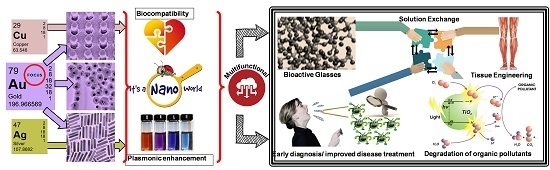
Surface Plasmon Resonance or Biocompatibility—Key Properties for Determining the Applicability of Noble Metal Nanoparticles
Abstract
:1. Introduction
- (1)
- development of innovative design of plasmonic biosensors with highly sensitive, selective, and reliable biomarker detection abilities to enable early diagnosis and improved disease treatment.
- (2)
- tissue engineering and drug delivery systems based on noble metal nanoparticles in bioactive glasses and glass ceramics: the specific biological effect of these materials such as silver nanoparticles are described by the antibacterial activity and healing enhancement effect of nano-silver, copper nanoparticles also demonstrated the size-dependent antibacterial activity with low toxicity, while bioactive glasses with gold nanoparticles have good proliferation rate of keratinocytes cells.
- (3)
- plasmonic effect-based photocatalysis: the surface plasmon resonance (SPR) of some noble metals can be exploited to activate photocatalytic materials, by injecting hot electrons in to the conduction band of semiconductors making possible visible- and near infrared light driven degradation of organic pollutants.
2. Optical Plasmonic Biosensors for Smart Disease Diagnostics
2.1. Solid Plasmonic Substrate-Based Immunoassays
2.2. Solution-Based Homogeneous Immunoassays
2.2.1. Immunoassays Based on the Intrinsic Optical Properties of AuNPs
2.2.2. Immunoassays Based on AuNPs as Signal Quenchers/Enhancers
3. Metallic Nanoparticles in Composites with Bioactive Glasses and Glass Ceramics
3.1. Bioactive Glasses and Glass-Ceramics
3.2. Role of Metallic Nanoparticles in Organism
3.2.1. Silver Nanoparticles in Bioactive Glass and Glass Ceramics
3.2.2. Gold Nanoparticles in Bioactive Glasses and Glass Ceramics
3.2.3. Copper Nanoparticles in Bioactive Glasses and Glass Ceramics
4. Photocatalytic Application of Gold Nanoparticles
5. Concluding Remarks and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nahavandi, S.; Baratchi, S.; Soffe, R.; Tang, S.Y.; Nahavandi, S.; Mitchell, A.; Khoshmanesh, K. Microfluidic platforms for biomarker analysis. Lab Chip 2014, 14, 1496–1514. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Reddy, P.J.; Choudhary, S.; Raghu, D.; Srivastava, S. Emerging nanoproteomics approaches for disease biomarker detection: A current perspective. J. Proteom. 2011, 74, 2660–2681. [Google Scholar] [CrossRef] [PubMed]
- Madu, C.O.; Lu, Y. Novel diagnostic biomarkers for prostate cancer. J. Cancer 2010, 1, 150–177. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mehta, G.; Srivastava, S. Label-free detection techniques for protein microarrays: Prospects, merits and challenges. Proteomics 2010, 10, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring (vol 8, pg 268, 2008). Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, S.T.; Fu, G.; Dou, M.; Xu, F.; Liu, R.; Qi, H.; Li, X. Biomarker detection for disease diagnosis using cost-effective microfluidic platforms. Analyst 2015, 140, 7062–7081. [Google Scholar] [CrossRef] [PubMed]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.M.; Liu, Q.J. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Volpetti, F.; Garcia-Cordero, J.; Maerkl, S.J. A microfluidic platform for high-throughput multiplexed protein quantitation. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, N.; Liu, H.; Zhou, X.; Knoll, W. Integrating plasmonic diagnostics and microfluidics. Biomicrofluidics 2015, 9, 052611. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodriguez-Fernandez, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzan, L.M.; Garcia de Abajo, F.J. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed]
- Truong, P.L.; Cao, C.; Park, S.; Kim, M.; Sim, S.J. A new method for non-labeling attomolar detection of diseases based on an individual gold nanorod immunosensor. Lab Chip 2011, 11, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, X.; Wang, Z.; Jin, A.; Sun, X.; Zhu, L.; Wang, F.; Ma, Y.; Niu, G.; Hight Walker, A.R.; et al. Gold Nanoparticle-Based Activatable Probe for Sensing Ultralow Levels of Prostate-Specific Antigen. ACS Nano 2013, 7, 5568–5576. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, X.; Xue, J.; Zhou, Z.; Zhou, J. Antibody modified gold nano-mushroom arrays for rapid detection of alpha-fetoprotein. Biosens. Bioelectron. 2015, 68, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Nguyen, A.H.; Sim, S.J. A nanoplasmonic biosensor for label-free multiplex detection of cancer biomarkers. Biosens. Bioelectron. 2015, 74, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.H.; Erdene, N.; Park, J.H.; Jeong, D.H.; Lee, H.Y.; Lee, S.K. Real-time label-free immunoassay of interferon-gamma and prostate-specific antigen using a Fiber-Optic Localized Surface Plasmon Resonance sensor. Biosens. Bioelectron. 2013, 39, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zeng, Z.; Lin, Y. Localized surface plasmon resonance (LSPR)-coupled fiber-optic nanoprobe for the detection of protein biomarkers. Methods Mol. Biol. 2017, 1571, 1–14. [Google Scholar] [PubMed]
- Liang, G.; Luo, Z.; Liu, K.; Wang, Y.; Dai, J.; Duan, Y. Fiber optic surface plasmon resonance-based biosensor technique: Fabrication, advancement, and application. Crit. Rev. Anal. Chem. 2016, 46, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Estevez, M.C.; Villar-Vazquez, R.; Casal, J.I.; Lechuga, L.M. Label-free nanoplasmonic sensing of tumor-associate autoantibodies for early diagnosis of colorectal cancer. Anal. Chim. Acta 2016, 930, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Marzo, A.M.; Merkoci, A. Paper-based sensors and assays: A success of the engineering design and the convergence of knowledge areas. Lab Chip 2016, 16, 3150–3176. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Tian, L.; Singamaneni, S. Paper-based SERS swab for rapid trace detection on real-world surfaces. ACS Appl. Mater. Interfaces 2010, 2, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Porta, A.L.; Novikov, S.M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Pen-on-paper approach toward the design of universal surface enhanced raman scattering substrates. Small 2014, 10, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Tong, L.; Tang, S.; Guo, Z.; Zhang, H.; Li, P.; Wang, H.; Du, C.; Yu, X.-F. PLLA nanofibrous paper-based plasmonic substrate with tailored hydrophilicity for focusing SERS detection. ACS Appl. Mater. Interfaces 2015, 7, 5391–5399. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; White, I.M. Inkjet printed surface enhanced raman spectroscopy array on cellulose paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Morrissey, J.J.; Kattumenu, R.; Gandra, N.; Kharasch, E.D.; Singamaneni, S. Bioplasmonic paper as a platform for detection of kidney cancer biomarkers. Anal. Chem. 2012, 84, 9928–9934. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Kuang, Z.; Jiang, Q.; Liu, K.-K.; Fisher, M.A.; Morrissey, J.J.; Kharasch, E.D.; Slocik, J.M.; Naik, R.R.; Singamaneni, S. Peptide functionalized gold nanorods for the sensitive detection of a cardiac biomarker using plasmonic paper devices. Sci. Rep. 2015, 5, 16206. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Ler, R.; Murakami, M.M. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin. Chem. 2012, 58, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.Y.; Koh, P.K.; Mal, M.; Cheah, P.Y.; Eu, K.W.; Backshall, A.; Cavill, R.; Nicholson, J.K.; Keun, H.C. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS). J. Proteome Res. 2009, 8, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Doering, W.E.; Nie, S. Single-molecule and single-nanoparticle SERS: Examining the roles of surface active sites and chemical enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- Baia, M.; Astilean, S.; Iliescu, T. Raman and SERS Investigations of Pharmaceuticals; Springer: New York, NY, USA, 2008. [Google Scholar]
- Li, M.; Cushing, S.K.; Zhang, J.; Suri, S.; Evans, R.; Petros, W.P.; Gibson, L.F.; Ma, D.; Liu, Y.; Wu, N. Three-dimensional hierarchical plasmonic nano-architecture enhanced surface-enhanced raman scattering immunosensor for cancer biomarker detection in blood plasma. ACS Nano 2013, 7, 4967–4976. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.H.; Granger, M.C.; Firpo, M.A.; Mulvihill, S.J.; Porter, M.D. Toward development of a surface-enhanced Raman scattering (SERS)-based cancer diagnostic immunoassay panel. Analyst 2013, 138, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhou, H.; Gu, J.; Liu, Q.; Zhang, W.; Su, H.; Su, Y.; Yao, Q.; Zhang, D. Tumor marker detection using surface enhanced Raman spectroscopy on 3D Au butterfly wings. J. Mater. Chem. B 2017, 5, 1594–1600. [Google Scholar] [CrossRef]
- Centeno, A.; Xie, F. Towards optimizing metal enhanced fluorescence (MEF) for improved detection of disease biomarkers. Biointerface Res. Appl. Chem. 2014, 4, 731–735. [Google Scholar]
- Zhou, X.; Wong, T.I.; Song, H.Y.; Wu, L.; Wang, Y.; Bai, P.; Kim, D.H.; Ng, S.H.; Tse, M.S.; Knoll, W. Development of localized surface plasmon resonance-based point-of-care system. Plasmonics 2014, 9, 835–844. [Google Scholar] [CrossRef]
- McPhee, S.J. Pocket Guide to Diagnostic Tests, 6th ed.; McGraw-Hill Publishing: New York, NY, USA, 2012. [Google Scholar]
- Preechaburana, P.; Gonzalez, M.C.; Suska, A.; Filippini, D. Surface plasmon resonance chemical sensing on cell phones. Angew. Chem. Int. Ed. 2012, 51, 11585–11588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Chen, P.; Tran, N.T.; Zhang, J.; Chia, W.S.; Boujday, S.; Liedberg, B. Smartphone spectrometer for colorimetric biosensing. Analyst 2016, 141, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.; Chen, H.; Huo, Q. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Rosenzweig, Z. Development of an aggregation-based immunoassay for anti-protein a using gold nanoparticles. Anal. Chem. 2002, 74, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wang, H.; Fu, Q.; Peng, J.; Wang, Y.; Du, J.; Zhou, Y.; Zhan, L. Gold nanorod-based localized surface plasmon resonance biosensor for sensitive detection of hepatitis B virus in buffer, blood serum and plasma. Biosens. Bioelectron. 2010, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, G.; Wu, Y.; Hao, P.; Zhou, W.; Zhang, Z. Gold nanoparticle amplified optical microfiber evanescent wave absorption biosensor for cancer biomarker detection in serum. Talanta 2014, 120, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Avan, A.; Ferns, G.A.; Pazoki-Toroudi, H. Enhanced detection sensitivity of prostate-specific antigen via PSA-conjugated gold nanoparticles based on localized surface plasmon resonance: GNP-coated anti-PSA/LSPR as a novel approach for the identification of prostate anomalies. Cancer Gene Ther. 2016, 23, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Salahvarzi, A.; Mahani, M.; Torkzadeh-Mahani, M.; Alizadeh, R. Localized surface plasmon resonance based gold nanobiosensor: Determination of thyroid stimulating hormone. Anal. Biochem. 2017, 516, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Huang, C.-C.; Chang, H.-T. Label-free colorimetric detection of picomolar thrombin in blood plasma using a gold nanoparticle-based assay. Biosens. Bioelectron. 2010, 25, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Zhang, H.; Yang, X. Label-free colorimetric immunoassay for the simple and sensitive detection of neurogenin3 using gold nanoparticles. Biosens. Bioelectron. 2011, 26, 4245–4248. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Rong, P.; Jia, H.; Yang, J.; Dong, B.; Dong, Q.; Yang, C.; Hu, P.; Wang, W.; Liu, H.; et al. A wash-free homogeneous colorimetric immunoassay method. Theranostics 2016, 6, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; He, Y.; Wang, L.; Huang, Y.; Jiang, B. Bifunctional Au@TiO2 core–shell nanoparticle films for clean water generation by photocatalysis and solar evaporation. Energy Convers. Manag. 2017, 132, 452–459. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wei, W.; Zhao, H.; Zhou, Z.; Zhang, Y.; Liu, S. Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst 2015, 140, 3989–3995. [Google Scholar] [CrossRef] [PubMed]
- Driskell, J.D.; Jones, C.A.; Tompkins, S.M.; Tripp, R.A. One-step assay for detecting influenza virus using dynamic light scattering and gold nanoparticles. Analyst 2011, 136, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Wang, J.; Dong, C.; Huang, X.; Ren, J. Homogeneous immunoassays by using photon burst counting technique of single gold nanoparticles. Talanta 2015, 132, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-H.; Yang, P.-H.; Feng, J.; Cai, J. Immunoassay detection using functionalized gold nanoparticle probes coupled with resonance Rayleigh scattering. Sens. Actuators B Chem. 2009, 135, 603–609. [Google Scholar] [CrossRef]
- Zhang, C.X.; Zhang, Y.; Wang, X.; Tang, Z.M.; Lu, Z.H. Hyper-Rayleigh scattering of protein-modified gold nanoparticles. Anal. Biochem. 2003, 320, 136–140. [Google Scholar] [CrossRef]
- Neely, A.; Perry, C.; Varisli, B.; Singh, A.K.; Arbneshi, T.; Senapati, D.; Kalluri, J.R.; Ray, P.C. Ultrasensitive and highly selective detection of alzheimer’s disease biomarker using two-photon rayleigh scattering properties of gold nanoparticle. ACS Nano 2009, 3, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, C.; Irudayaraj, J. Ultrasensitive protein detection in blood serum using gold nanoparticle probes by single molecule spectroscopy. BIOMEDO 2009, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, X.; Dong, C.; Ren, J. Fluorescence correlation spectroscopy of gold nanoparticles, and its application to an aptamer-based homogeneous thrombin assay. Microchim. Acta 2014, 181, 723–730. [Google Scholar] [CrossRef]
- Soller, T.; Ringler, M.; Wunderlich, M.; Klar, T.A.; Feldmann, J.; Josel, H.P.; Markert, Y.; Nichtl, A.; Kürzinger, K. Radiative and nonradiative rates of phosphors attached to gold nanoparticles. Nano Lett. 2007, 7, 1941–1946. [Google Scholar] [CrossRef]
- Ao, L.; Gao, F.; Pan, B.; He, R.; Cui, D. Fluoroimmunoassay for antigen based on fluorescence quenching signal of gold nanoparticles. Anal. Chem. 2006, 78, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Mayilo, S.; Kloster, M.A.; Wunderlich, M.; Lutich, A.; Klar, T.A.; Nichtl, A.; Kürzinger, K.; Stefani, F.D.; Feldmann, J. Long-range fluorescence quenching by gold nanoparticles in a sandwich immunoassay for cardiac troponin T. Nano Lett. 2009, 9, 4558–4563. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, B.S.S.; Sá e Cunha, C.; Gomes, I.; Cavadas, M.; Silva, I.; Doria, G.; Blatch, G.L.; Baptista, P.V.; Pereira, E.; Azzazy, H.M.E.; et al. Gold nanoparticle-based fluorescence immunoassay for malaria antigen detection. Anal. Bioanal. Chem. 2012, 402, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Cavadas, M.A.S.; Monopoli, M.P.; Cunha, C.S.E.; Prudêncio, M.; Pereira, E.; Lynch, I.; Dawson, K.A.; Franco, R. Unravelling malaria antigen binding to antibody-gold nanoparticle conjugates. Part. Part. Syst. Charact. 2016, 33, 906–915. [Google Scholar] [CrossRef]
- Chang, Y.F.; Chen, R.C.; Lee, Y.J.; Chao, S.C.; Su, L.C.; Li, Y.C.; Chou, C. Localized surface plasmon coupled fluorescence fiber-optic biosensor for alpha-fetoprotein detection in human serum. Biosens. Bioelectron. 2009, 24, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.P.W.; Aroca, R.F. Single molecule analysis by surfaced-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.; Lee, S.; Son, S.W.; Oh, C.H.; Choo, J. Highly sensitive immunoassay of lung cancer marker carcinoembryonic antigen using surface-enhanced raman scattering of hollow gold nanospheres. Anal. Chem. 2009, 81, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.; Lee, S.; Yoon, S.-Y.; Chang, S.-I.; Lim, D.W.; Choo, J. Simultaneous immunoassay for the detection of two lung cancer markers using functionalized SERS nanoprobes. Chem. Commun. 2011, 47, 12515–12517. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Harpster, M.H.; Wilson, W.C.; Johnson, P.A. Surface-enhanced Raman scattering (SERS) detection of multiple viral antigens using magnetic capture of SERS-active nanoparticles. Biosens. Bioelectron. 2013, 41, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, G.; Wei, F.; Zhang, A.; Yang, J.; Hu, Q. Detection of CEA in human serum using surface-enhanced Raman spectroscopy coupled with antibody-modified Au and γ-Fe2O3@Au nanoparticles. J. Pharm. Biomed. Anal. 2016, 121, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Harpster, M.H.; Zhang, H.; Mecham, J.O.; Wilson, W.C.; Johnson, P.A. A versatile SERS-based immunoassay for immunoglobulin detection using antigen-coated gold nanoparticles and malachite green-conjugated protein A/G. Biosens. Bioelectron. 2010, 26, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, L.-J.; Jiang, J.-H. Surface-enhanced raman spectroscopy-based, homogeneous, multiplexed immunoassay with antibody-fragments-decorated gold nanoparticles. Anal. Chem. 2013, 85, 9213–9220. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Kokubo, T. Properties of bioactive glasses and glass-ceramics. In Handbook of Biomaterial Properties; Springer: New York, NY, USA, 1998; pp. 335–363. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of bioactive glass: From hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Ragel, C.; Salinas, A.J. Glasses with medical applications. Eur. J. Inorg. Chem. 2003, 6, 1029–1042. [Google Scholar] [CrossRef]
- Hoppe, A.; Guldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, H.; Solla, E.L.; Serra, J.; González, P.; León, B.; Almeida, N.; Cachinho, S.; Davim, E.J.C.; Correia, R.; Oliveira, J.M.; et al. Orthophosphate nanostructures in SiO2–P2O5–CaO–Na2O–MgO bioactive glasses. J. Non-Cryst. Solids 2008, 354, 4075–4080. [Google Scholar] [CrossRef]
- Cacaina, D.; Ylanen, H.; Hupa, M.; Simon, S. Study of yttrium containing bioactive glasses behaviour in simulated body fluid. J. Mater. Sci. Mater.Med. 2006, 17, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of catalase mimetic activity in Ce(3+)/Ce(4+) doped bioactive glasses. J. Phys. Chem. B 2015, 119, 4009–4019. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Roman, J.; Carenas, A.; Vallet-Regi, M. The influence of the phosphorus content on the bioactivity of sol-gel glass ceramics. Biomaterials 2005, 26, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Riti, P.I.; Vulpoi, A.; Ponta, O.; Simon, V. The effect of synthesis route and magnesium addition on structure and bioactivity of sol–gel derived calcium-silicate glasses. Ceram. Int. 2014, 40, 14741–14748. [Google Scholar] [CrossRef]
- Ahmed, I.; Lewis, M.; Olsen, I.; Knowles, J.C. Phosphate glasses for tissue engineering: Part 1. Processing and characterisation of a ternary-based P2O5–CaO–Na2O glass system. Biomaterials 2004, 25, 491–499. [Google Scholar] [CrossRef]
- ElBatal, H.A.; Khalil, E.M.A.; Hamdy, Y.M. In vitro behavior of bioactive phosphate glass–ceramics from the system P2O5–Na2O–CaO containing titania. Ceram. Int. 2009, 35, 1195–1204. [Google Scholar] [CrossRef]
- Gayathri Devi, A.V.; Rajendran, V.; Rajendran, N. Structure, solubility and bioactivity in TiO2-doped phosphate-based bioglasses and glass–ceramics. Mater. Chem. Phys. 2010, 124, 312–318. [Google Scholar] [CrossRef]
- Navarro, M.; del Valle, S.; Martinez, S.; Zeppetelli, S.; Ambrosio, L.; Planell, J.A.; Ginebra, M.P. New macroporous calcium phosphate glass ceramic for guided bone regeneration. Biomaterials 2004, 25, 4233–4241. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, A.M. Novel method for early investigation of bioactivity in different borate bio-glasses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 100, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.F.; Rahaman, M.N.; Dwilewicz, A.B.; Huang, W.; Day, D.E.; Li, Y.; Bal, B.S. Effect of borate glass composition on its conversion to hydroxyapatite and on the proliferation of MC3T3-E1 cells. J. Biomed. Mater. Res. A 2009, 88, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Lusvardi, G.; Zaffe, D.; Menabue, L.; Bertoldi, C.; Malavasi, G.; Consolo, U. In vitro and in vivo behaviour of zinc-doped phosphosilicate glasses. Acta Biomater. 2009, 5, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gao, Q.; Bao, X.; Li, Y.; Teramoto, A.; Abe, K. Preparation and in vitro bioactivity of novel mesoporous borosilicate bioactive glass nanofibers. J. Am. Ceram. Soc. 2011, 94, 2841–2845. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Zhang, X.; Zhang, Y.; Zhang, C.; Liu, X.; Rahaman, M.N.; Huang, W.; Pan, H. Biodegradable borosilicate bioactive glass scaffolds with a trabecular microstructure for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Tu, Y.; Zhou, H.; Liu, C.; Rüssel, C. Borophosphate glass-ceramic scaffolds by a sodium silicate bonding process. J. Non-Cryst. Solids 2011, 357, 958–962. [Google Scholar] [CrossRef]
- Magyari, K.; Stefan, R.; Vulpoi, A.; Baia, L. Bioactivity evolution of calcium-free borophosphate glass with addition of titanium dioxide. J. Non-Cryst. Solids 2015, 410, 112–117. [Google Scholar] [CrossRef]
- Hidi, I.J.; Melinte, G.; Stefan, R.; Bindea, M.; Baia, L. The study of the structure and bioactivity of the B2O3-Na2O-P2O5system. J. Raman Spectrosc. 2013, 44, 1187–1194. [Google Scholar] [CrossRef]
- Lepry, W.C.; Nazhat, S.N. Highly bioactive sol-gel-derived borate glasses. Chem. Mater. 2015, 27, 4821–4831. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Pickup, D.M.; Valappil, S.P.; Newport, R.J.; Knowles, J.C. Bioactive functional materials: A perspective on phosphate-based glasses. J. Mater. Chem. 2009, 19, 690–701. [Google Scholar] [CrossRef]
- Scholze, H. Glass: Nature, Structure, and Properties; Springer: New York, NY, USA, 1991. [Google Scholar]
- Hench, L.L.; Splinter, R.; Allen, W.; Greenlee, T. Bonding mechanisms at the interface of ceramics prosthetic materials. J. Biomed. Mater. Res. 1971, 2, 117–147. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Gulf Professional Publishing: Woburn, MA, USA, 1990. [Google Scholar]
- Pierre, A.C. Introduction to Sol-Gel Processing; Springer: New York, NY, USA, 1998. [Google Scholar]
- Vulpoi, A.; Magyari, K.; Stefan, R.; Baia, L. Bioglass: Properties, Functions and Applications, Chapter 1 Overview of Properties of Bioactive Glasses and Ceramics Induced by Preparation Route; Nova Science Publishers, Inc.: New York, NY, USA, 2016; pp. 1–37. [Google Scholar]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Olsen-Claire, J.; Blaker, J.J.; Roether, J.A.; Boccaccini, A.R.; Schmack, G.; Gliesche, K. Bioglass® coatings on biodegradable poly(3-hydroxybutyrate) (P3HB) meshes for tissue engineering scaffolds. Materialwissenschaft und Werkstofftechnik 2006, 37, 577–583. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Grade, S.; Eberhard, J.; Neumeister, A.; Wagener, P.; Winkel, A.; Stiesch, M.; Barcikowski, S. Serum albumin reduces the antibacterial and cytotoxic effects of hydrogel-embedded colloidal silver nanoparticles. RSC Adv. 2012, 2, 7190. [Google Scholar] [CrossRef]
- Simon, V.; Albon, C.; Simon, S. Silver release from hydroxyapatite self-assembling calcium–phosphate glasses. J. Non-Cryst. Solids 2008, 354, 1751–1755. [Google Scholar] [CrossRef]
- Vulpoi, A.; Baia, L.; Simon, S.; Simon, V. Silver effect on the structure of SiO2-CaO-P2O5 ternary system. Mater. Sci. Eng. C 2012, 32, 178–183. [Google Scholar] [CrossRef]
- Vulpoi, A.; Gruian, C.; Vanea, E.; Baia, L.; Simon, S.; Steinhoff, H.J.; Goller, G.; Simon, V. Bioactivity and protein attachment onto bioactive glasses containing silver nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Chen, M.-S.; Wang, C.-W.; Shih, S.-J.; Chen, C.-Y.; Pan, Y.-N.; Lin, C.-K. Preparation and characterization of silver nanocrystals decorated mesoporous bioactive glass via synchrotron X-ray reduction. J. Non-Cryst. Solids 2016, 450, 128–134. [Google Scholar] [CrossRef]
- Magyari, K.; Stefan, R.; Vodnar, D.C.; Vulpoi, A.; Baia, L. The silver influence on the structure and antibacterial properties of the bioactive 10B2O3−30Na2O−60P2O2 glass. J. Non-Cryst. Solids 2014, 402, 182–186. [Google Scholar] [CrossRef]
- Goh, Y.-F.; Alshemary, A.Z.; Akram, M.; Abdul Kadir, M.R.; Hussain, R. Bioactive glass: Anin-vitrocomparative study of doping with nanoscale copper and silver particles. Int. J. Appl. Glass Sci. 2014, 5, 255–266. [Google Scholar] [CrossRef]
- Vulpoi, A.; Simon, V.; Ylanen, H.; Simon, S. Development and in vitro assessment of bioactive glass/polymer nanostructured composites with silver. J. Compos. Mater. 2014, 48, 63–70. [Google Scholar] [CrossRef]
- Lusvardi, G.; Malavasi, G.; Aina, V.; Bertinetti, L.; Cerrato, G.; Magnacca, G.; Morterra, C.; Menabue, L. Bioactive glasses containing Au nanoparticles. Effect of calcination temperature on structure, morphology, and surface properties. Langmuir ACS J. Surf. Colloids 2010, 26, 10303–10314. [Google Scholar] [CrossRef] [PubMed]
- Aina, V.; Cerrato, G.; Martra, G.; Bergandi, L.; Costamagna, C.; Ghigo, D.; Malavasi, G.; Lusvardi, G.; Menabue, L. Gold-containing bioactive glasses: A solid-state synthesis to produce alternative biomaterials for bone implantations. J. R. Soc. Interface 2013, 10, 20121040. [Google Scholar] [CrossRef] [PubMed]
- Magyari, K.; Nagy-Simon, T.; Vulpoi, A.; Popescu, R.A.; Licarete, E.; Stefan, R.; Hernádi, K.; Papuc, I.; Baia, L. Novel bioactive glass-AuNP composites for biomedical applications. Mater. Sci. Eng. C 2017, 76, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Ciceo-Lucacel, R.; Radu, T.; Baia, L.; Ponta, O.; Iepure, A.; Simon, V. Gold nanoparticles developed in sol-gel derived apatite--bioactive glass composites. J. Mater. Sci. Mater. Med. 2012, 23, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Jayalekshmi, A.C.; Sharma, C.P. Gold nanoparticle incorporated polymer/bioactive glass composite for controlled drug delivery application. Colloids Surf. B Biointerfaces 2015, 126, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Bonici, A.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Piva, A. Synthesis and characterization of bioactive glasses functionalized with Cu nanoparticles and organic molecules. J. Eur. Ceram. Soc. 2012, 32, 2777–2783. [Google Scholar] [CrossRef]
- Aina, V.; Cerrato, G.; Martra, G.; Malavasi, G.; Lusvardi, G.; Menabue, L. Towards the controlled release of metal nanoparticles from biomaterials: Physico-chemical, morphological and bioactivity features of Cu-containing sol–gel glasses. Appl. Surf. Sci. 2013, 283, 240–248. [Google Scholar] [CrossRef]
- Popescu, R.A.; Magyari, K.; Vulpoi, A.; Trandafir, D.L.; Licarete, E.; Todea, M.; Stefan, R.; Voica, C.; Vodnar, D.C.; Simon, S.; et al. Bioactive and biocompatible copper containing glass-ceramics with remarkable antibacterial properties and high cell viability designed for future in vivo trials. Biomater. Sci. 2016, 4, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Tejeda, L.; Malpartida, F.; Esteban-Cubillo, A.; Pecharroman, C.; Moya, J.S. Antibacterial and antifungal activity of a soda-lime glass containing copper nanoparticles. Nanotechnology 2009, 20, 505701. [Google Scholar] [CrossRef] [PubMed]
- Baheiraeia, N.; Moztarzadeha, F.; Hedayatib, M. Preparation and antibacterial activity of Ag/SiO2 thin film on glazed ceramic tiles by sol–gel method. Ceram. Int. 2012, 38, 2921–2925. [Google Scholar] [CrossRef]
- Magyari, K.; Gruian, C.; Varga, B.; Ciceo-Lucacel, R.; Radu, T.; Steinhoff, H.-J.; Váró, G.; Simon, V.; Baia, L. Addressing the optimal silver content in bioactive glass systems in terms of BSA adsorption. J. Mater. Chem. B 2014, 2, 5799–5808. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Liu, X. Silver nanoparticles-modified films versus biomedical device-associated infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles—The real “silver bullet” in clinical medicine? MedChemComm 2010, 1, 125–131. [Google Scholar] [CrossRef]
- Austin, L.A.; Kang, B.; Yen, C.W.; El-Sayed, M.A. Nuclear targeted silver nanospheres perturb the cancer cell cycle differently than those of nanogold. Bioconjug. Chem. 2011, 22, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.A.; Zapata, P.A.; Vejar, N.D.; Azocar, M.I.; Gulppi, M.A.; Zhou, X.; Thompson, G.E.; Rabagliati, F.M.; Paez, M.A. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Boccaccini, A.R. Silver-Containing Bioactive Glasses for Tissue Engineering Applications; Woodhead Publishing Limited: Cambridge, UK, 2014. [Google Scholar]
- Srinivasan, S.; Kumar, P.T.S.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Antibacterial and bioactive α- and β-chitin hydrogel/nanobioactive glass ceramic/nano silver composite scaffolds for periodontal regeneration. J. Biomed. Nanotechnol. 2013, 9, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Seuss, S.; Heinloth, M.; Boccaccini, A.R. Development of bioactive composite coatings based on combination of PEEK, bioactive glass and Ag nanoparticles with antibacterial properties. Surf. Coat. Technol. 2016, 301, 100–105. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ali, A.A.; Mahmoud, D.A.R.; El-Fiqi, A.M. Study on the preparation and properties of silver-doped phosphate antibacterial glasses (Part I). Solid State Sci. 2011, 13, 981–992. [Google Scholar] [CrossRef]
- Baia, L.; Baia, M.; Kiefer, W.; Popp, J.; Simon, S. Structural and morphological properties of silver nanoparticles–phosphate glass composites. Chem. Phys. 2006, 327, 63–69. [Google Scholar] [CrossRef]
- Radu, T.; Benea, D.; Ciceo-Lucacel, R.; Barbu-Tudoran, L.; Simon, S. X-ray Photoelectron Spectroscopic Characterization of Ag Nanoparticles Embedded Bioglasses. J. Phys. Chem. C 2012, 116, 17975–17979. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications Volume 2: New Trends and Technologies; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Baia, L.; Muresan, D.; Baia, M.; Popp, J.; Simon, S. Structural properties of silver nanoclusters–phosphate glass composites. Vib. Spectrosc. 2007, 43, 313–318. [Google Scholar] [CrossRef]
- Lu, J.; Bravosuarez, J.; Takahashi, A.; Haruta, M.; Oyama, S. In situ UV–vis studies of the effect of particle size on the epoxidation of ethylene and propylene on supported silver catalysts with molecular oxygen. J. Catal. 2005, 232, 85–95. [Google Scholar] [CrossRef]
- Popescu, R.A.; Magyari, K.; Papuc, I.; Baia, L. Synthesis, structural characterization and in vitro evaluation of bioactivity of silver containing bioactive glasses. Stud. Univ. Babes-Bolyai Phys. 2015, 60, 89–96. [Google Scholar]
- Jiménez, J.A.; Liu, H.; Fachini, E. X-ray photoelectron spectroscopy of silver nanoparticles in phosphate glass. Mater. Lett. 2010, 64, 2046–2048. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Borsarelli, C.D.; Rey, V.; Veglia, A.V. Synthetic Routes for the Preparation of Silver Nanoparticles. Silver Nanoparticle Applications; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–46. [Google Scholar]
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar]
- Sasai, J.; Hirao, K. Relaxation behavior of nonlinear optical response in borate glasses containing gold nanoparticles. J. Appl. Phys. 2001, 89, 4548. [Google Scholar] [CrossRef] [Green Version]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Sendova, M.; Jimenez, J.A.; Smith, R.; Rudawski, N. Kinetics of copper nanoparticle precipitation in phosphate glass: An isothermal plasmonic approach. Phys. Chem. Chem. Phy. PCCP 2015, 17, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Véron, O.; Blondeau, J.P.; Meneses, D.D.S.; Vignolle, C.A. Characterization of silver or copper nanoparticles embedded in Soda-lime glass after a staining process. Surf. Coat. Technol. 2013, 227, 48–57. [Google Scholar] [CrossRef]
- Gurin, V.S.; Prokopenko, V.B.; Alexeenko, A.A.; Wang, S.; Yumashev, K.V.; Prokoshin, P.V. Sol–gel silica glasses with nanoparticles of copper selenide: Synthesis, optics and structure. Int. J. Inorg. Mater. 2001, 3, 493–496. [Google Scholar] [CrossRef]
- Li, Y.; Chopra, N. Fabrication of nanoscale heterostructures comprised of graphene-encapsulated gold nanoparticles and semiconducting quantum dots for photocatalysis. Phys. Chem. Chem. Phys. 2015, 17, 12881–12893. [Google Scholar] [CrossRef] [PubMed]
- Biroju, R.K.; Choudhury, B.; Giri, P.K. Plasmon-enhanced strong visible light photocatalysis by defect engineered CVD graphene and graphene oxide physically functionalized with Au nanoparticles. Catal. Sci. Technol. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Bonmatí, E.; Casanovas, A.; Angurell, I.; Llorca, J. Hydrogen photoproduction from ethanol-water mixtures over Au-Cu alloy nanoparticles supported on TiO2. Top. Catal. 2015, 58, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Xia, Y.; Zhu, S.; Zheng, S.; He, Y.; Bi, J.; Liu, M.; Wu, L. Au and Pt co-loaded g-C3N4 nanosheets for enhanced photocatalytic hydrogen production under visible light irradiation. Appl. Surf. Sci. 2015, 358, 304–312. [Google Scholar] [CrossRef]
- Mogyorósi, K.; Kmetykó, Á.; Czirbus, N.; Veréb, G.; Sipos, P.; Dombi, A. Comparison of the substrate dependent performance of Pt-, Au- and Ag-doped TiO2 photocatalysts in H2-production and in decomposition of various organics. React. Kinet. Catal. Lett. 2009, 98, 215–225. [Google Scholar] [CrossRef]
- Zeng, D.; Chen, Y.; Wang, Z.; Wang, J.; Xie, Q.; Peng, D.L. Synthesis of Ni-Au-ZnO ternary magnetic hybrid nanocrystals with enhanced photocatalytic activity. Nanoscale 2015, 7, 11371–11378. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, A.; Naji, M. Degradation photocatalysis of tetrodotoxin as a poison by gold doped PdO nanoparticles supported on reduced graphene oxide nanocomposites and evaluation of its antibacterial activity. J. Photochem. Photobiol. B Biol. 2017, 167, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.L.; Liu, J.; Jiang, W.J.; Zhang, X.; Hu, J.S.; Wan, L.J. Urchin-like Au@CdS/WO3 micro/nano heterostructure as a visible-light driven photocatalyst for efficient hydrogen generation. Chem. Commun. 2015, 51, 13842–13845. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jiao, J.; Zhao, Z.; Liu, J.; Li, J.; Jiang, G.; Wang, Y.; Duan, A. Fabrication of inverse opal TiO2-supported Au@CdS core-shell nanoparticles for efficient photocatalytic CO2 conversion. Appl. Catal. B Environ. 2015, 179, 422–432. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Q.; Guo, W.; Zheng, M.; Xu, W.; Ma, F.; Hou, B. Fabrication of g-C3N4/Au/CdZnS Z-scheme photocatalyst to enhance photocatalysis performance. RSC Adv. 2016, 6, 28263–28269. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Selvaraj, R.; Kim, Y. Microwave-assisted synthesis of Au/CdS nanorods for a visible-light responsive photocatalyst. RSC Adv. 2015, 5, 52737–52742. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, S.; Xin, Y. Synthesis of Au-CuS-TiO2 nanobelts photocatalyst for efficient photocatalytic degradation of antibiotic oxytetracycline. Chem. Eng. J. 2016, 302, 377–387. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Que, W.; Du, Y. Synthesis of high quality CuO nanoflakes and CuO-Au nanohybrids for superior visible light photocatalytic behavior. RSC Adv. 2016, 6, 81607–81613. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, M.; Wu, J.; Ma, Y.; Ma, Z. In situ synthesis of TiO2/SnO2-Au ternary heterostructures effectively promoting visible-light photocatalysis. Dalton Trans. 2015, 44, 11901–11910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, W.; Sun, S.; Zhang, L.; Zheng, Y. Equilibrating the plasmonic and catalytic roles of metallic nanostructures in photocatalytic oxidation over Au-modified CeO2. ACS Catal. 2015, 5, 613–621. [Google Scholar] [CrossRef]
- Bi, J.; Fang, W.; Li, L.; Li, X.; Liu, M.; Liang, S.; Zhang, Z.; He, Y.; Lin, H.; Wu, L.; et al. Ternary reduced-graphene-oxide/Bi2MoO6/Au nanocomposites with enhanced photocatalytic activity under visible light. J. Alloys Compd. 2015, 649, 28–34. [Google Scholar] [CrossRef]
- Hu, X.; Tian, J.; Xue, Y.; Li, Y.; Cui, H. Bi2WO6 nanosheets decorated with Au nanorods for enhanced near-infrared photocatalytic properties based on surface plasmon resonance effects and wide-range near-infrared light harvesting. ChemCatChem 2017, 9, 1511–1516. [Google Scholar] [CrossRef]
- Hirakawa, H.; Shiota, S.; Shiraishi, Y.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Au nanoparticles supported on BiVO4: Effective inorganic photocatalysts for H2O2production from water and O2 under visible light. ACS Catal. 2016, 6, 4976–4982. [Google Scholar] [CrossRef]
- Van, C.N.; Chang, W.S.; Chen, J.W.; Tsai, K.A.; Tzeng, W.Y.; Lin, Y.C.; Kuo, H.H.; Liu, H.J.; Chang, K.D.; Chou, W.C.; et al. Heteroepitaxial approach to explore charge dynamics across Au/BiVO4 interface for photoactivity enhancement. Nano Energy 2015, 15, 625–633. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Cui, W.; Zhang, Y.; Dong, F. In situ growth of Au nanoparticles on 3D Bi2O2CO3 for surface plasmon enhanced visible light photocatalysis. New J. Chem. 2015, 39, 8446–8453. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, W.; Zhu, L.; Li, G.; Yang, K.; Jin, R. Integrating plasmonic Au nanorods with dendritic like α-Bi2O3/Bi2O2CO3 heterostructures for superior visible-light-driven photocatalysis. Appl. Catal. B Environ. 2016, 184, 1–11. [Google Scholar] [CrossRef]
- Gan, J.; Rajeeva, B.B.; Wu, Z.; Penley, D.; Zheng, Y. Hydrogen-reduced bismuth oxyiodide nanoflake arrays with plasmonic enhancements for efficient photoelectrochemical water reduction. Electrochim. Acta 2016, 219, 20–27. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; Tian, N.; Guo, Y.; Zhang, Y. Plasmon induced Au particle and surface oxidation co-decorated BiOIO3 heteronanostructures with highly promoted photocatalysis and photoelectrochemical properties. RSC Adv. 2015, 5, 81078–81086. [Google Scholar] [CrossRef]
- Ha, E.; Lee, L.Y.; Man, H.W.; Tsang, S.C.; Wong, K.Y. Morphology-Controlled Synthesis of Au/Cu(2)FeSnS(4) Core-Shell Nanostructures for Plasmon-Enhanced Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2015, 7, 9072–9077. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kang, Y.; Luo, D. Synthesis of novel Au/FeVO4/Bi2O3 heterojunction for efficient visible-light-driven photocatalysis. Mater. Lett. 2016, 185, 189–192. [Google Scholar] [CrossRef]
- She, P.; Xu, K.; He, Q.; Zeng, S.; Sun, H.; Liu, Z. Controlled preparation and visible light photocatalytic activities of corn cob-like Au–ZnO nanorods. J. Mater. Sci. 2017, 52, 3478–3489. [Google Scholar] [CrossRef]
- Huang, X.; Chen, R.; Zhang, C.; Chai, J.; Wang, S.; Chi, D.; Chua, S.J. Ultrafast and Robust UV Luminescence from Cu-Doped ZnO Nanowires Mediated by Plasmonic Hot Electrons. Adv. Opt. Mater. 2016, 4, 960–966. [Google Scholar] [CrossRef]
- She, P.; Xu, K.; Zeng, S.; He, Q.; Sun, H.; Liu, Z. Investigating the size effect of Au nanospheres on the photocatalytic activity of Au-modified ZnO nanorods. J. Colloid Interface Sci. 2017, 499, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; You, N.; Yu, Z.; Zhou, G.; Xu, X. Two-dimensional ZnO ultrathin nanosheets decorated with Au nanoparticles for effective photocatalysis. J. Appl. Phys. 2016, 120. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Miao, Y.; Feng, G.; Zhang, R. Facet-selective photodeposition of gold nanoparticles on faceted ZnO crystals for visible light photocatalysis. J. Colloid Interface Sci. 2016, 475, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, Y.; Xu, T.; Wang, X.; Ahmad, M.; Sun, H. Hierarchical nanoflowers assembled with Au nanoparticles decorated ZnO nanosheets toward enhanced photocatalytic properties. Mater. Lett. 2017, 190, 185–187. [Google Scholar] [CrossRef]
- Ranasingha, O.K.; Wang, C.; Ohodnicki, P.R.; Lekse, J.W.; Lewis, J.P.; Matranga, C. Synthesis, characterization, and photocatalytic activity of Au-ZnO nanopyramids. J. Mater. Chem. A 2015, 3, 15141–15147. [Google Scholar] [CrossRef]
- Jiang, T.; Qin, X.; Sun, Y.; Yu, M. UV photocatalytic activity of Au@ZnO core-shell nanostructure with enhanced UV emission. RSC Adv. 2015, 5, 65595–65599. [Google Scholar] [CrossRef]
- Pap, Zs.; Tóth, Zs.R.; Danciu, V.; Baia, L.; Kovács, G. Differently shaped au nanoparticles: A case study on the enhancement of the photocatalytic activity of commercial TiO2. Materials 2015, 8, 162–180. [Google Scholar] [CrossRef]
- Gołabiewska, A.; Malankowska, A.; Jarek, M.; Lisowski, W.; Nowaczyk, G.; Jurga, S.; Zaleska-Medynska, A. The effect of gold shape and size on the properties and visible light-induced photoactivity of Au-TiO2. Appl. Catal. B Environ. 2016, 196, 27–40. [Google Scholar] [CrossRef]
- Xu, Z.; Quintanilla, M.; Vetrone, F.; Govorov, A.O.; Chaker, M.; Ma, D. Harvesting lost photons: Plasmon and upconversion enhanced broadband photocatalytic activity in core@shell microspheres based on lanthanide-doped NaYF4, TiO2, and Au. Adv. Funct. Mater. 2015, 25, 2950–2960. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Y.; Wang, G.; Zhou, H.; Zhang, H. Enhanced photocatalytic activity of a hollow TiO2-Au-TiO2 sandwich structured nanocomposite. RSC Adv. 2016, 6, 18958–18964. [Google Scholar] [CrossRef]




















| NP | Composites | Biological Response In Vitro/In Vivo | References |
|---|---|---|---|
| AgNP | phosphate glasses | in vitro bioactivity | [108] |
| silicate bioactive glass | in vitro bioactivity; protein adsorption | [109,110] | |
| mesoporous bioactive glass | antibacterial activity | [111] | |
| borophosphate glass | antibacterial activity | [112] | |
| bioactive glass | in vitro bioactivity; antibacterial activity | [113] | |
| bioactive glass-polymer | in vitro bioactivity; antibacterial activity | [114] | |
| AuNP | bioactive glass | in vitro bioactivity; cytotoxic effect, cell viability | [115,116,117,118] |
| cytotoxic effect, cell viability | [116,117] | ||
| polymer-bioactive glass | biocompatibility, cell viability | [119] | |
| CuNP | bioactive glass | in vitro bioactivity | [120] |
| bioactive glass, | in vitro bioactivity | [121] | |
| bioactive glass-ceramics | in vitro bioactivity; biocompatibility; cell viability, antibacterial activity | [122] | |
| soda-lime glass | antibacterial activity | [123] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craciun, A.M.; Focsan, M.; Magyari, K.; Vulpoi, A.; Pap, Z. Surface Plasmon Resonance or Biocompatibility—Key Properties for Determining the Applicability of Noble Metal Nanoparticles. Materials 2017, 10, 836. https://doi.org/10.3390/ma10070836
Craciun AM, Focsan M, Magyari K, Vulpoi A, Pap Z. Surface Plasmon Resonance or Biocompatibility—Key Properties for Determining the Applicability of Noble Metal Nanoparticles. Materials. 2017; 10(7):836. https://doi.org/10.3390/ma10070836
Chicago/Turabian StyleCraciun, Ana Maria, Monica Focsan, Klara Magyari, Adriana Vulpoi, and Zsolt Pap. 2017. "Surface Plasmon Resonance or Biocompatibility—Key Properties for Determining the Applicability of Noble Metal Nanoparticles" Materials 10, no. 7: 836. https://doi.org/10.3390/ma10070836