Karachalios, T.; Koutalos, A.; Komnos, G. Management strategies for infected total hip arthroplasty. A critical appreciation of problems and techniques. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2014, 24 (Suppl. 10), S44–S47. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.; Rechtenbach, A.; Buchner, H.; Vogt, S.; Hahn, M. Articulating spacers used in two-stage revision of infected hip and knee prostheses abrade with time. Clin. Orthop. Relat. Res. 2011, 469, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Wimhurst, J.A.; Brooks, R.A.; Rushton, N. The effects of particulate bone cements at the bone-implant interface. J. Bone Jt. Surg. Br. Vol. 2001, 83, 588–592. [Google Scholar] [CrossRef]
- Sun, S.G.; Ma, B.A.; Zhou, Y.; Zhang, M.H.; Fan, Q.Y. Effects of bone cement particles on the function of pseudocapsule-derived fibroblasts. Acta Orthop. 2006, 77, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Gomoll, A.H.; Fitz, W.; Scott, R.D.; Thornhill, T.S.; Bellare, A. Nanoparticulate fillers improve the mechanical strength of bone cement. Acta Orthop. 2008, 79, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Grupp, T.M.; Fritz, B.; Schilling, C.; Chevalier, Y.; Utzschneider, S.; Jansson, V. The influence of third-body particles on wear rate in unicondylar knee arthroplasty: A wear simulator study with bone and cement debris. J. Mater. Sci. Mater. Med. 2013, 24, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Zietz, C.; Bergschmidt, P.; Lange, R.; Mittelmeier, W.; Bader, R. Third-body abrasive wear of tibial polyethylene inserts combined with metallic and ceramic femoral components in a knee simulator study. Int. J. Artif. Organs 2013, 36, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, R.G.; Kretzer, J.P.; Vogt, S.; Buchner, H.; Thomsen, M.N.; Lehner, B. Increased antibiotic release and equivalent biomechanics of a spacer cement without hard radio contrast agents. Diag. Microbiol. Infect. Dis. 2015, 83, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Mattarozzi, A.; Taddei, P.; Robotti, P.; Soffiatti, R.; Sudanese, A.; Toni, A. Investigations on the wear behaviour of the temporary PMMA-based hip Spacer-G®. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2003, 217, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.; Carnelli, D. Experimental evaluation of the biomechanical performances of a PMMA-based knee spacer. Knee 2007, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.; Fisher, J. Biological reactions to wear debris in total joint replacement. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2000, 214, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Noordin, S.; Schmalzried, T.P.; Campbell, P.; Amstutz, H.C. Synovial fluid from patients with prosthetic joint arthroplasty: Protein concentration and in vivo wear of polyethylene. Trans. Orthop. Res. Soc. 1997, 21, 1022–1023. [Google Scholar]
- Kinkel, S.; Wollmerstedt, N.; Kleinhans, J.A.; Hendrich, C.; Heisel, C. Patient activity after total hip arthroplasty declines with advancing age. Clin. Rthop. Relat. Res. 2009, 467, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.E.; Whiteside, L.A.; Tilden, D.S.; Noel, O.F. Reduced UHMWPE wear using magnesia-stabilized zirconia instead of CoCr femoral components in a knee simulator. J. Arthroplast. 2015, 30, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Reinders, J.; Sonntag, R.; Kretzer, J.P. How do gait frequency and serum-replacement interval affect polyethylene wear in knee-wear simulator tests? J. Mater. Sci. Mater. Med. 2014, 24, 2463. [Google Scholar] [CrossRef] [PubMed]
- Reinders, J.; Sonntag, R.; Kretzer, J.P. Synovial fluid replication in knee wear testing: An investigation of the fluid volume. J. Orthop. Res. 2014, 33, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kretzer, J.P.; Reinders, J.; Sonntag, R.; Hagmann, S.; Streit, M.; Jeager, S.; Moradi, B. Wear in total knee arthroplasty—Just a question of polyethylene?: Metal ion release in total knee arthroplasty. Int. Orthop. 2014, 38, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kretzer, J.P.; Jakubowitz, E.; Reinders, J.; Lietz, E.; Moradi, B.; Hofmann, K.; Sonntag, R. Wear analysis of unicondylar mobile bearing and fixed bearing knee systems: A knee simulator study. Acta Biomater. 2011, 7, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Grupp, T.M.; Kaddick, C.; Schwiesau, J.; Maas, A.; Stulberg, S.D. Fixed and mobile bearing total knee arthroplasty--influence on wear generation, corresponding wear areas, knee kinematics and particle composition. Clin. Biomech. 2009, 24, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S.M.; Frondoza, C.G.; Lennox, D.W. Effects of polymethylmethacrylate exposure upon macrophages. J. Orthop. Res. 1988, 6, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Glant, T.T.; Jacobs, J.J.; Molnar, G.; Shanbhag, A.S.; Valyon, M.; Galante, J.O. Bone resorption activity of particulate-stimulated macrophages. J. Bone Miner. Res. 1993, 8, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.D.; Cuckler, J.M.; Schumacher, H.R., Jr.; Ducheyne, P.; Baker, D.G. Comparison of the inflammatory response to particulate polymethylmethacrylate debris with and without barium sulfate. J. Orthop. Res. 1994, 12, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.; Bridget Matthews, J.; Stone, M.H.; Fisher, J.; Ingham, E. Comparison of the response of human peripheral blood mononuclear cells to challenge with particles of three bone cements in vitro. Biomaterials 2003, 24, 737–748. [Google Scholar] [CrossRef]
- Sieving, A.; Wu, B.; Mayton, L.; Nasser, S.; Wooley, P.H. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J. Biomed. Mater. Res. Part A 2003, 64, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Que, L.; Topoleski, L.D.; Parks, N.L. Surface roughness of retrieved CoCrMo alloy femoral components from PCA artificial total knee joints. J. Biomed. Mater. Res. 2000, 53, 111–118. [Google Scholar] [CrossRef]
- Arnholt, C.M.; MacDonald, D.W.; Malkani, A.L.; Klein, G.R.; Rimnac, C.M.; Kurtz, S.M.; Kocagoz, S.B.; Gilbert, J.L. Corrosion Damage and Wear Mechanisms in Long-Term Retrieved CoCr Femoral Components for Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, R.C.; Galante, J.O.; Leighty, R.M.; Natarajan, R.N.; Rosenberg, A.G. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin. Orthop. Relat. Res. 1994, 299, 31–43. [Google Scholar] [CrossRef]
- Hirakawa, K.; Bauer, T.W.; Yamaguchi, M.; Stulberg, B.N.; Wilde, A.H. Relationship between wear debris particles and polyethylene surface damage in primary total knee arthroplasty. J. Arthroplast. 1999, 14, 165–171. [Google Scholar] [CrossRef]




| Characteristics | CC-Cement (New Material) | BS-Cement (Commercial Available) |
|---|---|---|
| Manufacturer | Heraeus Medical, Germany | Spacer K, TECRES, Italy |
| Cement | Polymethylmethacrylate (PMMA) | Polymethylmethacrylate (PMMA) |
| Radio-opaque substance | Calcium carbonate (15% w/w) | Barium sulphate (10% w/w) |
| Antibiotic | Gentamicin (2.5% w/w) | Gentamicin (2.5% w/w) |
| Condition for application | Preformed | Preformed |
| Type | Mobile articulating spacer | Mobile articulating spacer |
| Spacer cement | Condyle | Tibia | ||
|---|---|---|---|---|
| Ra (in μm) | Rz (in μm) | Ra (in μm) | Rz (in μm) | |
| CC–cement | 1.166 ± 0.359 | 6.176 ± 1.613 | 1.560 ± 0.463 | 7.998 ± 2.219 |
| BS–cement | 1.127 ± 0.074 | 9.856 ± 0.457 | 1.086 ± 0.174 | 9.361 ± 1.523 |
| Particle parameters | CC-Cement | BS-Cement | p |
|---|---|---|---|
| Estimated total number of released particles | 7.07 × 1011 ± 1.33 × 1011 | 10.38 × 1011 ± 1.17 × 1011 | 0.136 |
| Equivalent circle diameter (ECD) in μm | 0.378 ± 0.009 | 0.427 ± 0.010 | 0.020 |
| Aspect ratio (AR) | 1.730 ± 0.017 | 1.655 ± 0.0288 | 0.087 |
| Roundness (R) | 0.592 ± 0.005 | 0.592 ± 0.005 | 0.962 |
| Form factor (FF) | 0.674 ± 0.003 | 0.677 ± 0.005 | 0.574 |
| Study | Gravimetric Wear in mg per Million Cycles | Estimated Total Number of Particles Released per Million Cycles |
|---|---|---|
| Current study | CC-cement: 272.63 (±75.17) 1 BS-cement: 751.06 (±322.04) 1 | CC-cement: 14.14 × 1011 (±4.62 × 1011) 1 BS-cement: 20.75 × 1011 (±4.06 × 1011) 1 |
| Roy et al. [19] | 0.06 (±0.06) | - |
| Reinders et al. [20] | 8.0 (±0.9) | 7.1 × 1011 (±1.0 × 1011) |
| Reinders et al. [21] | 9.7 (±1.2) | 3.93 × 1011 1 |
| Kretzer et al. [22] | 7.28 (±0.27) | - |
| Kretzer et al. [23] | 10.55–16.08 | 2.63 × 1011–3.36 × 1011 |
| Grupp et al. [24] | 6.6 (±2.0)–9.7 (±1.3) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, U.; Reinders, J.; Smith-Romanski, S.; Kretzer, J.P. Wear Performance of Calcium Carbonate-Containing Knee Spacers. Materials 2017, 10, 805. https://doi.org/10.3390/ma10070805
Mueller U, Reinders J, Smith-Romanski S, Kretzer JP. Wear Performance of Calcium Carbonate-Containing Knee Spacers. Materials. 2017; 10(7):805. https://doi.org/10.3390/ma10070805
Chicago/Turabian StyleMueller, Ulrike, Joern Reinders, Sydney Smith-Romanski, and Jan Philippe Kretzer. 2017. "Wear Performance of Calcium Carbonate-Containing Knee Spacers" Materials 10, no. 7: 805. https://doi.org/10.3390/ma10070805





