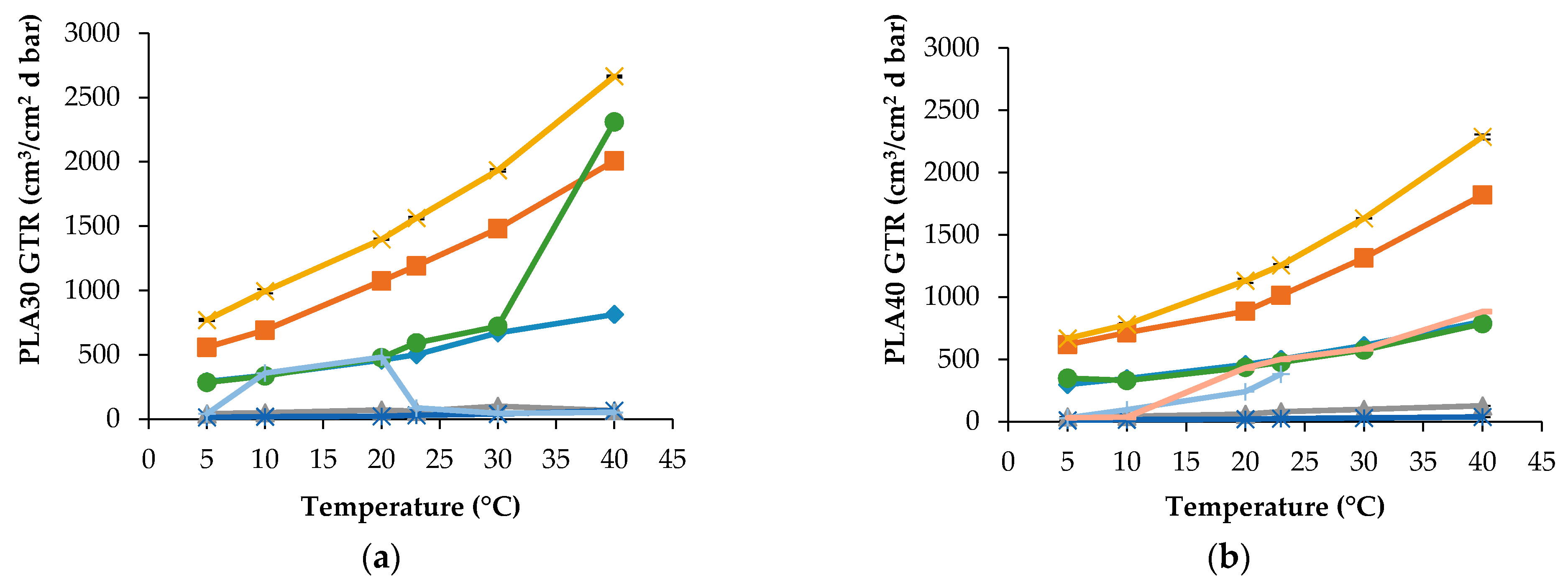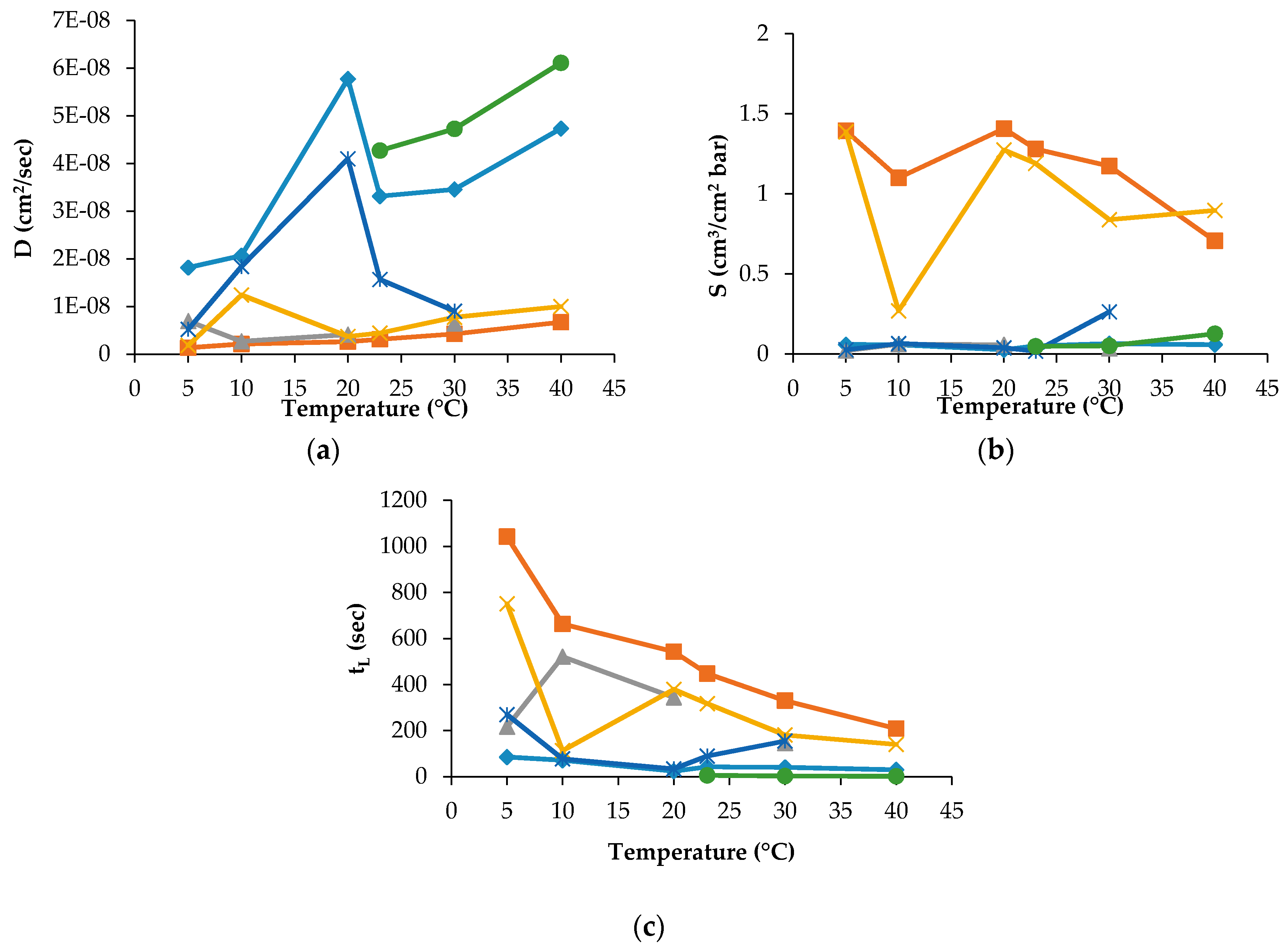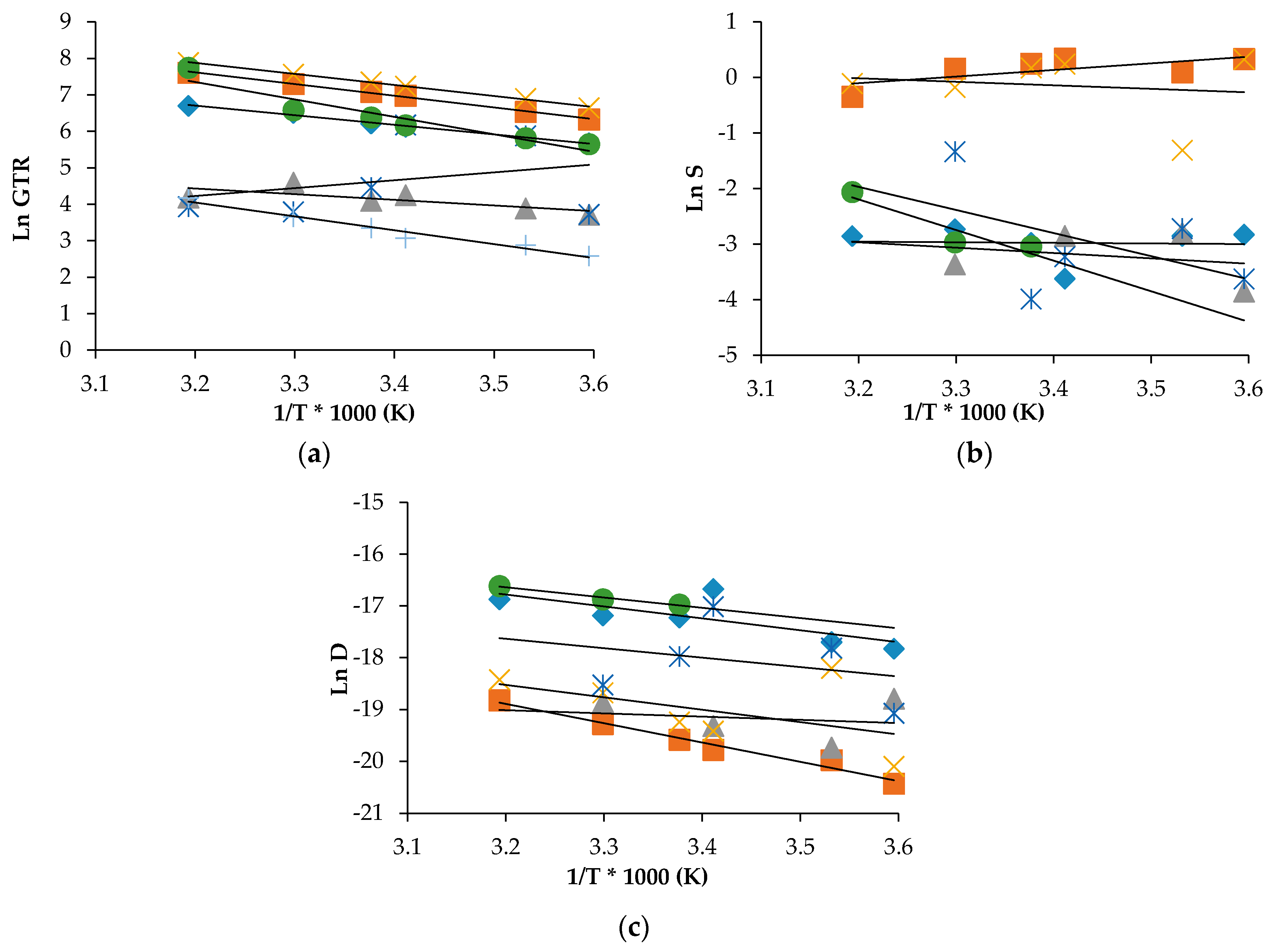2.1. Barrier Behavior
Gas transmission in polymer materials depends strongly on the polymer structure, on the gas type, as well as on the temperature. The theoretical models that have been proposed and reported in the literature [
16,
17] describe the transport mechanism of molecular species in polymers by diffusion, involving a temperature-dependent diffusion coefficient. However, it should be observed that it is not easy to identify this dependency exactly and to provide exact information on both the diffusion coefficient and the solubility coefficients, in particular under real condition [
18,
19,
20]. Therefore, in-depth analyses of the relationship between such parameters are needed to get the best market solution and to enable better understanding of the underlying transport phenomenon that occur in real conditions. The primary mechanism for gas flow through a film or coating is an activated diffusion. This means that the permeate dissolves in the film matrix at the higher concentration side, diffuses through the film, driven by a concentration gradient and evaporates from the other surface. Differences in the solubility of specific gases may influence the diffusivity of gases across the film. The second step of the permeability phenomenon, the diffusion, depends on the size, shape and polarity of the penetrating molecule of the permeant and on the crystallinity, degree of cross-linking and polymer chain segmental motion of the polymer matrix. Permeability of polymers to an organic compound or water is presented using the Gas Transmission Rate (GTR), which is in common use as well just reported in literature [
12,
21,
22].
In our work, we correlate the GTR values of five PLA films with the main influencing factors such as temperature, gases and chemical structure. GTR values (cm
3/cm
2 d bar), together with Solubility S (cm
3/cm
2 bar), Diffusivity D (cm
2/s) and time Lag t
L (s), were recorded for pure gases, Air and MA, together with thickness (in micron) and perm-selectivity ratio. In
Table 1 are reported all the collected data recorded with O
2 gas test, at 23 °C, while in
Table 2 are reported the corresponding perm-selectivity data, recorded with all gases used in the experiment and at all temperature investigated (the value recorded at 23 °C is in bold style to be used as standard value).
Perm-selectivity values represent the permeability ratio of different permeants As reported by Schmid et al. [
23], for many polymers, this ratio is in the range 1:4:16 (N
2:O
2:CO
2). The calculated perm-selectivity ratios are shown in
Table 2. The results are very different from the simplified ratio. These data are useful because they give the possibility to determine the unknown GTR data, knowing the GTR value of another gas. For example, obtaining the CO
2 GTR data is enough to multiply the O
2 GTR data for the corresponding perm-selectivity ratio, at the desired temperature. It will be an error to use the tabulated values present in the literature because it was scientifically demonstrated that the perm-selectivity ratio are not constant for every type of polymer materials but it changes depending on several factors, first of all the chemical structure and experimental temperature [
23,
24,
25].
As expected, the surface modification effectively retarded the transport of gas molecules through the PLA matrix, resulting in a substantial decrease in GTR value. PLA samples with larger amount of oxygen-containing functional groups were found to yield more prominent improvement in barrier performance, giving rise to better compatibility with PLA resins, ultimately resulting in effective suppression of gas molecule permeation through the films.
CO
2 and N
2O gases were more permeable through the polymer film, with respect to O
2 and N
2, despite their different molecular diameters being larger than of for oxygen and nitrogen molecules. The C
2H
4 gas transmission is quite low, very similar to the N
2 gas. GTR behavior in a modified atmosphere (Air and MA) is instead strictly dependent from the superficial treatment of the PLA film. It is well known that the mechanisms that drive the adsorption/desorption permeability, solubility and diffusion phenomena are all closely dependent on the temperature [
21,
22] so, as expected, temperature has a considerable influence on the transmission of the gas through the material. Considering polymeric film, which performs optimally at a given temperature, may increase/decrease the rate of food respiration or material permeability at a higher/lower temperature, it is important to understand the PLAs gas barrier performance under a range of temperature generally used for food storage. In fact, limited information is still available on most film’s permeability properties at varying storage temperature, as well as on the ability of the polymeric film to withstand mechanical stress during storage and transport. Gas transmission behavior was then described in detail.
In
Figure 1 GTR data for PLA30 and PLA40 films obtained at different temperature and for various gases are reported.
Both samples have differences in thickness and crystalline percentage, therefore their barrier characteristics differ each other due to change in the gas diffusion pathway and the amorphous state in the intercrystalline area of PLA films. For the PLA30 specimen, the transport of CO
2 and N
2O has a similar behavior, however N
2O is characterized by higher gas transmission in comparison with CO
2. If compare the transfer rates of principal atmospheric gases, such as O
2 and N
2, the former has essentially higher GTR than the latter. Additionally, it is worth noting that C
2H
4 exhibits GTR values practically similar to the N
2 values. Especially, the difference in transport behavior is noticeable for the modified atmospheric composition of the gases (MA). In this situation, the temperature dependences of GTR for both PLA samples displays the different forms, namely the curve with a sharp inflection point at 30 °C for PLA30 and the monotonic temperature curve for PLA40. Besides,
Figure 1 shows that the MA-GTR values are close to the O
2 values in whole temperature interval for the PLA40 film but for the PLA30 film the given proximity occurs only by 23 °C. The analogous comparison between MA and N
2 permeabilities shows that, for both specimens, while for N
2 the analogous behavior is observed at T < 23 °C and only for the PLA40 film. Qualitatively, the analogous domination in MA-GTR is observed for N
2O transferring. The next step in estimation of gaseous mix behavior represents the measuring of air permeability. In
Figure 1b, the transfer behavior of air is reported as: Air
1 (the first 10 minutes of GTR measurement) and Air
2 (after 10 minutes of GTR measurement). In other words, there are short and long terms of measurement. In
Figure 1b, Air
1-GTR values for the PLA40 film are increased until 23 °C. The Air
2-GTR values reach constant values similar to N
2 and MA, respectively. The quantitative description of MA transport and its comparison with the single gas transport seems to us extremely important because similar modeling brings above results closer to the real conditions when using eco-friendly PLA packaging.
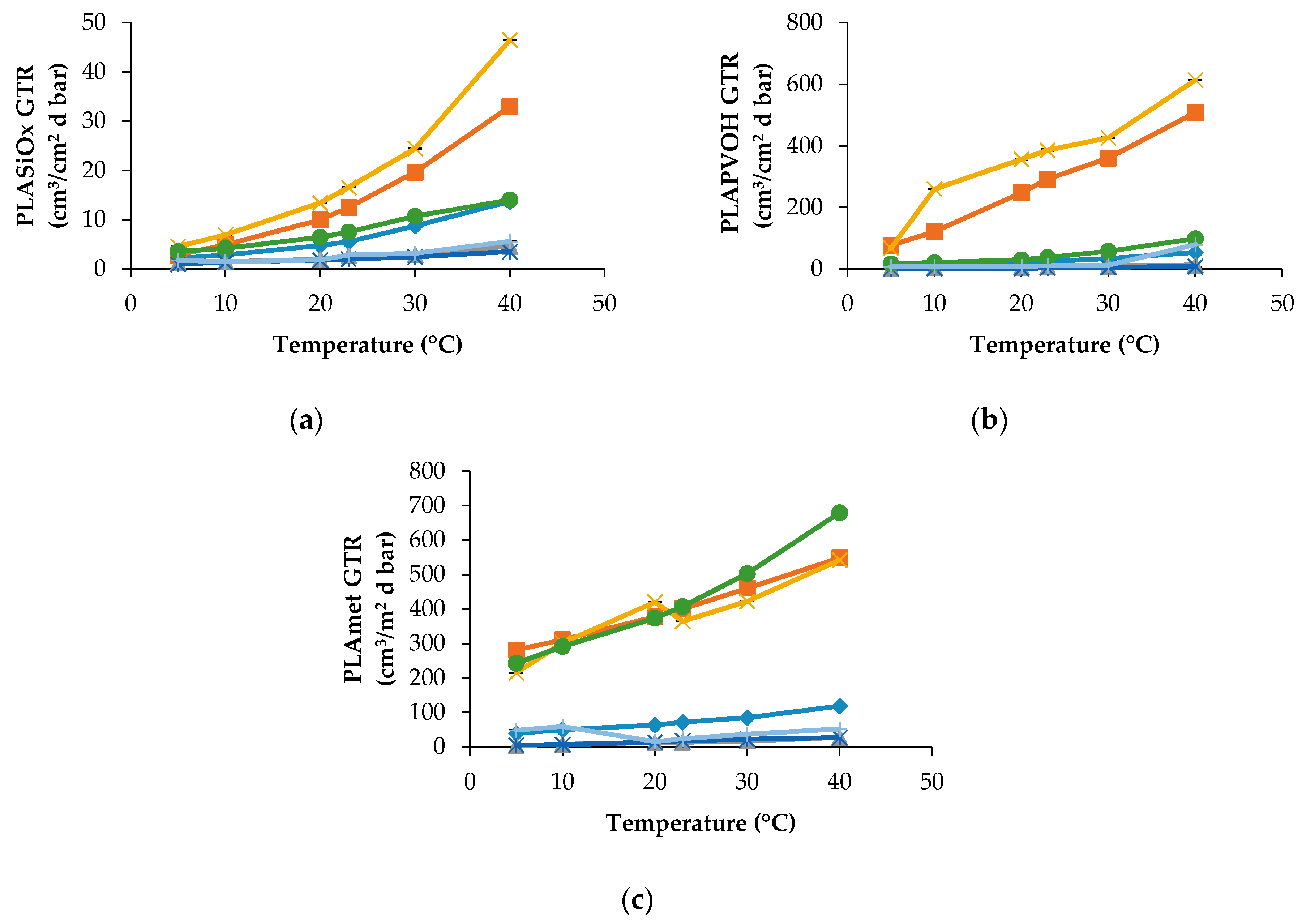
PLA modified films show a very different trend. As can be observed in
Figure 2a, the PLASiO
x specimen displays the best barrier characteristics for all penetrants including the pure gases and MA.
At all temperatures, the gaseous mixtures, such as MA and Air, have trends and GTR values that are quite close to O2 GTR and N2 GTR, respectively. Even when the multicomponent penetrant is applied, its transport phenomenon could be approximated by a simple gaseous penetrant what facilitates the prognosis of barrier properties of biopolymers.
PLAPVOH film shows a dual behavior, being dependent on the chemical nature of gaseous penetrant. CO2 and N2O show the higher GTR value relatively to the other gases (C2H4, N2 and O2) and modified atmosphere (Air and MA). Analogous results were obtained for N2 gas and Air at all temperatures, while a small GTR difference was observed between O2 gas and MA atmosphere at temperatures up to 20 °C.
PLAmet showed a different behavior. GTR recorded for CO2, N2O and MA demonstrate the same tendency with higher recorded value, while GTR data obtained for C2H4, O2, N2 and Air are characterized by lower GTR value.
In general, superficial treatment lowered the GTR value, especially when SiOx was used as a coating.
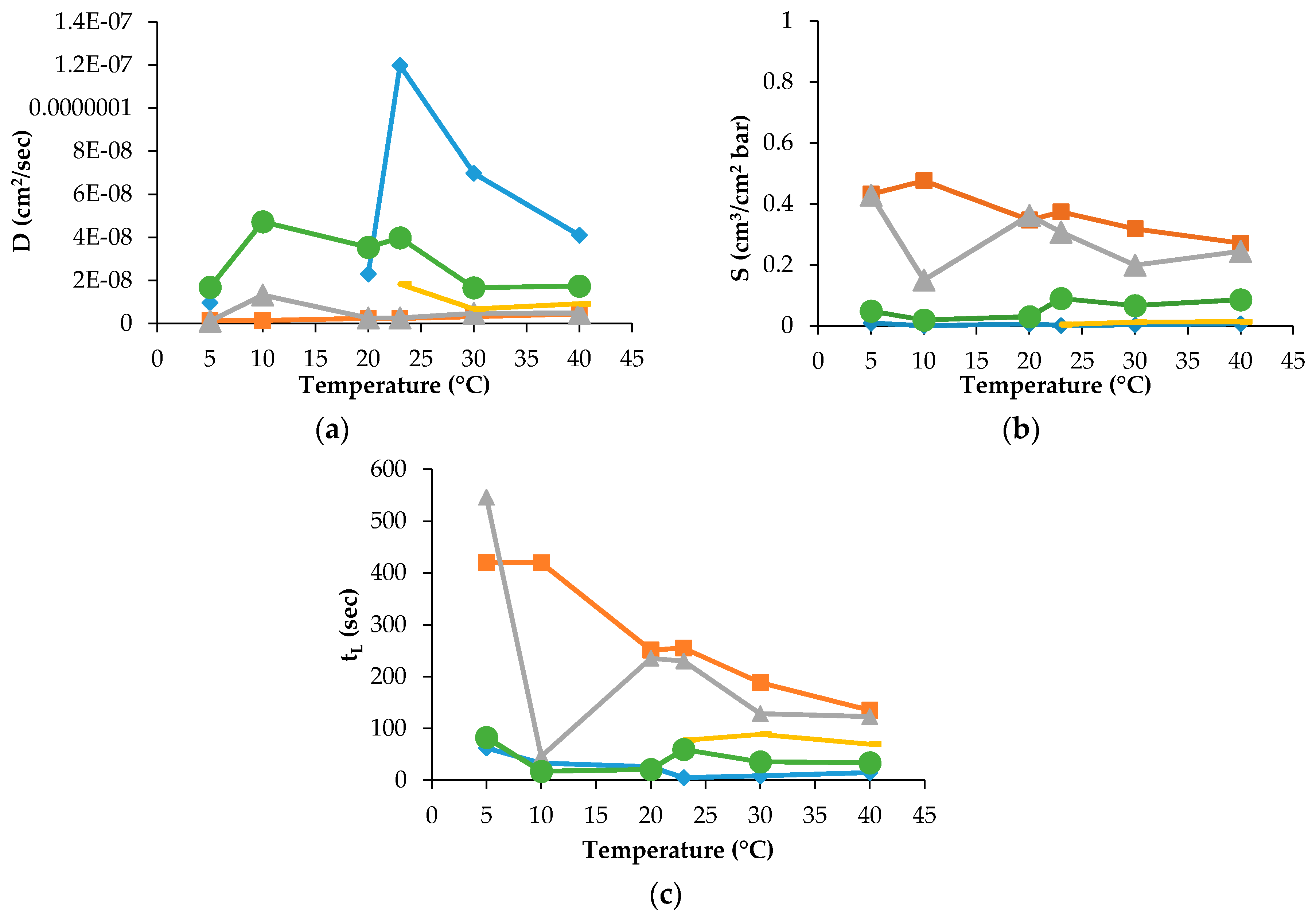
The D, S and t
L data were recorded for PLA30, PLA40 and PLAmet samples, but not for all gases and temperature employed in the experiment. For PLAPVOH, it was possible only with CO
2 and N
2O gases, while, for PLASiO
x, no data were recorded because it was not possible to fit the slope of the linear portion of the GTR curves [
26]. The D, S and t
L are reported in
Figure 3 and
Figure 4 for PLA30 and PLAmet, respectively.
The other data recorded for the other samples were not reported for the sake of simplicity.
The D value, correlated to the kinetic parameters, showed a similar trend for all gases. This means that the diffusion was the same into the film for all tested gases and gas mixture. PLA coated films showed a high barrier behavior because of a very low diffusion coefficient. The surface modification decreased the gas transmission through the films thanks to the reduction of the gas diffusion [
13,
27].
The effect of temperature on the solubility coefficient differs depending on the chemical structure of penetrant. S values of used gases increased according to the following order: N2 < O2 = Air = MA < CO2 < N2O, in the range of temperature investigated. The oxygen-containing composite molecules of gases (N2O and CO2) have higher thermodynamic affinities to the polymer segments with the ester groups. Simultaneously, the lowest equilibrium solubility and, respectively, the small thermodynamic affinity belongs to N2 and O2 molecules. The intermediate position is occupied by the gas mixtures on the base of molecular oxygen enriched with molecular nitrogen (MA) and Air. Thus, the gas permeability of the PLA films as the product of the diffusion mobility and solubility determined by the size of the diffusing particles (on the kinetic level) and the gas equilibrium sorption in polymer (on the thermodynamic level). The latter is necessary for the determination and recording of the boundary conditions for a correct solution of a Fick diffusion equation and gives its own independent contribution to the overall gas transport.
According to a widely known classical diffusion–solubility model [
26,
27] of gas permeability through polymer films and membranes, gaseous penetrant solubility determine the mathematical border conditions of the principal diffusivity equation (Fick’s diffusivity) describing the diffusion impact upon gas permeation. Furthermore, the GTR is generally determined by the product of gas diffusivity (D) by its coefficient solubility (S). Therefore, gas solubility evaluation in PLA and PLA modified films with different crystallinity and morphology provides the key information on barrier characteristics so essential for packaging.
The t
L values as the basic experimental parameters required for gas diffusivity evaluation correlate with the GTR values: as GTR meanings are increased, t
L meanings are decreased in accordance with the relation
where L is the film thickness, k is the numerical coefficient reflecting the dimension of GTR, and S is gas coefficient solubility. For PLA30, the t
L values are decreased with temperature increasing from the highest values at 5 °C from ~1350 s to ~750 s for CO
2 and N
2O, respectively, to the lowest ones at 40 °C for the same gases. The t
L values of O
2, N
2, Air and MA have a sufficiently weak trend of decrement ranging from 85 s to 40 s, from 216 s to 145 s, from 269 s to 154 s and from 33 s to 22 s, respectively. Analogous tendency is observed for the PLA40 specimen where the highest values were recorded at 5 °C for CO
2 (780 s), for N
2 (700 s) and for N
2O (1650 s). For O
2, Air, and MA the t
L decrease is observed as well, when the corresponding values range from 211 s to 97 s, from 60 s to 29 s, 106 s to 84 s, respectively. For PLAPVOH, the highest value was observed at 5 °C for CO
2 (1852 s) and N
2O (2629 s). For PLAmet, the highest value was recorded at 5 °C for CO
2 (420 s), for N
2O (546 s), while, for O
2, Air and MA, were quite constant, ranging from 62 s to 26 s, from 77 s to 69 s, from 82 s to 59 s, respectively.
2.2. Activation Energy of Gas Transport Process
In order to describe the permeation dependence to the temperature, the Arrhenius model was utilized to calculate the activation energy for gas transmission (E
GTR), heat of solution (H
S) and diffusion (E
D) processes. The mathematical relations used are well described in the related scientific literature [
15,
19,
23]. The activation energy is obtained by calculating the value of the slope (−E
a/R) of the Arrhenius equation, where R is the gas constant (8.314 J/mol K). Natural logarithmic (ln) of GTR, S and D compared with reciprocal of the absolute temperature (1/T) were reported as an example in
Figure 5 for PLA30, together with the indication of the calculated linear regression of the corrected experimental points fittings.
Table 3 and
Table 4 contain the corresponding energy characteristics such as activation energies for the gas transmission rate (E
GTR), the specific heat of gas solution (H
S) and activation energy of gas diffusivities (E
D) in the temperature range of 5 °C–40 °C.
As can be observed, the data satisfactory fitted the theoretical relation with the high values of regression coefficient (
R2) that indicates a good linear correlation between permeability and reciprocal temperature for all simple gases. Practically all individual gases and mixes, excluding Air-PLAmet system, have the negative slopes of the semilogarithmic temperature function of GTR that correspond to the positive meanings of E
GTR for both pristine and modified PLA films (see the respective data in
Table 3 and
Table 4).
The corresponding EGTR characteristics have the highest values for N2O and CO2 gases containing oxygen in the molecular formula as compared with the permeability characteristics of unpolarized gas molecules such as C2H4, N2 and O2. When the gaseous mixes such as Air and MP are used, different tendencies for GTR, S and D are observed, depending on the way of modification of PLA. For the solution and diffusion coefficients some deviations were observed. According to N2O and CO2 gas test data, the solubility increased with the temperature while N2 and O2 solubilities were almost constant. Simultaneously, for MA and Air, there is not the linear trend that confirms the difficulty in measuring the permeability of modified atmosphere as the multicomponent medium.
Generally speaking, gas solubility is the parameter correlated to the polymer composition so this trend is a confirmation that the gases interact differently with the matrix. From low to high temperature, the lnS value shifts with a scissors trend, and the corresponding H
S shows a fluctuating value, positive for N
2O and CO
2 gas test and negative for the others, comprising Air and MA. LnD shows a standard behavior with all gases and mixture, thus meaning that the diffusion was the same for the tested gas, despite the different solubility. Consequently, E
D shows a positive value for all pure gas tests but not for modified atmosphere (Air, MA). Similar results were recorded for the PLA40 and PLAmet samples. For PLASiO
x sample, no S and D data were recorded, while, for PLAPVOH sample, only few data with CO
2 and N
2O gases were recorded (data not reported for the sake of simplicity). As it is well known from the literature [
28,
29,
30], high activation energies imply more sensitivity to temperature variation. The permeation process is very well correlated to the temperature variation, while the sorption/diffusion process shows consistent deviation, more correlated to polymer structure. Further, the corresponding selectivity ratio shows different value depending on the temperature, confirming that also this parameter is not a constant and is correlated not only with the chemical structure of the materials, but depends also from the (analysis) temperature. Values were reported in
Table 2. The behavior could be explained with the crystalline percentage calculated after annealing of the sample. As the crystalline phase increased, the E
GTR and E
D increased while the H
S decreased. It means that, if the gas transmission increases (less barrier behavior), the solubility increase (major interaction with the polymer matrix) and the diffusivity decrease (the gas molecules spend less time in moving inside the polymer matrix). Consequently, if E
GTR decrease (less energy required for the gas transmission process), H
S decrease (the gas solubility is enhanced) and E
D increase (more difficult by the gas molecules to move inside the polymer matrix). This trend varies with gas and temperature and underlain the importance to perform the determination of the barrier characteristics at different storage temperature.
2.3. Thermal Behavior
Samples have been stored at ambient temperature for 30 days in order to provide the same heat treatment for all the samples investigated, erasing their previous thermal history. In order to calculate the PLA crystalline degree in percentage, the following formula was used: x
c = 100 ((ΔH
m − ΔH
c)/ΔH
m*), where ΔH
m is the fusion enthalpy calculated from the experimental DSC curve, ΔH
c is the crystallization enthalpy calculated from the experimental curve as well, and ΔH
m* = 93 J/g is the fusion enthalpy for the entirely 100% crystalline PLA [
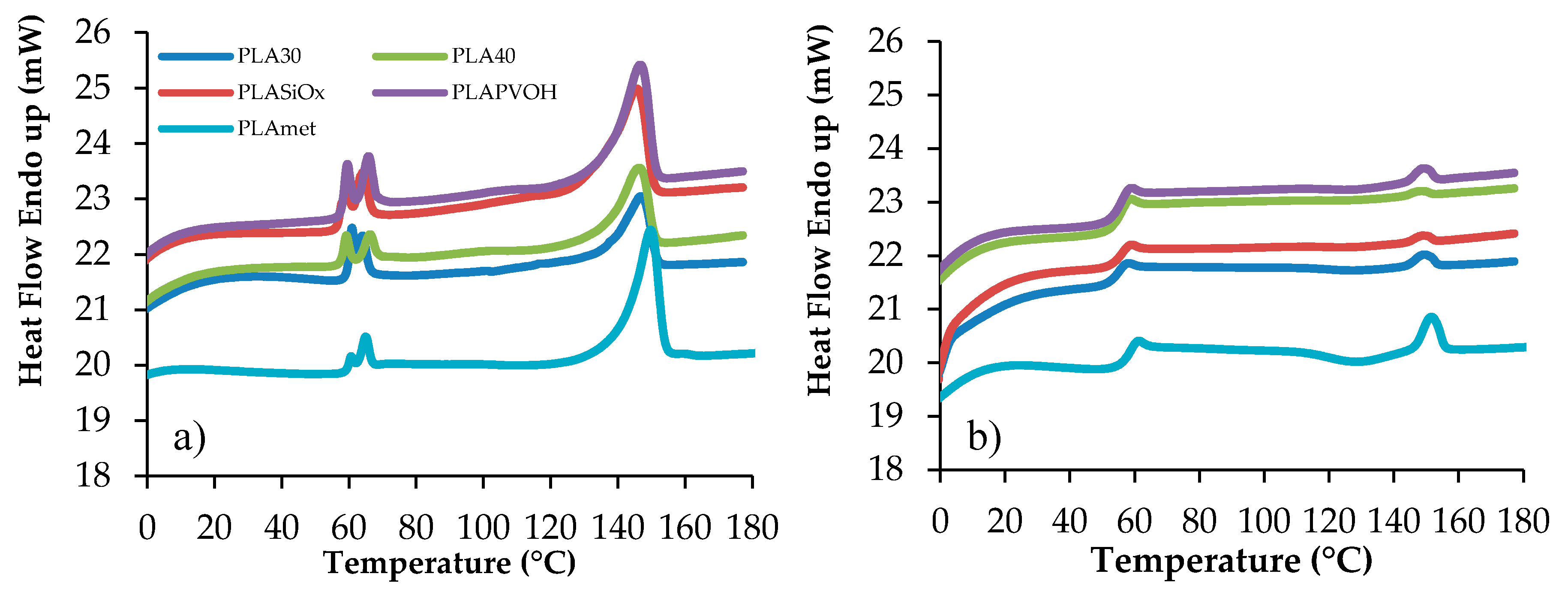
13]. The corresponding Differential Scanning Calorimetry (DSC) curves for the neat and surface-modified specimens of PLA are represented in
Figure 6. Using these experimental data, the principal thermal transition characteristics are collected in
Table 5.
T
g value was not observed from the first heating run due to the presence of the crystalline phase, the corresponding calorimetric traces characterized by a conspicuous melting endotherm. Although PLA is an ideal polymer for environmental application, it has some disadvantages such as high crystallinity and consequently high brittleness, at ambient temperature. This T
g was relatively weak and difficult to determine. Furthermore, it can be seen from the first heating run that the PLA film is a multilayer material, showing a first transition phase, represented by two endothermic peaks, corresponding to the fusion of the polymer substrate material on which PLA is added [
13]. The first two T
m values, represented by endothermic phenomena, are so correlated to the substrate polymer film behavior and are recorded in the range where the T
g is detected. In order to detect the T
g, an amorphous polymer was obtained by rapid cooling from the melt. In this case, during the second heating run, a consistent transition was detected, associated to the glass-to-rubber transition, with the corresponding Δc
p value, with a small endothermic peak corresponding to a residual crystalline PLA, with a T
m value slightly higher due to more perfect crystallites.
As can be observed, the T
g value varies from 54 °C to 58 °C and the melting temperature is in the range from 145 °C to 150 °C value, in accordance with the results reported in literature [
29]. To determine the possible change in PLA crystallinity during the gas transfer measurements, the DSC curves were recorded for the samples being stored for a different time. Under isothermal conditions, the storage time was chosen on the base of GTR measurement duration, namely from 1 h to 4 h (see
Table 6). Crystallinity percentage for the uncoated and coated films is weakly dependent on the temperature of gas permeability, while the way of surface modification alters the PLA crystallization by about 30%. After quenching and DSC rescanning, the degree of crystallinity was very small evidencing the low ability of PLA to recrystallize during cooling, except for PLA metallized sample. As reported also from Kim and Choi on its paper on PLA/graphene films [
30], modification of neat PLA matrix was found to increase the T
g value. Such a reinforcing effect in the thermal property may be ascribed to the reduced mobility of PLA chain molecules in the modified materials, resulting from the interaction between the surface ester groups of the polymer molecules and the groups of modifying layer. Similar results have likewise been reported for a number of nanocomposites incorporated with various nanofillers such as nanosilica particles, nanoclays, cellulose nanofiber and graphene nanosheets [
30,
31,
32,
33,
34,
35], and PLA blend with other polymers (biodegradable or not) [
11]. It should be noted that the metallization led to a more substantial increase in T
g of the PLA composite, compared to neat PLA, because the metallization could yield more interaction between the two phases, leading a more homogeneous composite material. As regards crystallization behavior, the χ
c of PLA in the modified films was revealed to slightly change with the exception of the metallized PLA. It could be seen that the incorporation of a metallized layer led to a remarkable increase in χ
c. The aluminum incorporated into the PLA matrix may be considered as extraneous nucleating agents promoting the crystallization rate, and thus yielded the noticeable improvement in the degree of crystallinity for PLA film. Additionally, the PLA metallized exhibited a more pronounced effect on the crystallization behavior compared to the other films, which may be associated with uniform dispersion in the PLA matrix. The crystallization process acceleration caused by the incorporation of fillers has also been reported in a variety of crystalline polymer-based nanocomposites [
36,
37].
In order to understand if during the gas barrier measurements, performed at different temperature, the crystallinity of the sample changed, influencing consequently the barrier behavior measurements, DSC scans were recorded after 1 h to 4 h of sample maintained in isothermal conditions (annealing treatment) at different temperatures. The isothermally keeping time of the samples was chosen considering the time required to obtain a representative GTR curve in its equilibrium state (steady state), with the different gases and at different temperatures. Data recorded are reported in
Table 6.
As can be observed, a different crystalline percentage was recorded, confirming that temperature plays a key role in the final performance of the tested films. The crystallinity changes are different, depending on the PLA film type. The influence of change into the crystalline/amorphous phase was much more evident in the solubility coefficient value (S). Therefore, the influence of the annealing on the thermal properties of the PLA films can be summarized as follows:
- (1)
Annealing was responsible for an increase or decrease of the crystallinity compared to that of raw material. On the other hand, melting temperature and shape of the melting endothermic signal do not seem to be influenced by the annealing.
- (2)
After the second heating run, a higher tendency to crystallize was recorded for the samples. Similar conclusion was obtained by Carrasco and collaborators [
38] on their thermal and mechanical study on the processed poly(lactic acid). In addition, glass transition temperature was approximately the same for raw and annealed materials (T
g = 54−58 °C; Δ
cp = 0.5−0.6 J/g °C), determined during the second heating scan. The mid-point T
g value was not affected by an increase of the crystalline fraction, which act as a physical entanglement, limiting the molecular mobility of amorphous zones. A broadening of the melting transition signal was thus recorded.
- (3)
A visible cold crystallization exothermic signal was recorded only for annealed samples.
- (4)
Endothermic shoulder located at about 140 °C was recorded for annealed samples, attributed to a lamellar population generated during heat treatment.
- (5)
No detectable change in opacity of the films was detected after annealing.
2.4. Mechanical Behavior
Food packaging applications put specific demands on the properties of the materials. Besides barrier behavior, the mechanical characteristics such as modulus and elasticity of the material are of decisive importance for the suitability of the materials as packaging. To provide insight into the mechanical properties of the PLA polymer films, tensile measurements and cycling loading tests were performed, to assess the extent of mechanical reinforcement resulting from the surface modification of PLA resin. The results of tensile testing are summarized in
Table 7.
In general, the tensile modulus as a measure of stiffness is improved at the expense of elongation representing the degree of ductility when the reinforcing particles are incorporated into the polymer matrix. In this study, the surface modification of PLA film also caused the decrease in elongation at break of the resultant films, but the extent of reduction was revealed to be slight for PLASiOx film, which may be attributed to easily deformable nature of this molecule in polymer matrix. The extent of reduction was instead remarkable when the PLA film was modified by PVOH and metallization. The tensile properties of elongation at break for the PLASiOx and PLAmet films were measured to very low, demonstrating a low level of ductility. Based on sustainability of elongation property after the surface modification of PLA resins, it is believed that the films analyzed in this study may be utilized for the application of practical packaging film where a required proper level of ductility must be well-defined. All PLA films showed anisotropic behavior, with a lower tensile strength and Young modulus in the MD direction than in the CD direction.
In particular, although PLA40 is thicker than PLA30, it shows less mechanical strain due to the less crystallinity percentage, with a consequently higher elasticity. For the three other samples, an increase of E modulus, together with σ
y and σ
M was recorded, showing a strain hardening, induced by an increase of the crystalline portion and by the surface treatment. PLAPVOH and PLA metallized showed the highest tensile strength due to their higher crystallinity. In the same samples, σ
B is lower than σ
M, showing defect on the chemical microstructure [
39].
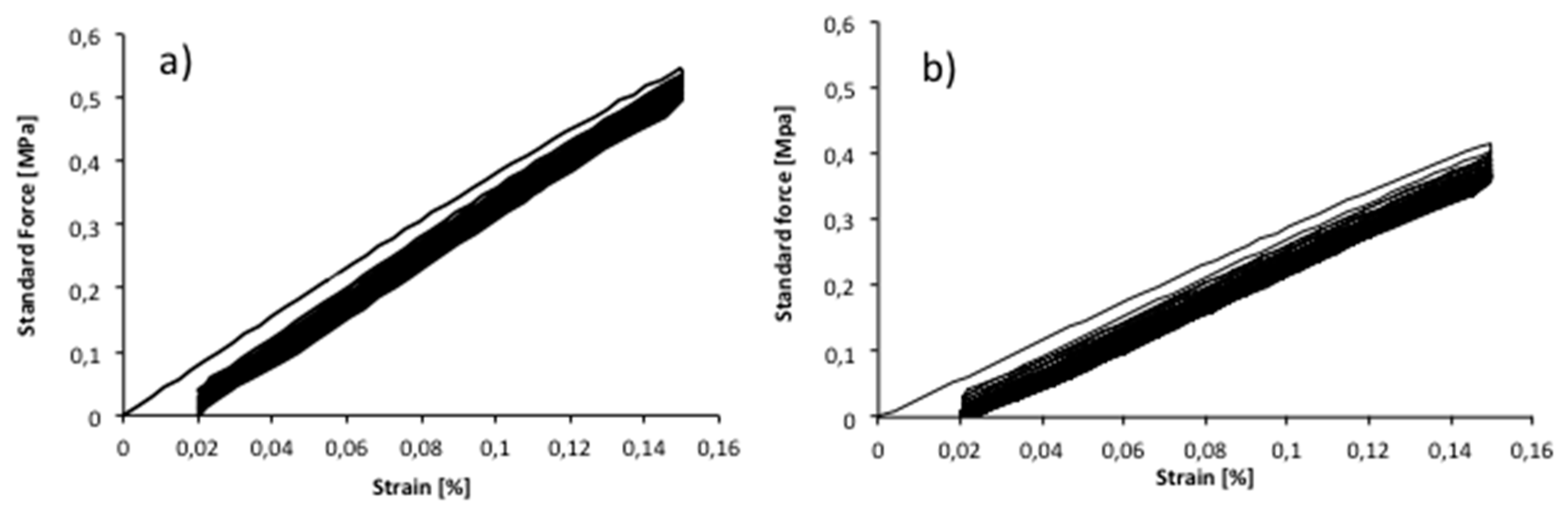
The cycle-loading tests demonstrated that the mechanical properties of the copolymers were affected by the repeated loading, especially after the first recorded cycle. In
Figure 7, an example of the cycle stress–strain curves of PLA metallized film, in MD and CD direction, strained upon 0.15% of strain are reported.
This value was chosen in order to perform the test in the elastic region, taking into consideration the ε
y recorded value (linear elastic field). The same value was used for all PLA films. The sample was very little elastic, showing ca. 77.5% recovery for MD direction and CD direction, after 25 cycles. A mechanical hysteresis was characteristic of the first cycle resulting in a relatively small initial set. As can be observed in
Figure 7, during the cycles, the narrowed hysteresis shape was repeated, producing smaller additional set after each cycle, until the 25 cycles. The stress softening in the ascending curve in the 1st cycle can be caused by a rearrangement in the crystalline micro-phase, formed during the film casting [
40]. The hysteresis area decreased starting from the 2nd cycle, showing a less additional set area. The difference between the ascending curves can be attributed to a reorganization of the macromolecules during straining. Other PLA films showed a similar cyclic tensile behavior, with about 80% elastic recovery for both MD and CD direction, after 1 and 25 cycles. It must be underlined that the elasticity is very low for all films and that the cyclic stress–strain measurement was done just to have an idea of the elastic recovery of the polymer matrix after repeated cyclic stress.