Preparation and Properties of a Novel Microcrystalline Cellulose-Filled Composites Based on Polyamide 6/High-Density Polyethylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.3.1. Differential Scanning Calorimetry
2.3.2. Thermogravimetric Analysis
2.3.3. Mechanical Testing
2.3.4. Morphological Analysis
2.3.5. Dynamic Mechanical Analysis
3. Results and Discussion
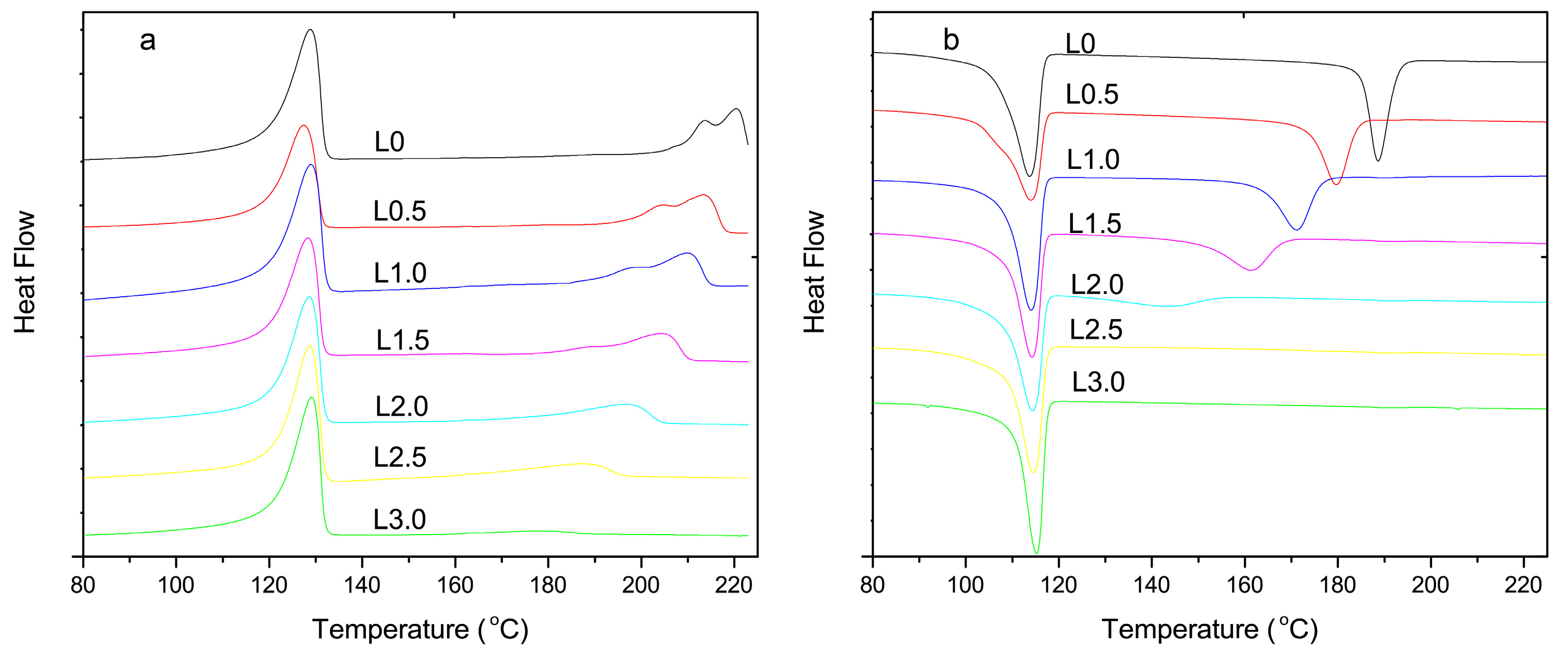
3.1. Differential Scanning Calorimetry Analysis
3.2. Thermal Analysis
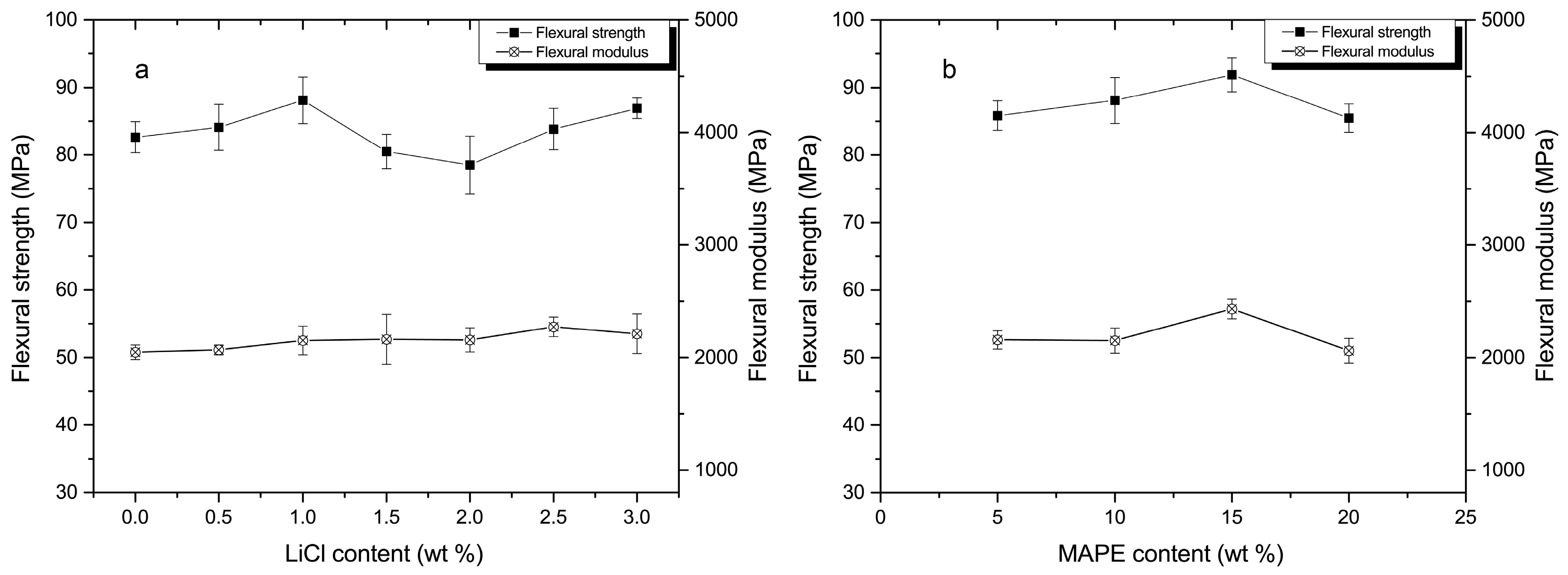
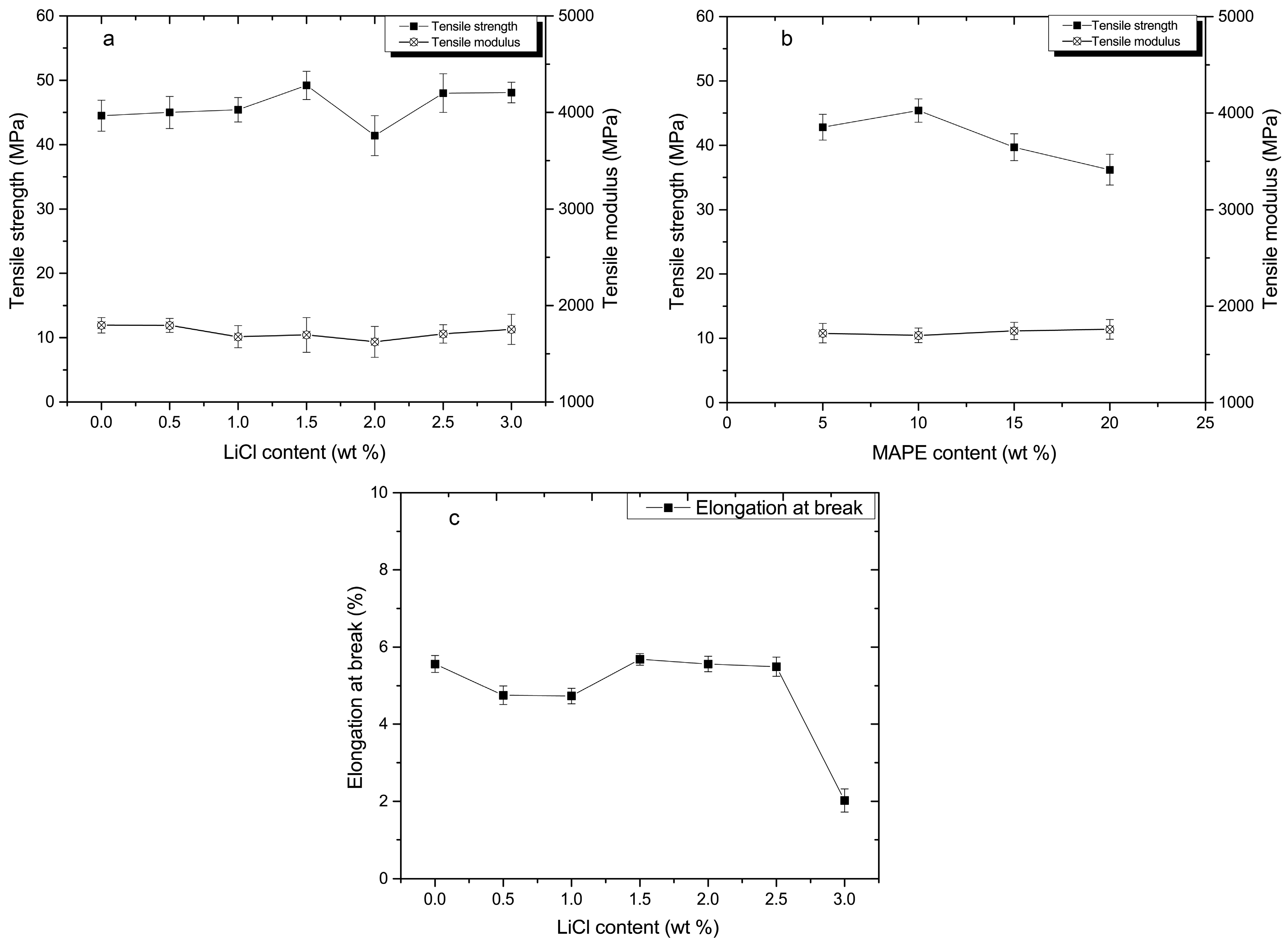
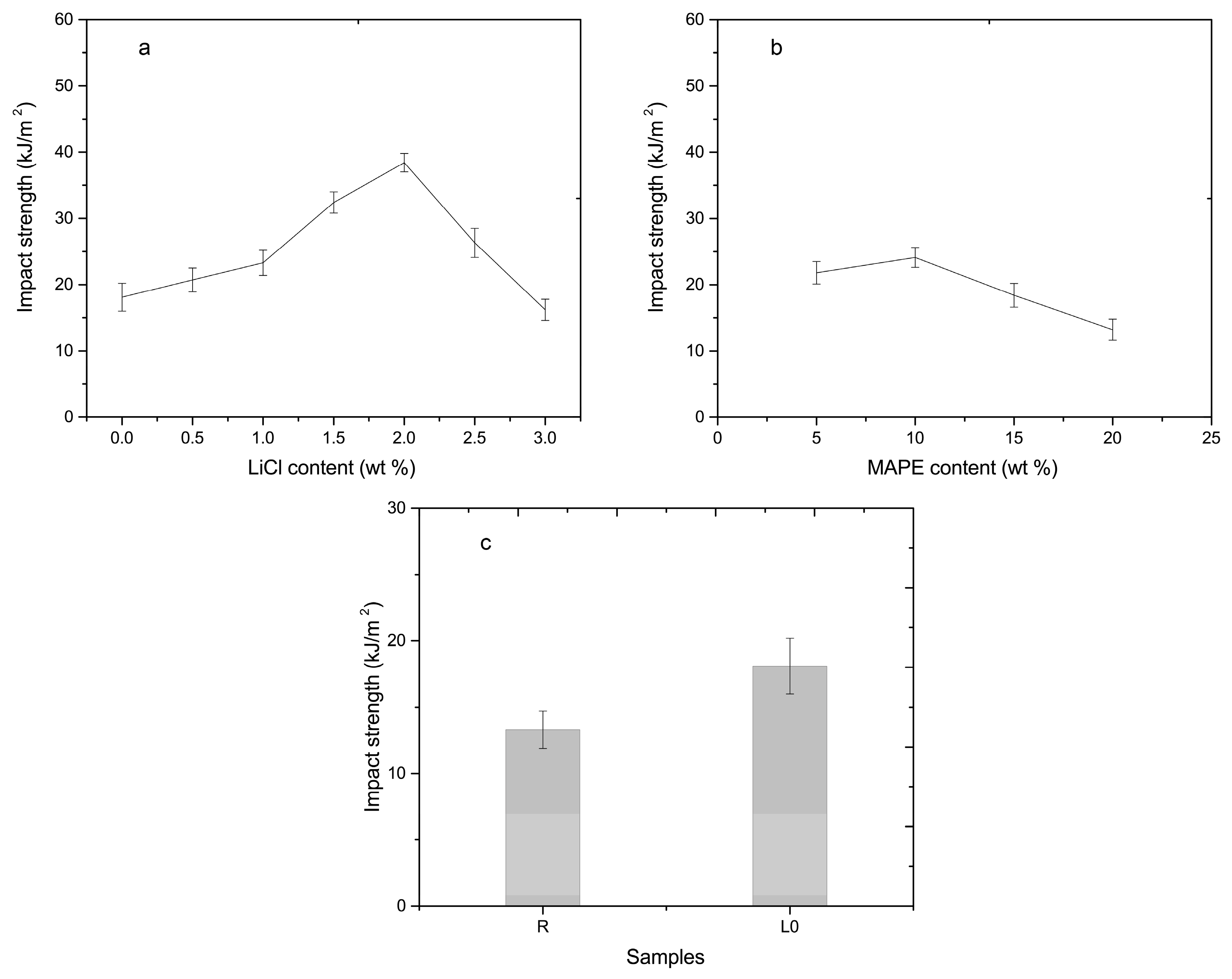
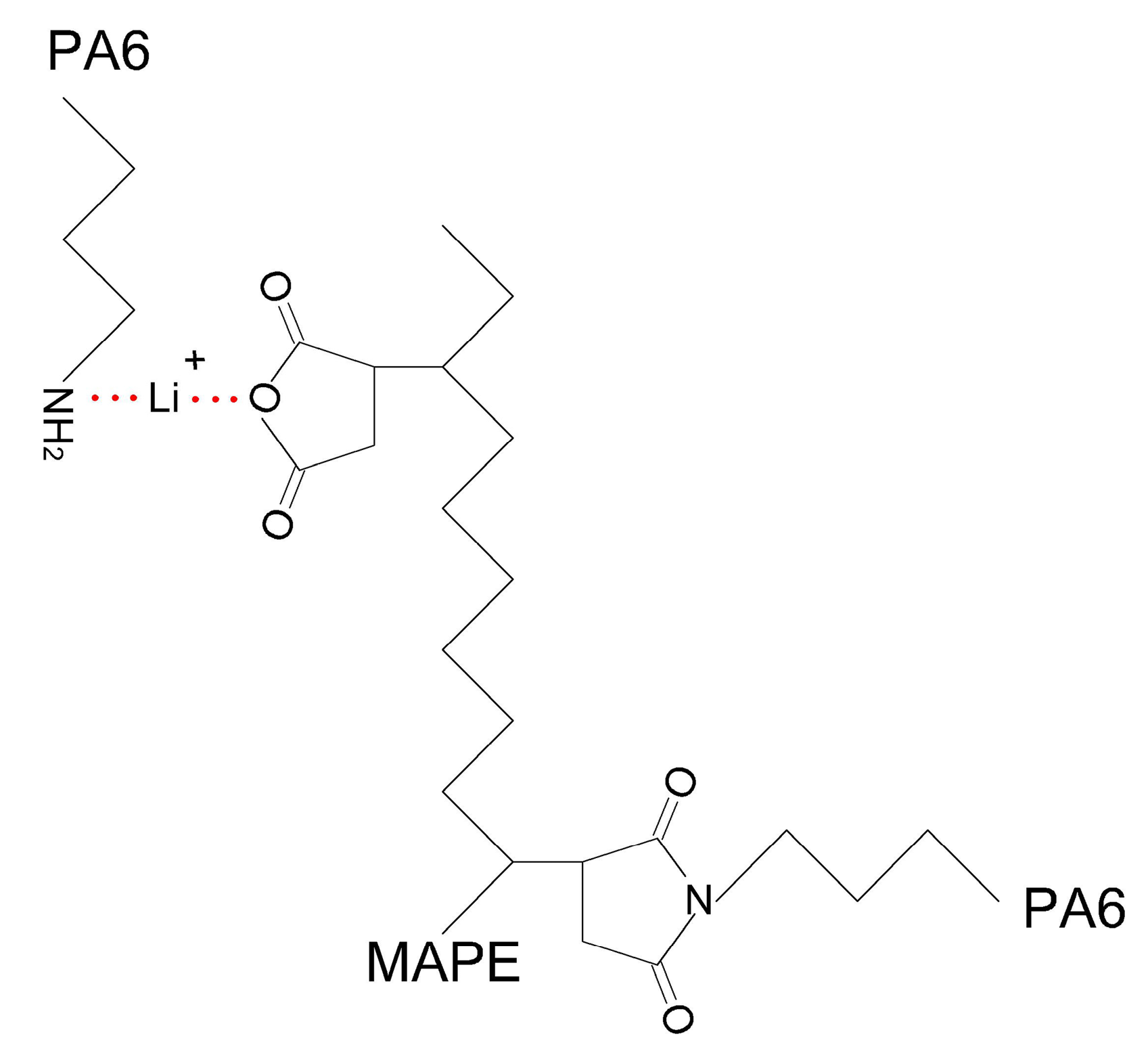
3.3. Mechanical Analysis
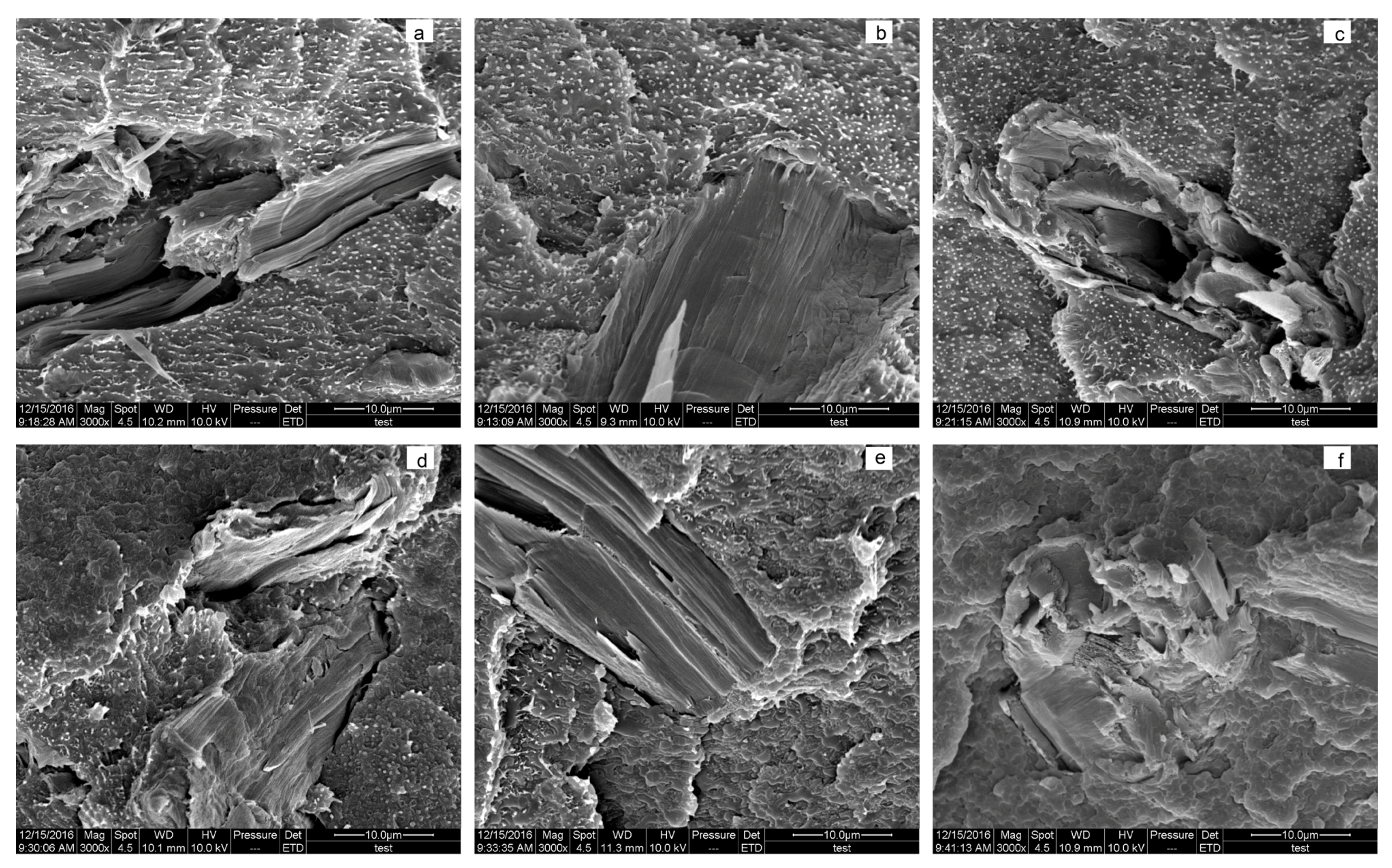
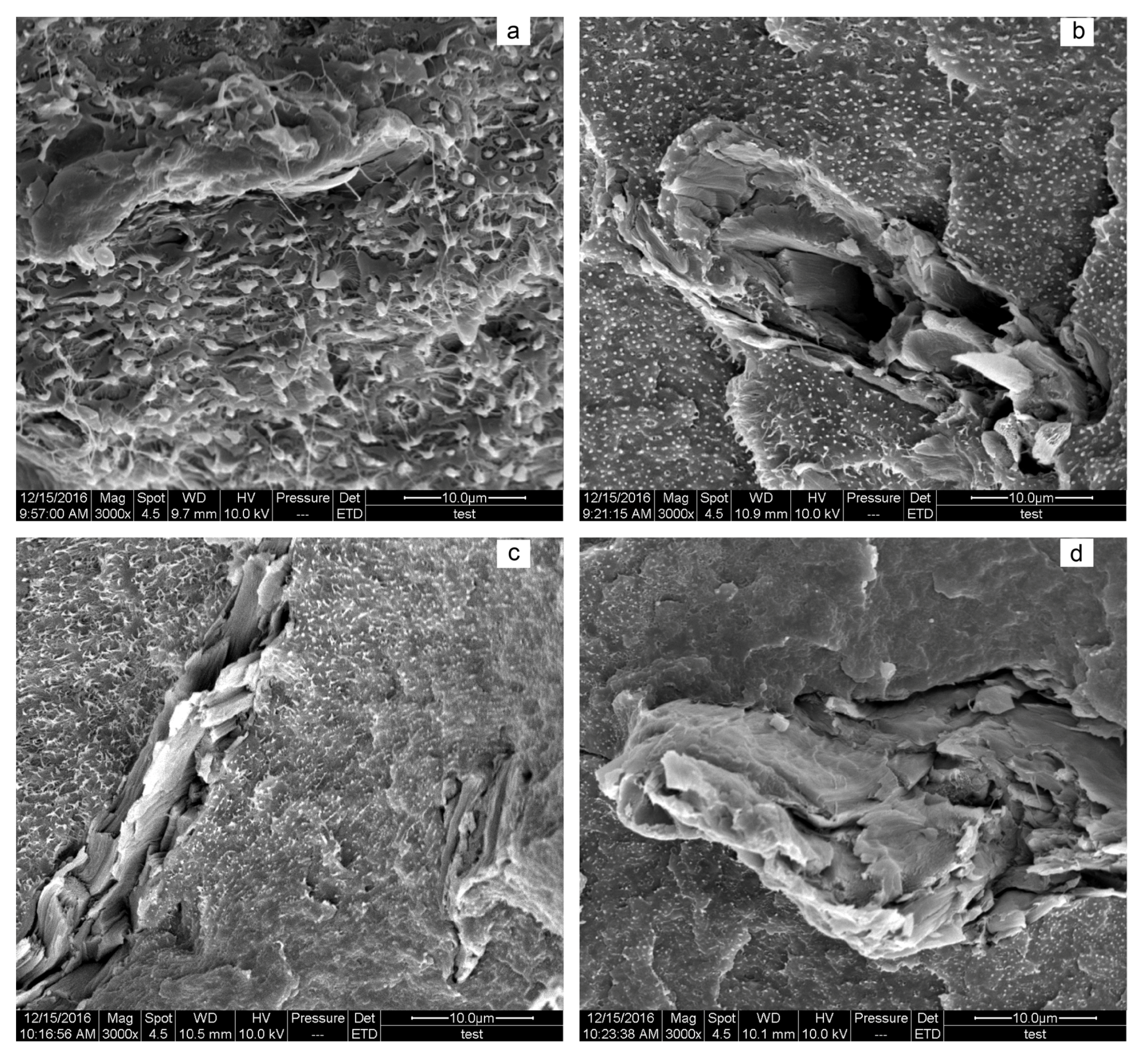
3.4. Morphology Analysis
3.5. Dynamic Mechanical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kossentini-Kallel, T.; Houichi, H. Reactive blending of PE-GMA/PA6 effect of composition and processing conditions. Polym. Adv. Technol. 2015, 26, 539–545. [Google Scholar] [CrossRef]
- Chow, W.S.; Ishak, Z.A.M. Polyamide blended-based nanocomposites: A review. Express Polym. Lett. 2015, 9, 211–232. [Google Scholar] [CrossRef]
- Dasdemir, M.; Maze, B.; Anantharamaiah, N.; Pourdeyhimi, B. Reactive compatibilization of polyamide 6/polyethylene nonwoven based thermoplastic composites. Eur. Polym. J. 2015, 63, 194–206. [Google Scholar] [CrossRef]
- Attari, M.; Arefazar, A.; Bakhshandeh, G. Mechanical and thermal properties of toughened PA6/HDPE/SEBS-g-MA/Clay nanocomposite. Polym. Eng. Sci. 2015, 55, 29–33. [Google Scholar] [CrossRef]
- Wang, W.; Dou, R.; Zhou, Y.; Li, L.P.; Yin, B.; Yang, M.B. The influence of mixing order on the phase inversion and crystallization behavior of polyamide 6/maleic anhydride-g-ethylene-propylene-diene rubber/high-density polyethylene blend. J. Elastom. Plast. 2015, 47, 608–624. [Google Scholar] [CrossRef]
- Apollonio, C.; Covas, D.I.C.; de Marinis, G.; Leopardi, A.; Ramos, H.M. Creep functions for transients in HDPE pipes. Urban Water J. 2014, 11, 160–166. [Google Scholar] [CrossRef]
- Covas, D.; Stoianov, I.; Mano, J.F.; Ramos, H.; Graham, N.; Maksimovic, C. The dynamic effect of pipe-wall viscoelasticity in hydraulic transients. Part I—Experimental analysis and creep characterization. J. Hydraul. Res. 2004, 42, 516–530. [Google Scholar] [CrossRef]
- Covas, D.; Stoianov, I.; Mano, J.F.; Ramos, H.; Graham, N.; Maksimovic, C. The dynamic effect of pipe-wall viscoelasticity in hydraulic transients. Part II—Model development, calibration and verification. J. Hydraul. Res. 2005, 43, 56–70. [Google Scholar] [CrossRef]
- Pappu, A.; Patil, V.; Jain, S.; Mahindrakar, A.; Haque, R.; Thakur, V.K. Advances in industrial prospective of cellulosic macromolecules enriched banana biofibre resources: A review. Int. J. Biol. Macromol. 2015, 79, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Rapid synthesis of graft copolymers from natural cellulose fibers. Carbohydr. Polym. 2013, 98, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Li, K.C. Partial replacement of silica with microcrystalline cellulose in rubber composites. Compos. Part A-Appl. Sci. Manuf. 2009, 40, 1597–1605. [Google Scholar] [CrossRef]
- Lamnawar, K.; Baudouin, A.; Maazouz, A. Interdiffusion/reaction at the polymer/polymer interface in multilayer systems probed by linearviscoelasticity coupled to FTIR and NMR measurements. Eur. Polym. J. 2010, 46, 1604–1622. [Google Scholar] [CrossRef]
- Yordanov, C.; Minkova, L. Fractionated crystallization of compatibilized LDPE/PA6 blends. Eur. Polym. J. 2005, 41, 527–534. [Google Scholar] [CrossRef]
- Argoud, A.; Trouillet-Fonti, L.; Ceccia, S.; Sotta, P. Morphologies in polyamide 6/high-density polyethylene blends with high amounts of reactive compatibilizer. Eur. Polym. J. 2014, 50, 177–189. [Google Scholar] [CrossRef]
- Amintowlieh, Y.; Sardashti, A.; Simon, L.C. Polyamide 6-wheat straw composites: Degradation kinetics. Polym. Compos. 2012, 33, 985–989. [Google Scholar] [CrossRef]
- Amintowlieh, Y.; Sardashti, A.; Simon, L.C. Polyamide 6-wheat straw composites: Effects of additives on physical and mechanical properties of the composite. Polym. Compos. 2012, 33, 976–984. [Google Scholar] [CrossRef]
- Amintowlieh, Y. Nylon-6/Agricultural Filler Composites. Ph.D. Dissertation, University of Waterloo, Waterloo, ON, Canada, 2010. [Google Scholar]
- Cai, H.Z.; Yi, W.M.; Bai, X.Y. Effect of coupling agents on the mechanical properties of wheat straw flour & polyethylene composites. In Proceedings of the 2011 International Conference on Manufacturing Science and Technology, Singapore, 16–18 September 2011. [Google Scholar]
- Liu, H.; Wu, Q.; Han, G.; Yao, F.; Kojima, Y.; Suzuki, S. Compatibilizing and toughening bamboo flour-filled HDPE composites: Mechanical properties and morphology. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1891–1900. [Google Scholar] [CrossRef]
- Shao, H.J.; Wu, B.; Yang, J.K.; Zhang, K.Z.; Qin, S.H. Effect of LiCl on the miscibility and crystallization behavior of a hydrophilic PP/PP-g-MAH/PA6 blend. J. Polym. Res. 2015, 22. [Google Scholar] [CrossRef]
- Sallem-Idrissi, N.; Sclavons, M.; Debecker, D.P.; Devaux, J. Miscible raw lignin/nylon 6 blends: Thermal and mechanical performances. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Verghese, K.N.E.; Jensen, R.E.; Lesko, J.J.; Ward, T.C. Effects of molecular relaxation behavior on sized carbon fiber-vinyl ester matrix composite properties. Polymer 2001, 42, 1633–1645. [Google Scholar] [CrossRef]
- Taividi, M.; Falk, R.H.; Hermanson, J.C. Effect of natural fibers on thermal and mechanical properties of natural fiber polypropylene composites studied by dynamic mechanical analysis. J. Appl. Polym. Sci. 2006, 101, 4341–4349. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.W.; Xie, Y.J.; Lee, S.; Wu, Q.L. Effects of use of coupling agents on the properties of microfibrillar composite based on high-density polyethylene and polyamide-6. Polym. Bull. 2014, 71, 685–703. [Google Scholar] [CrossRef]


| Samples | LiCl (wt %) | MAPE (wt %) | Processing Temperature (°C) |
|---|---|---|---|
| L0 | 0 | 10 | 240 |
| L0.5 | 0.5 | 10 | 235 |
| L1.0 | 1 | 10 | 230 |
| L1.5 | 1.5 | 10 | 225 |
| L2.0 | 2 | 10 | 220 |
| L2.5 | 2.5 | 10 | 210 |
| L3.0 | 3 | 10 | 200 |
| M5 | 1 | 5 | 230 |
| M15 | 1 | 15 | 230 |
| M20 | 1 | 20 | 230 |
| R | 0 | 0 | 240 |
| Samples | Tm (°C) | Tp (°C) | Crystallization Time (s) | Xc (%) |
|---|---|---|---|---|
| L0 | 220.4 | 188.6 | 47 | 20.0 |
| L0.5 | 213.4 | 179.7 | 64 | 19.2 |
| L1.0 | 209.9 | 171.1 | 78 | 17.8 |
| L1.5 | 204.5 | 161.3 | 107 | 15.3 |
| L2.0 | 197.0 | 143.3 | 169 | 10.9 |
| L2.5 | 187.5 | - | - | 9.4 |
| L3.0 | - | - | - | - |
| Samples | Tp (°C) | ||
|---|---|---|---|
| Peak A | Peak B | Peak C | |
| L0 | 366.2 | 442.5 | 482.7 |
| L0.5 | 350.8 | 429.9 | 483.7 |
| L1.0 | 343.0 | 422.9 | 480.8 |
| L1.5 | 336.2 | 420.6 | 480.6 |
| L2.0 | 319.8 | 418.5 | 479.8 |
| L2.5 | 317.1 | 423.2 | 482.5 |
| L3.0 | 314.6 | 421.0 | 483.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Yi, S.; He, J.; Wang, H.; Fang, Y.; Wang, Q. Preparation and Properties of a Novel Microcrystalline Cellulose-Filled Composites Based on Polyamide 6/High-Density Polyethylene. Materials 2017, 10, 808. https://doi.org/10.3390/ma10070808
Xu S, Yi S, He J, Wang H, Fang Y, Wang Q. Preparation and Properties of a Novel Microcrystalline Cellulose-Filled Composites Based on Polyamide 6/High-Density Polyethylene. Materials. 2017; 10(7):808. https://doi.org/10.3390/ma10070808
Chicago/Turabian StyleXu, Shihua, Shunmin Yi, Jun He, Haigang Wang, Yiqun Fang, and Qingwen Wang. 2017. "Preparation and Properties of a Novel Microcrystalline Cellulose-Filled Composites Based on Polyamide 6/High-Density Polyethylene" Materials 10, no. 7: 808. https://doi.org/10.3390/ma10070808






