Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Printing of 3D Scaffolds and Surface Treatment
2.2. Preparation of Platelet-Rich Plasma
2.3. Coating Scaffolds with PRP
2.4. Cell Culture
2.5. In Vitro Experiments
2.5.1. Scaffold Characterization
2.5.2. Cell Attachment
2.5.3. Cell Migration
2.5.4. Cell Proliferation
2.5.5. Alkaline Phosphatase (ALP) Activity Assay
2.5.6. RNA Isolation and Analysis by Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.6. In Vivo Bone Regeneration
2.6.1. Surgery Procedures
2.6.2. Harvesting of Samples
2.6.3. Micro-CT Analysis
2.6.4. Histological Staining
2.6.5. Statistical Analysis
3. Results
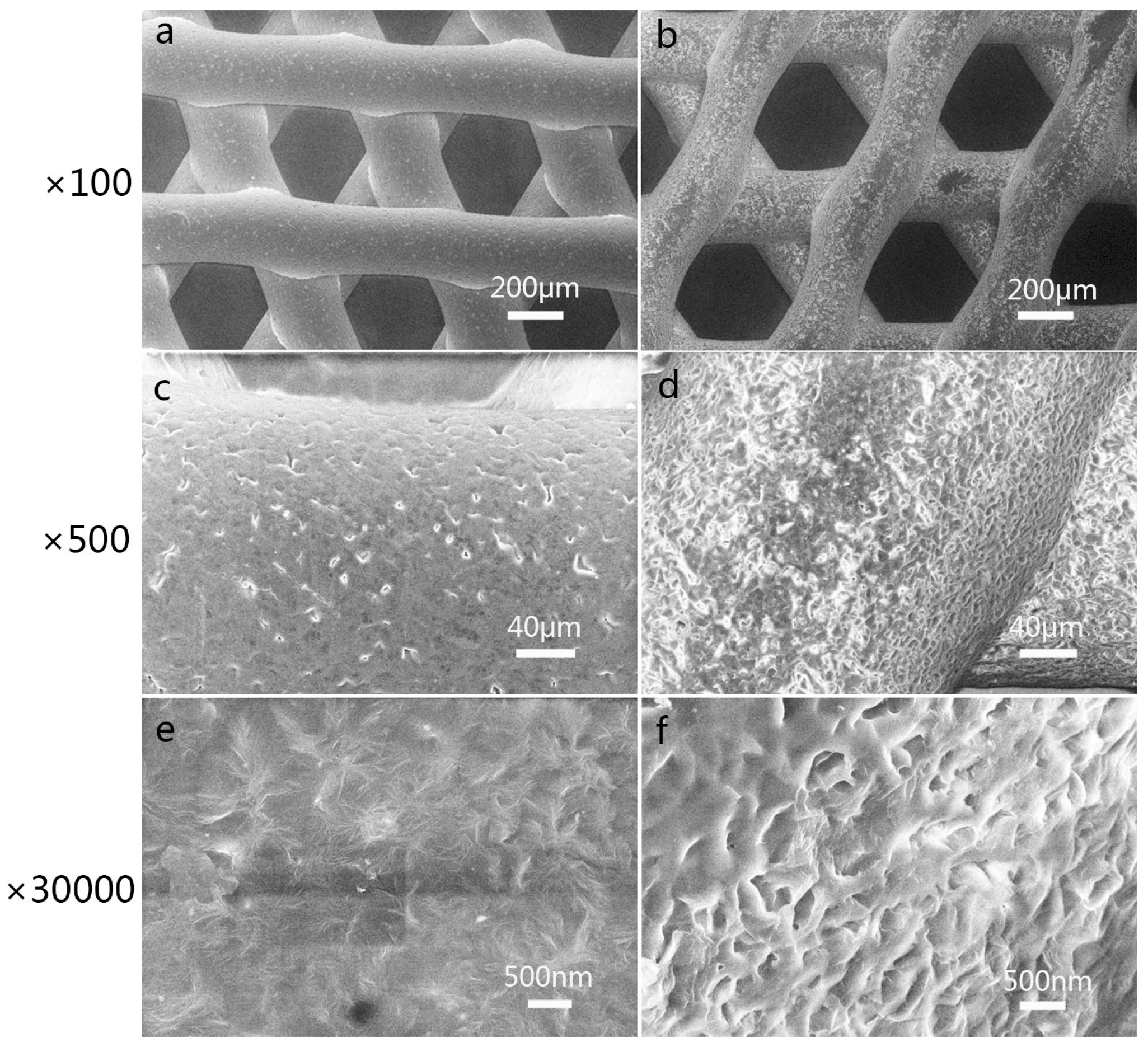
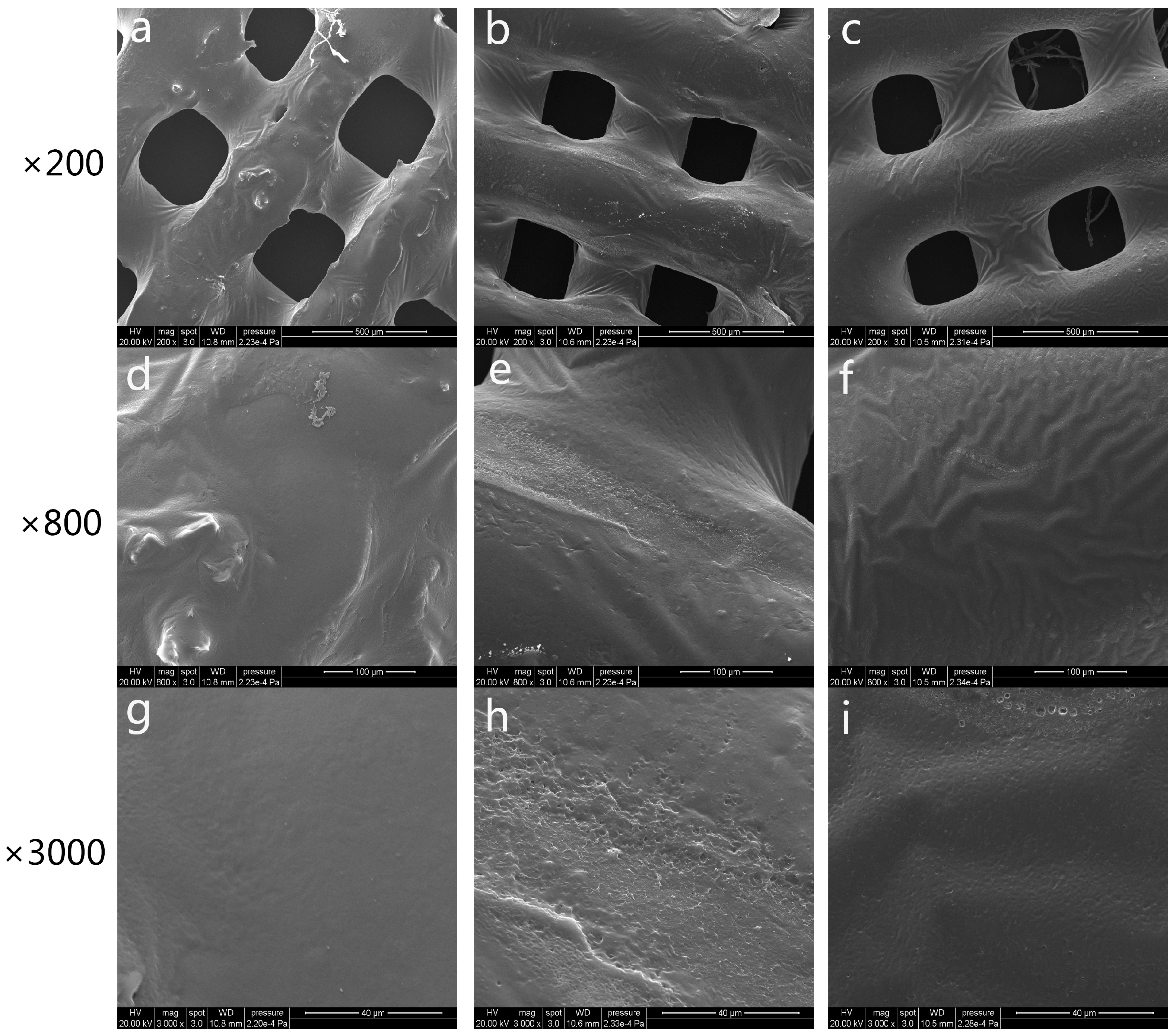
3.1. Characterization of 3D-Printed PCL Scaffolds after Surface Treatment
3.2. Characterization of 3D-Printed PRP-PCL Scaffolds
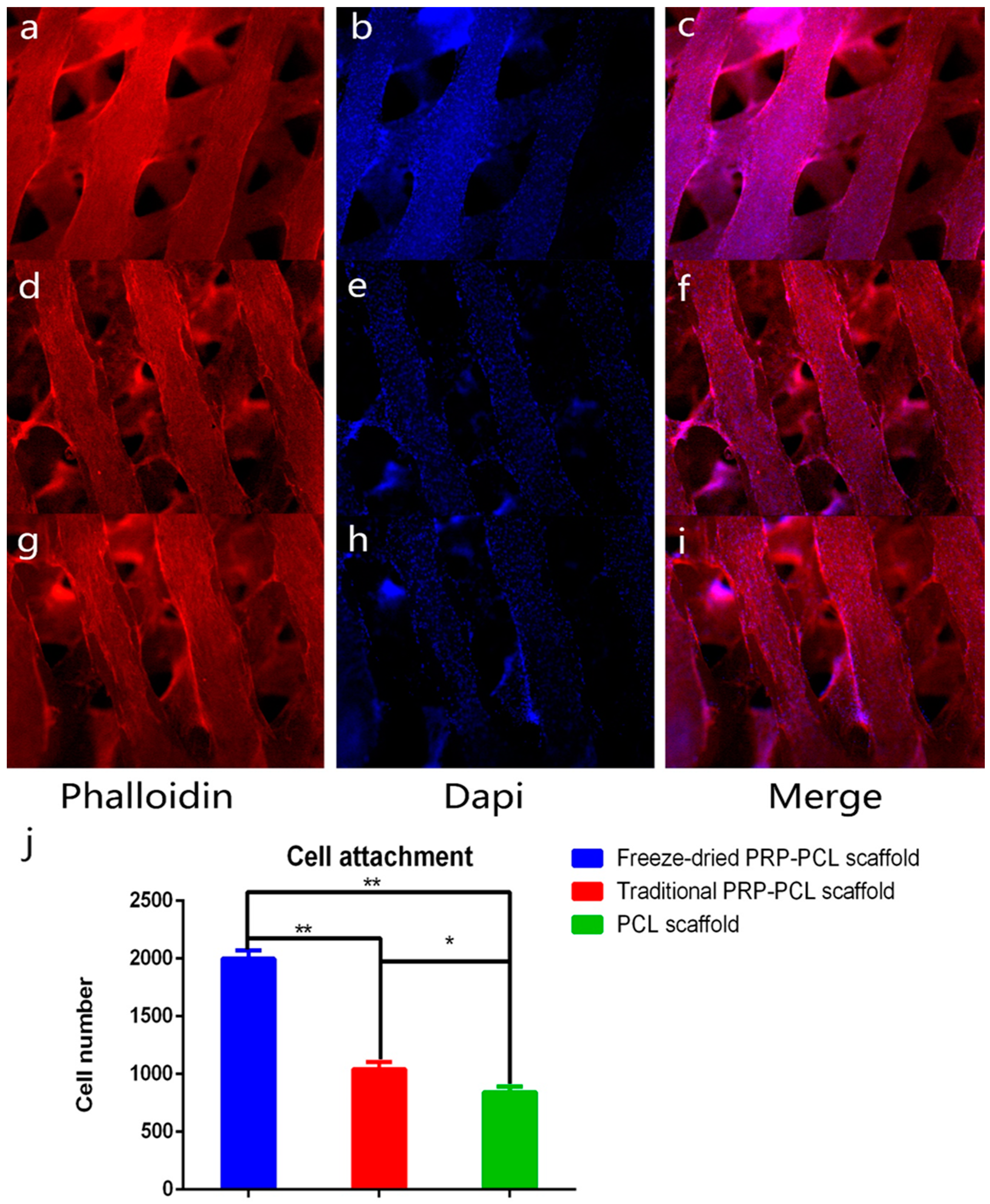
3.3. Effect of the PRP-PCL Scaffold on Cell Attachment
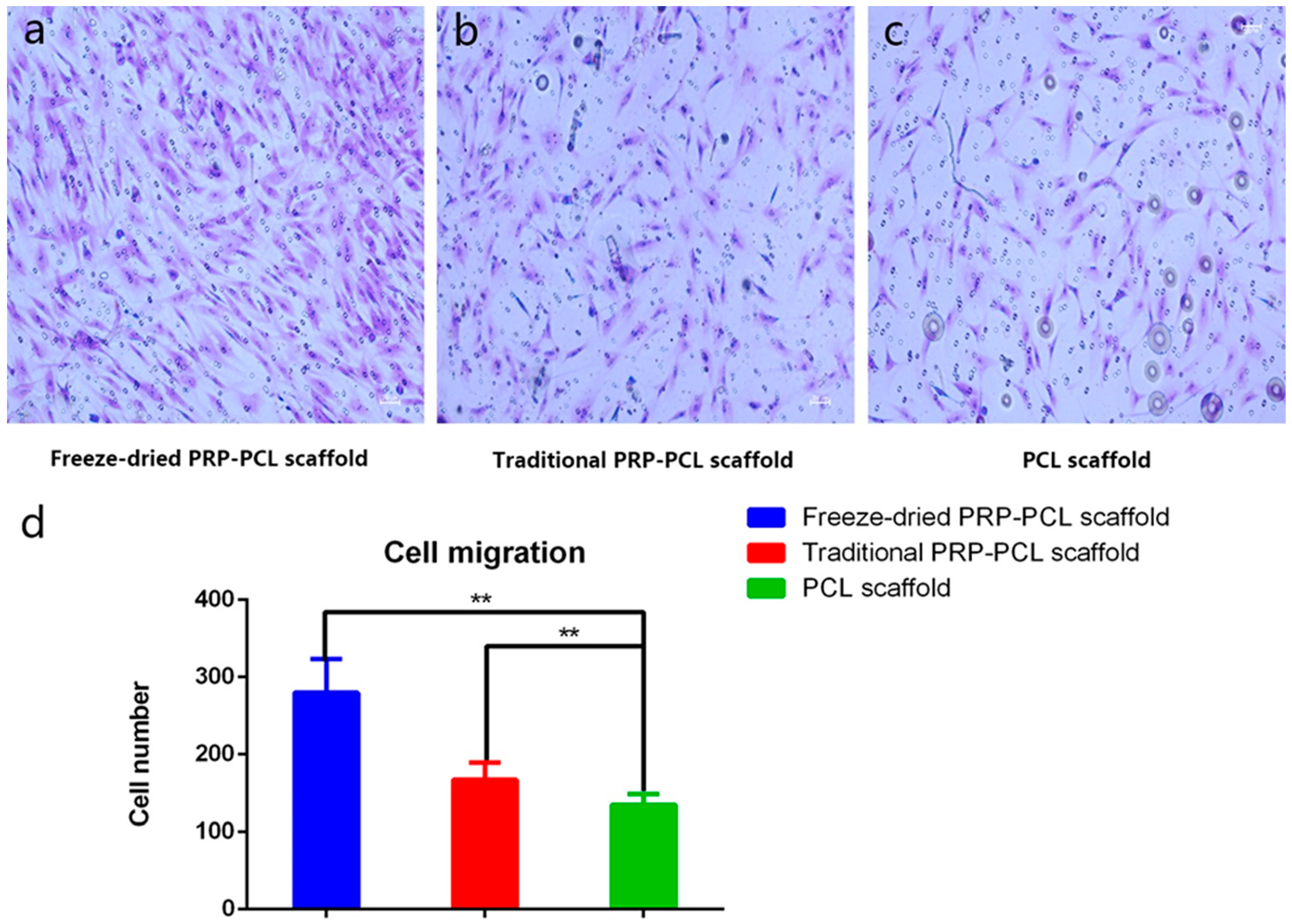
3.4. Effect of the PRP-PCL Scaffold on Cell Migration
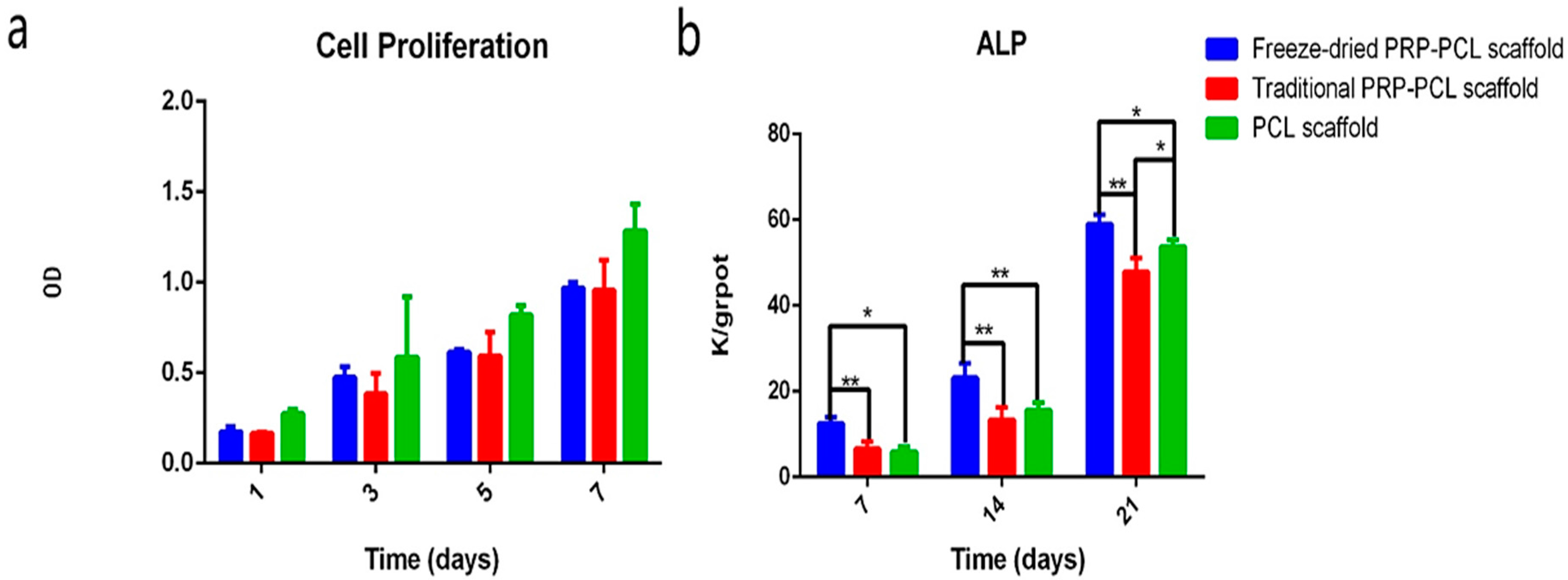
3.5. Effect of the PRP-PCL Scaffold on Cell Proliferation
3.6. ALP Activity Assay
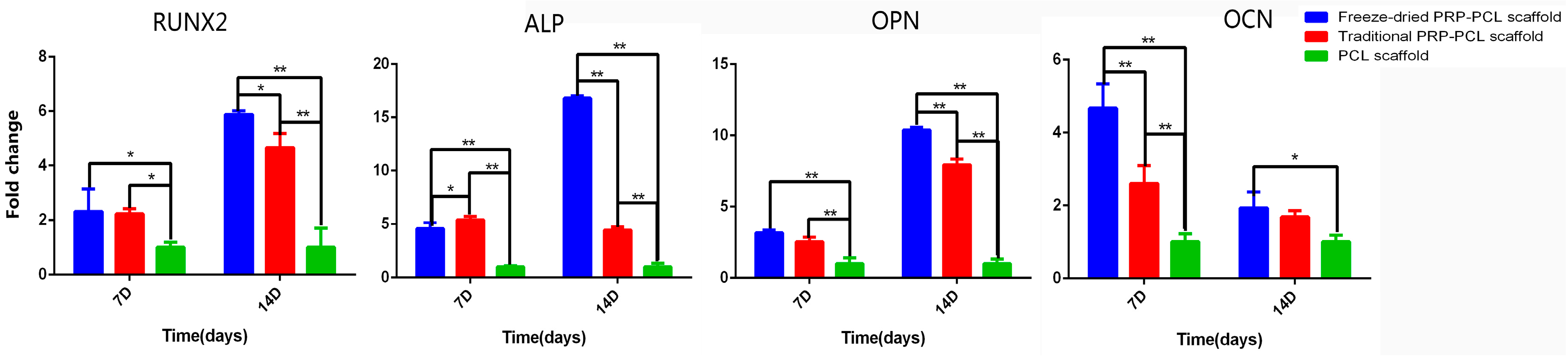
3.7. Differentiation of DPSCs: RT-PCR
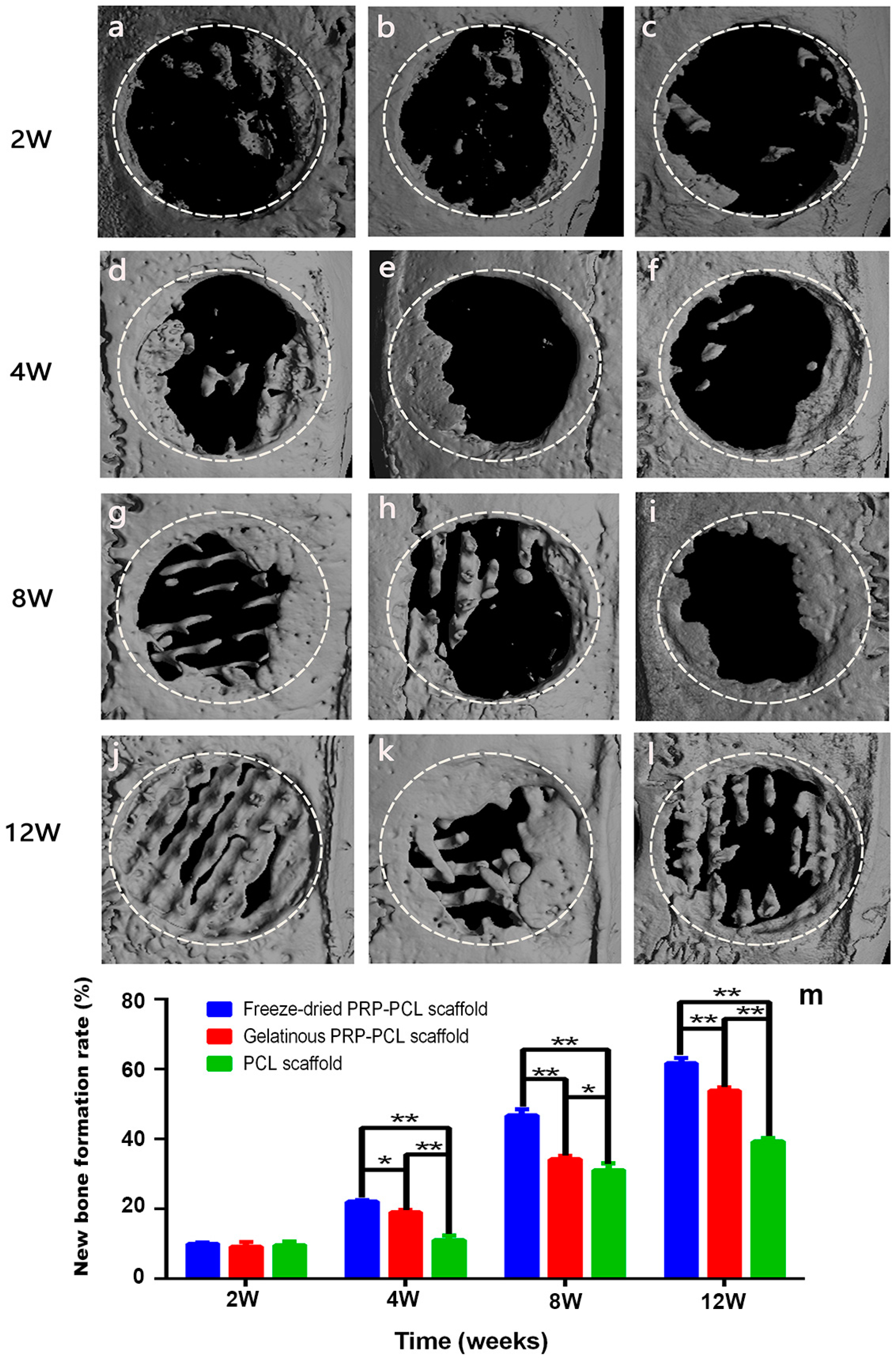
3.8. Micro-CT Analysis of the Critical-Size Calvarial Bone Defect in Rats
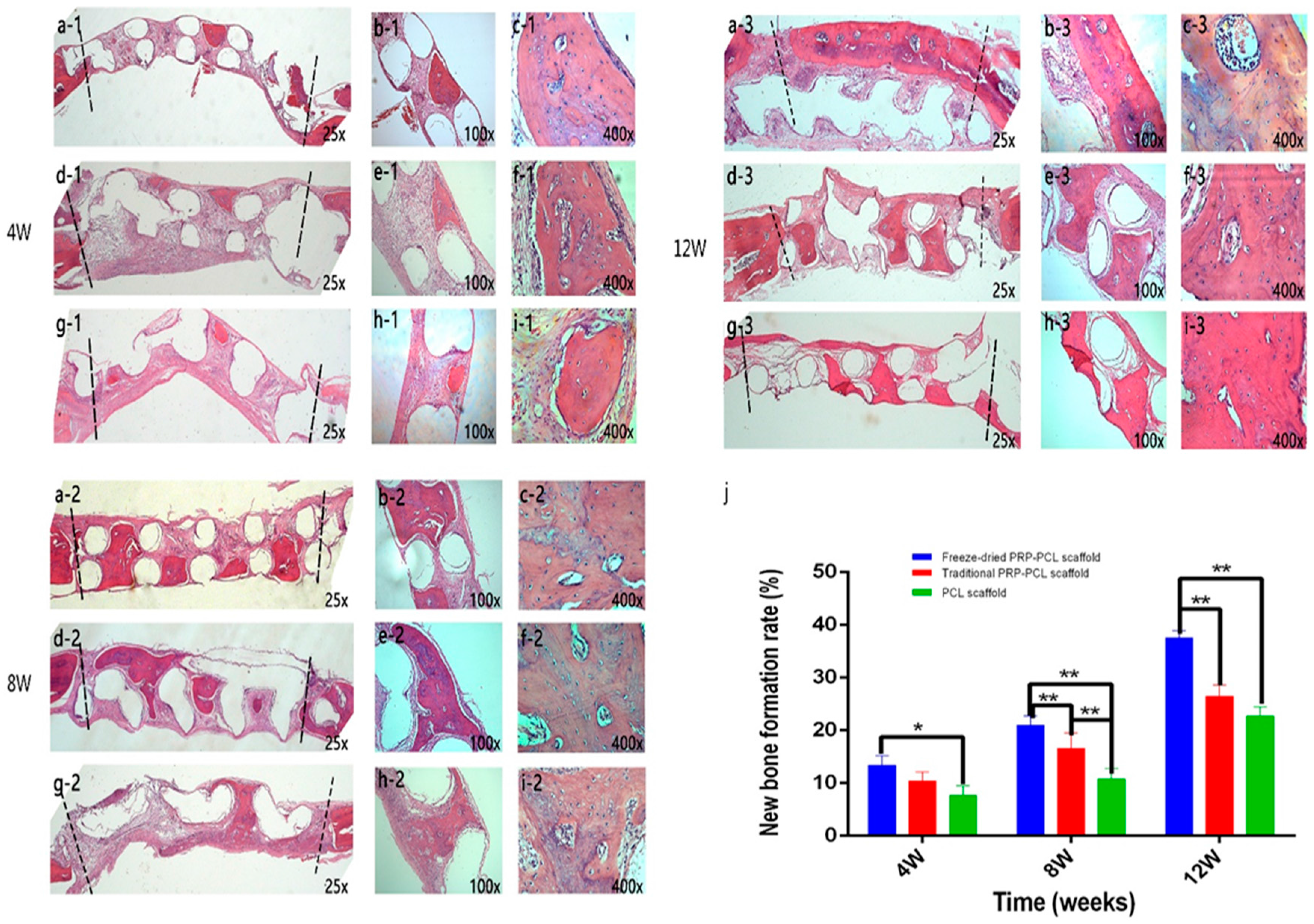
3.9. Histological Analysis Using H&E Staining
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone Grafting, Orthopaedic Biomaterials, and the Clinical Need for Bone Engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Czernuszka, J.T. Making Tissue Engineering Scaffolds Work. Review: The Application of Solid Freeform Fabrication Technology to the Production of Tissue Engineering Scaffolds. Eur. Cells Mater. 2003, 5, 29–39, discussion 39–40. [Google Scholar] [CrossRef]
- Jeong, C.G.; Hollister, S.J. A Comparison of the Influence of Material on in Vitro Cartilage Tissue Engineering with PCL, PGS, and POC 3D Scaffold Architecture Seeded with Chondrocytes. Biomaterials 2010, 31, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, J.M.; Hollister, S.J. Differential Effects of Designed Scaffold Permeability on Chondrogenesis by Chondrocytes and Bone Marrow Stromal Cells. Biomaterials 2010, 31, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Petrie, A.C.; Cooper, J.J.; Sefcik, L.S.; Tholpady, S.S.; Ogle, R.C.; Botchwey, E.A. Osteogenic Differentiation of Dura Mater Stem Cells Cultured in Vitro on Three-Dimensional Porous Scaffolds of Poly(Epsilon-Caprolactone) Fabricated via Co-Extrusion and Gas Foaming. Acta Biomater. 2008, 4, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Im, G.I. Chondrogenesis Using Mesenchymal Stem Cells and PCL Scaffolds. J. Biomed. Mater. Res. A 2010, 92, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, R.; Garcia-Giralt, N.; Rodriguez, M.T.; Caceres, E.; Garcia, S.J.; Gomez, R.J.; Monleon, M.; Monllau, J.C.; Suay, J. Biodegradable PCL Scaffolds with an Interconnected Spherical Pore Network for Tissue Engineering. J. Biomed. Mater. Res. A 2008, 85, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A Three-Dimensional Nanofibrous Scaffold for Cartilage Tissue Engineering Using Human Mesenchymal Stem Cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive Electrospun Scaffolds Delivering Growth Factors and Genes for Tissue Engineering Applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Luong-Van, E.; Grondahl, L.; Chua, K.N.; Leong, K.W.; Nurcombe, V.; Cool, S.M. Controlled Release of Heparin from Poly (Epsilon-Caprolactone) Electrospun Fibers. Biomaterials 2006, 27, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.; Madihally, S.V. Characterization of Chitosan-Polycaprolactone Blends for Tissue Engineering Applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kang, S.M.; Park, S.A.; Kim, W.D.; Kwak, J.; Lee, H. Enhanced Adhesion of Preosteoblasts inside 3D PCL Scaffolds by Polydopamine Coating and Mineralization. Macromol. Biosci. 2013, 13, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wei, B.; Liu, N.; Li, C.; Guo, Y.; Shamie, A.N.; Chen, J.; Tang, C.; Jin, C.; Xu, Y.; et al. Chondrogenic Regeneration Using Bone Marrow Clots and a Porous Polycaprolactone-Hydroxyapatite Scaffold by Three-Dimensional Printing. Tissue Eng. Part A 2015, 21, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Bae, J.H.; Park, S.A.; Kim, W.D.; Park, M.S.; Ko, Y.J.; Jang, H.S.; Park, J.H. Combination Therapy with BMP-2 and BMSCs Enhances Bone Healing Efficacy of PCL Scaffold Fabricated Using the 3D Plotting System in a Large Segmental Defect Model. Biotechnol. Lett. 2012, 34, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.L. Platelet Secretory Mechanisms. Semin. Thromb. Hemost. 2004, 30, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The Biology of Platelet-Rich Plasma and its Application in Trauma and Orthopaedic Surgery: A Review of the Literature. J. Bone Jt. Surg. Br. 2009, 91, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet Quantification and Growth Factor Analysis from Platelet-Rich Plasma: Implications for Wound Healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Son, S.R.; Sarkar, S.K.; Nguyen-Thuy, B.L.; Padalhin, A.R.; Kim, B.R.; Jung, H.I.; Lee, B.T. Platelet-Rich Plasma Encapsulation in Hyaluronic acid/gelatin-BCP Hydrogel for Growth Factor Delivery in BCP Sponge Scaffold for Bone Regeneration. J. Biomater. Appl. 2015, 29, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Oest, M.E.; Dupont, K.M.; Ho, K.H.; Teoh, S.H.; Guldberg, R.E. Combination of Platelet-Rich Plasma with Polycaprolactone-Tricalcium Phosphate Scaffolds for Segmental Bone Defect Repair. J. Biomed. Mater. Res. A 2007, 81, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Chatterjee, A.; Gokhale, S.; Singh, H.P.; Kandwal, A. Evaluation of Platelet-Rich Plasma Alone Or in Combination with Demineralized Freeze Dried Bone Allograft in Treatment of Periodontal Infrabony Defects: A Comparative Clinical Trial. J. Indian Soc. Periodontol. 2016, 20, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Horimizu, M.; Kawase, T.; Nakajima, Y.; Okuda, K.; Nagata, M.; Wolff, L.F.; Yoshie, H. An Improved Freeze-Dried PRP-coated Biodegradable Material Suitable for Connective Tissue Regenerative Therapy. Cryobiology 2013, 66, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Tayalia, P.; Mooney, D.J. Controlled Growth Factor Delivery for Tissue Engineering. Adv. Mater. 2009, 21, 3269–3285. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-Rich Plasma: Evidence to Support its Use. J. Oral Maxil. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef]
- Yamamiya, K.; Okuda, K.; Kawase, T.; Hata, K.; Wolff, L.F.; Yoshie, H. Tissue-Engineered Cultured Periosteum Used with Platelet-Rich Plasma and Hydroxyapatite in Treating Human Osseous Defects. J. Periodontol. 2008, 79, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Yamamiya, K.; Kawase, T.; Mizuno, H.; Ueda, M.; Yoshie, H. Treatment of Human Infrabony Periodontal Defects by Grafting Human Cultured Periosteum Sheets Combined with Platelet-Rich Plasma and Porous Hydroxyapatite Granules: Case Series. J. Int. Acad. Periodontol. 2009, 11, 206–213. [Google Scholar] [PubMed]
- Betsch, M.; Schneppendahl, J.; Thuns, S.; Herten, M.; Sager, M.; Jungbluth, P.; Hakimi, M.; Wild, M. Bone Marrow Aspiration Concentrate and Platelet Rich Plasma for Osteochondral Repair in a Porcine Osteochondral Defect Model. PLoS ONE 2013, 8, e71602. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Silva, M.; Dominguez, F.; Sheikh, F.A.; Cantu, T.; Desai, R.; Garcia, V.L.; Macossay, J. Biodegradable Electrospun Nanofibers Coated with Platelet-Rich Plasma for Cell Adhesion and Proliferation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wang, C.G.; Yin, K.; Liao, Q.; Zhou, X.; Liu, A.S.; Kong, L.Y. In Vivo Ossification of a Scaffold Combining Beta-Tricalcium Phosphate and Platelet-Rich Plasma. Exp. Ther. Med. 2014, 8, 1381–1388. [Google Scholar] [PubMed]
- Kumar, K.R.; Genmorgan, K.; Abdul, R.S.; Rajan, M.A.; Kumar, T.A.; Prasad, V.S. Role of Plasma-Rich Fibrin in Oral Surgery. J. Pharm. Bioallied Sci. 2016, 8, S36–S38. [Google Scholar] [PubMed]
- Hamzacebi, B.; Oduncuoglu, B.; Alaaddinoglu, E.E. Treatment of Peri-Implant Bone Defects with Platelet-Rich Fibrin. Int. J. Periodontics Restor. Dent. 2015, 35, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Kawase, T.; Kobayashi, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. Bioactivity of Freeze-Dried Platelet-Rich Plasma in an Adsorbed Form On a Biodegradable Polymer Material. Platelets 2012, 23, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated Craniofacial Bone Regeneration through Dense Collagen Gel Scaffolds Seeded with Dental Pulp Stem Cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, X.; Ling, J.; Huang, Y.; Gong, Q.; Huo, Y. Identification and Characterization of Side Population Cells from Adult Human Dental Pulp after Ischemic Culture. J. Endod. 2012, 38, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, X.; Ling, J.; Huang, Y.; Gong, Q. Side Population Increase After Simulated Transient Ischemia in Human Dental Pulp Cell. J. Endod. 2010, 36, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Kraft, D.C.; Lysdahl, H.; Foldager, C.B.; Chen, M.; Kristiansen, A.A.; Rolfing, J.H.; Bunger, C.E. Functionalization of Polycaprolactone Scaffolds with Hyaluronic Acid and beta-TCP Facilitates Migration and Osteogenic Differentiation of Human Dental Pulp Stem Cells in Vitro. Tissue Eng. Part A 2015, 21, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Kraft, D.C.; Bindslev, D.A.; Melsen, B.; Abdallah, B.M.; Kassem, M.; Klein-Nulend, J. Mechanosensitivity of Dental Pulp Stem Cells is Related to their Osteogenic Maturity. Eur. J. Oral Sci. 2010, 118, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Tvedesoe, C.; Rolfing, J.H.; Foldager, C.B.; Lysdahl, H.; Kraft, D.C.; Chen, M.; Baas, J.; Le, D.Q.; Bunger, C.E. Dental Pulp-Derived Stromal Cells Exhibit a Higher Osteogenic Potency than Bone Marrow-Derived Stromal Cells in Vitro and in a Porcine Critical-Size Bone Defect Model. SICOT J. 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human Mandible Bone Defect Repair by the Grafting of Dental Pulp Stem/Progenitor Cells and Collagen Sponge Biocomplexes. Eur. Cells Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef]
- Rentsch, C.; Rentsch, B.; Heinemann, S.; Bernhardt, R.; Bischoff, B.; Forster, Y.; Scharnweber, D.; Rammelt, S. ECM Inspired Coating of Embroidered 3D Scaffolds Enhances Calvaria Bone Regeneration. Biomed. Res. Int. 2014, 2014, 217078. [Google Scholar] [CrossRef] [PubMed]
- Qutachi, O.; Vetsch, J.R.; Gill, D.; Cox, H.; Scurr, D.J.; Hofmann, S.; Muller, R.; Quirk, R.A.; Shakesheff, K.M.; Rahman, C.V. Injectable and Porous PLGA Microspheres that Form Highly Porous Scaffolds at Body Temperature. Acta Biomater. 2014, 10, 5090–5098. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Song, T.H.; Rijal, G.; Jang, J.; Kim, S.W.; Cho, D.W. Ornamenting 3D Printed Scaffolds with Cell-Laid Extracellular Matrix for Bone Tissue Regeneration. Biomaterials 2015, 37, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kim, G.H.; Koh, Y.H. A Micro-Scale Surface-Structured PCL Scaffold Fabricated by a 3D Plotter and a Chemical Blowing Agent. J. Biomater. Sci. Polym. Ed. 2010, 21, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Koch, A.; Jun, Y.; Chou, C.; Awadallah, M.R.; Lee, C.H. Micro-Precise Spatiotemporal Delivery System Embedded in 3D Printing for Complex Tissue Regeneration. Biofabrication 2016, 8, 025003. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Cho, Y.B.; Choi, C.H.; Jang, Y.S.; Jung, W.; Lee, H.; Kim, G.H. Effect of Umbilical Cord Serum Coated 3D PCL/alginate Scaffold for Mastoid Obliteration. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-Epsilon-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part A 2016, 23, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Mishra, A.; Lin, P.T.; Ng, S.H.; Yeong, W.Y.; Kim, Y.J.; Yoon, Y.J. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Rampichova, M.; Chvojka, J.; Buzgo, M.; Prosecka, E.; Mikes, P.; Vyslouzilova, L.; Tvrdik, D.; Kochova, P.; Gregor, T.; Lukas, D.; et al. Elastic Three-Dimensional Poly (Epsilon-Caprolactone) Nanofibre Scaffold Enhances Migration, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Cell Prolif. 2013, 46, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.A.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering Anatomically Shaped Vascularized Bone Grafts with hASCs and 3D-printed PCL Scaffolds. J. Biomed. Mater. Res. A 2014, 102, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Sadiasa, A.; Thi, H.N.; Lee, B. In Vitro and in Vivo Evaluation of Porous PCL-PLLA 3D Polymer Scaffolds Fabricated via Salt Leaching Method for Bone Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2014, 25, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; O’Brien, J.; Zhang, L.G. Integrating Biologically Inspired Nanomaterials and Table-Top Stereolithography for 3D Printed Biomimetic Osteochondral Scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Ericksen, J.J.; Simpson, D.G.; Bowlin, G.L. Incorporating Platelet-Rich Plasma Into Electrospun Scaffolds for Tissue Engineering Applications. Tissue Eng. Part A 2011, 17, 2723–2737. [Google Scholar] [CrossRef] [PubMed]
- Pietramaggiori, G.; Kaipainen, A.; Czeczuga, J.M.; Wagner, C.T.; Orgill, D.P. Freeze-Dried Platelet-Rich Plasma Shows Beneficial Healing Properties in Chronic Wounds. Wound Repair Regen. 2006, 14, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yong, Z.; Yuk, K.S.; Hoon, K.Y.; Yuedong, S.; Xu, J. Growth Factor Release from Lyophilized Porcine Platelet-Rich Plasma: Quantitative Analysis and Implications for Clinical Applications. Aesthetic Plast. Surg. 2016, 40, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Agata, H.; Sumita, Y.; Koga, T.; Asahina, I. Efficacy of Freeze-Dried Platelet-Rich Plasma in Bone Engineering. Arch. Oral Biol. 2017, 73, 172–178. [Google Scholar] [CrossRef] [PubMed]

| Gene | Prime Sequence (F, Forward; R, Reverse; 50–30) | Product Size (bp) |
|---|---|---|
| ALP | F: GCAACTTCCAGACCATTGGC R: TCCCACTGACTTCCCTGCTT | 119 |
| RUNX2 | F: CGTGGCCTTCAAGGTGGTAG R: GAGGCATTCCGGAGCTCAG | 105 |
| OCN | F: AGCAAAGGTGCAGCCTTTGT R: GCGCCTGGGTCTCTTCACT | 63 |
| OPN | F: ACATCCAGTACCCTGATGCTACAG R: TGGCCTTGTATGCACCATTC | 81 |
| GAPDH | F: GATTCCACCCATGGCAAATT R: TCTCGCTCCTGGAAGATGGT | 95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration. Materials 2017, 10, 831. https://doi.org/10.3390/ma10070831
Li J, Chen M, Wei X, Hao Y, Wang J. Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration. Materials. 2017; 10(7):831. https://doi.org/10.3390/ma10070831
Chicago/Turabian StyleLi, Junda, Meilin Chen, Xiaoying Wei, Yishan Hao, and Jinming Wang. 2017. "Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration" Materials 10, no. 7: 831. https://doi.org/10.3390/ma10070831




