Production of High-Value Nanoparticles via Biogenic Processes Using Aquacultural and Horticultural Food Waste
Abstract
:1. Introduction
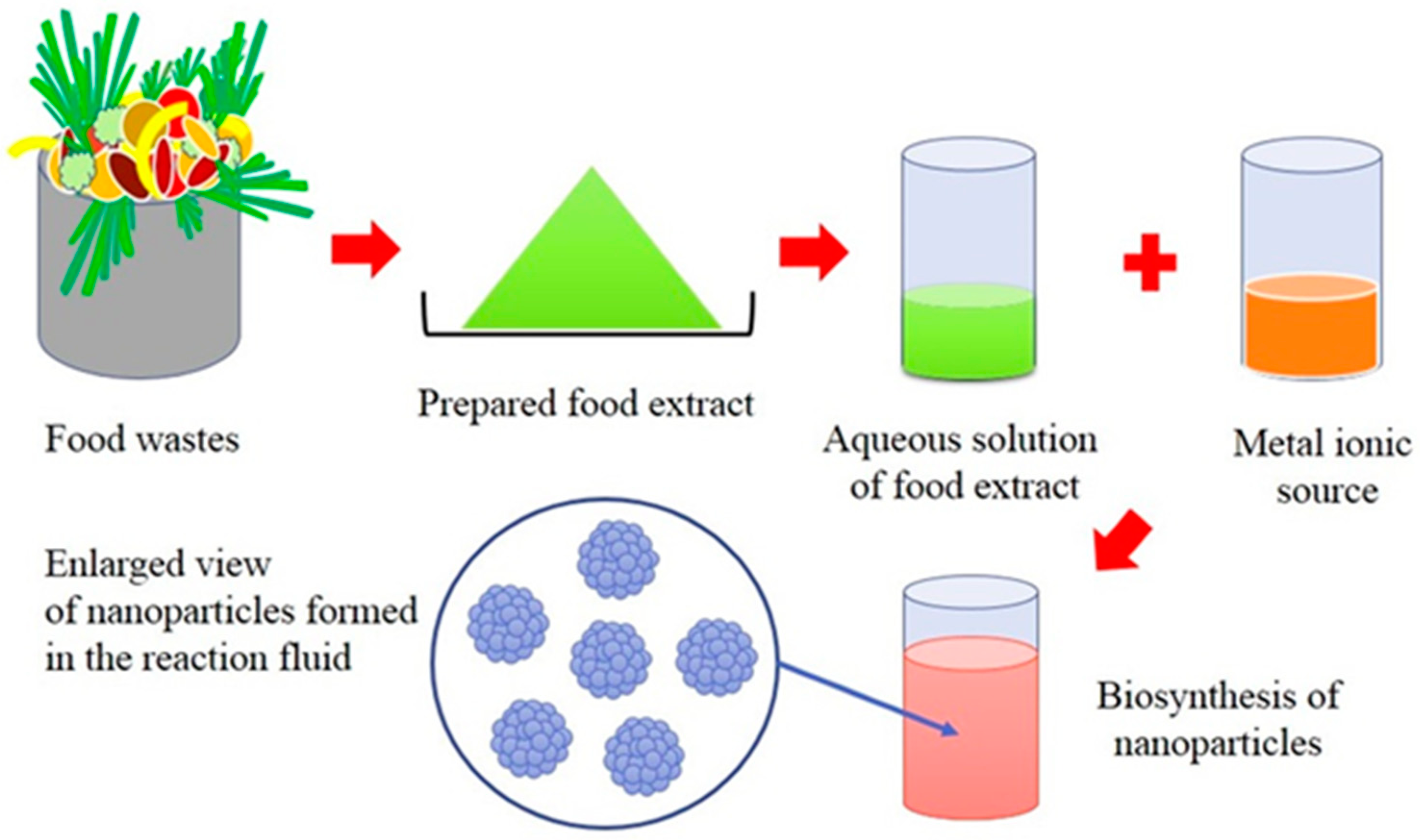
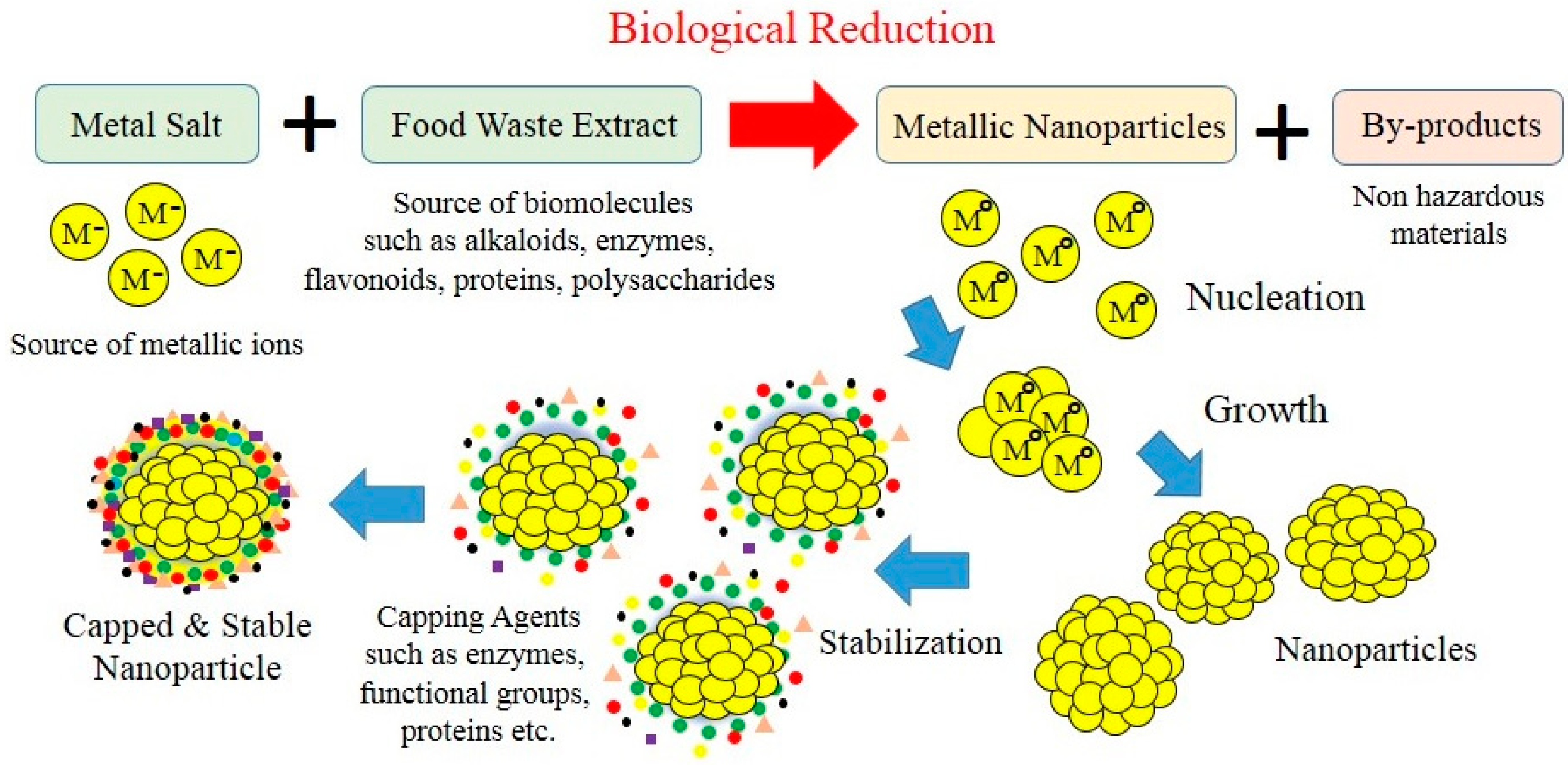
2. Biogenic Synthesis of Nanoparticles Using Aquacultural and Horticultural Food Waste
3. Types of Nanoparticles Produced by Aquacultural and Horticultural Food Waste
3.1. Silver (Ag) Nanoparticles
3.2. Gold (Au) Nanoparticles
3.3. Other Types of Nanoparticles
4. Applications and Future Perspectives
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Kantor, L.S.; Lipton, K.; Manchester, A.; Oliveira, V. Estimating and addressing America’s food losses. Food Rev. 1997, 20, 2–12. [Google Scholar]
- Beretta, C.; Stoessel, F.; Baier, U.; Hellweg, S. Quantifying food losses and the potential for reduction in Switzerland. Waste Manag. 2013, 33, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Gerland, P.; Raftery, A.E.; Sevcikova, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Dosdick, B.K.; Chunn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Cicero, N.; Salvo, A.; Palombieri, D.; Zaccone, D.; Dugo, G.; Bruno, M.; Vadala, R.; Lauriano, E.R.; Perggolizzi, S. Extracts deriving from olive mill wastewater and their effects on the liver of goldfish Carassius auratus with hypercholesterolemic diet. Nat. Prod. Res. 2014, 28, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Ghosh, P.R.; Sharma, S.B.; Haigh, Y.T.; Barbara Evers, A.L.; Ho, G. An overview of food loss and waste: Why does it matter? COSMOS 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Buzby, J.C.; Wells, H.F.; Hyman, J. The Estimated Amount, Value, and Calories of Postharvest Food Losses at the Retail and Consumer Levels in the United States; EIB-121; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Segre, A.; Falasconi, L. Save Food: Global Initiative on Food Loss and Waste Reduction, Background Paper on the Economics of Food Loss and Waste; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Hirsch, J.; Harmanci, R. Food Waste: The Next Food Revolution. 2013. Available online: http://modernfarmer.com/2013/09/next-food-revolution-youre-eating/ (accessed on 19 July 2017).
- Hindustan Times. India Wastes Rs. 44,000 cr of Fruits, Vegetables and Grains Annually; Hindustan Times: Delhi, India, 2013. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of Food Insecurity in the World High Food Prices and Food Security—Threats and Opportunities; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef] [PubMed]
- Waarts, Y.; Eppink, M.M.; Oosterkamp, E.B.; Hiller, S.; van der Sluis, A.; Timmermans, T. Reducing Food Waste: Obstacles Experienced in Legislation and Regulations; LEI Report 2011-059; Wageningen University & Research: Wageningen, The Netherlands, 2011. [Google Scholar]
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Progress towards sustainable utilisation and management of food wastes in the global economy. Int. J. Food Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Evans, F.D.; Critchley, A.T. Seaweeds for animal production use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Salvo, A.; Giuffrida, D.; Rotondo, A.; De Pasquuale, P.; LaTorre, G.L.; Dugo, G. Determine and quantification of carotenoids in marine sponges Raspaciona aculeate and Dictyonelia marsilii present in the Ganzirri Lake (Messina) Italy. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Herrero, M.; Ibanez, E.; Senorans, J.; Cifuentes, A. Accelerated solvent extracts from Spirulina platensis microalga: Determination of their antioxidant activity and analysis by Micellar Electrokinetic Chromatography. J. Chromatogr. A 2003, 1047, 195–203. [Google Scholar]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends. Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Renn, D. Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends Biotechnol. 1997, 15, 9–14. [Google Scholar] [CrossRef]
- Chanda, S.; Dave, R.; Kaneria, M.; Nagani, K. Seaweeds: A novel, untapped source of drugs from sea to combat infectious diseases. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Research Centre: Badajoz, Spain, 2010; pp. 473–480. [Google Scholar]
- Miyashita, K. The carotenoid fucoxanthin from brown seaweed affects obesity. Lipid Technol. 2009, 21, 186–190. [Google Scholar] [CrossRef]
- Zuercher, A.W.; Fritsche, R.; Corthesy, B.; Mercenier, A. Food products and allergy development, prevention and treatment. Curr. Opin. Biotechnol. 2006, 17, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Choi, J.S.; Lee, M.C.; Kim, E.; Nam, T.J.; Fuji, H.; Hong, Y.K. Anti-inflammatory activities of methanol extracts from various seaweed species. J. Environ. Biol. 2008, 29, 465–469. [Google Scholar] [PubMed]
- Wada, K.; Nakamura, K.; Tamai, Y. Seaweed intake and blood pressure levels in healthy pre-school Japanese children. Nutrition 2011, 10, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.; Vonshak, A. Fatty acid composition of Spirulina and Spirulina-like cyanobacteria in relation to their chemotaxonomy. Phytochemistry 1991, 30, 205–206. [Google Scholar] [CrossRef]
- Nishino, T.; Fukuda, A.; Nagumo, T.; Fujihara, T.; Kaji, E. Inhibition of the generation of thrombin and factor Xa by a fucoidan from the brown seaweed Ecklonia kurome. Thromb. Res. 1999, 96, 37–49. [Google Scholar] [CrossRef]
- Namvar, F.; Suhaila, M.; Gasemi Fard, S.; Behravan, J. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Fish matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Metian, A.; Taconand, M. Fishing for feed or fishing for food: Increasing global competition for small pelagic forage fish. AMBIO J. Hum. Environ. 2009, 38, 294–302. [Google Scholar] [CrossRef]
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Pierera, D.; Poinern, G.E.J. Survey of food waste generated by Western Australian fruit and vegetable producers: Options for minimization and utilization. Food Pub. Health 2016, 6, 115–122. [Google Scholar]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mudliar, S.; Sen, R.G.; Giri, B.; Satpute, D.; Chakrabatarti, T.; Pandey, R.A. Commercializing lignocellulosic bioethanol: Technology bottlenecks and possible remedies. Biofuels Bioprod. Biorefin. 2010, 4, 77–93. [Google Scholar] [CrossRef]
- Galbe, M.; Sassner, P.; Wingren, A.; Zacchi, G. Process engineering economics of bioethanol production. Adv. Biochem. Eng. Biotechnol. 2007, 108, 303–327. [Google Scholar] [PubMed]
- Loehr, R. Agricultural Waste Management: Problems, Processes, and Approaches; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Langeveld, J.W.A.; Dixon, J.; Jaworski, J.F. Development perspectives of the bio-based economy: A review. Crop Sci. 2010, 50, S142–S151. [Google Scholar] [CrossRef]
- Balagopalan, C. Cassava utilization in food, feed and industry. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Eds.; CABI: Oxfordshire, UK, 2001; pp. 301–318. [Google Scholar]
- Gunstone, F. Vegetable Oils in Food Technology: Composition, Properties and Uses; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Johansson, D. Renewable Raw Materials—A Way to Reduced Greenhouse Gas Emissions for the EU Industry. DG Enterprise; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Dahl, J.A.; Maddux, B.L.; Hutchison, J.E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 1–8. [Google Scholar] [CrossRef]
- Madhumitha, G.; Rajakumar, G.; Roopan, S.M.; Rahuman, A.A.; Priya, K.M.; Khan, F.R.; Khanna, V.G.; Saral, A.M.; Velayutham, K.; Jayaseelan, C.; et al. Acaricidal, insecticidal, and larvicidal efficacy of fruit peel aqueous extract of Annona squamosa and its compounds against blood-feeding parasites. Parasitol. Res. 2012, 111, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wei-Hong, L.; Hao, L. Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and it’s in vitro cytotoxic effect on two normal cells. Mater. Lett. 2014, 134, 67–70. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Mosaddik, A.; Gyawall, R.; Ahn, K.S.; Cho, S.K. Induction of apoptosis by ethanolic extract of mango peel and comparative analysis of the chemical constitutes of mango peel and flesh. Food Chem. 2012, 133, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of Current Research into the Biogenic Synthesis of Metal and Metal Oxide Nanoparticles via Marine Algae and Seagrasses. J. Nanosci. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Perez, J.; Bax, L.; Escolano, C. Roadmap Report on Nanoparticles; Willems & van den Wildenberg: Barcelona, Spain, 2005. (In Espana) [Google Scholar]
- Torres-Chavolla, E.; Ranasinghe, R.J.; Alocilja, E.C. Characterization and functionalization of biogenic gold nanoparticles for biosensing enhancement. IEEE Trans. Nanobiotechnol. 2010, 9, 533–538. [Google Scholar] [CrossRef]
- Liu, X.Q.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.H.; Chen, H.; Huo, Q. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Puvanakrishnan, P.; Park, J.; Chatterjee, D.; Krishnan, S.; Tunnell, J.W. In vivo tumor targeting of gold nanoparticles: Effect of particle type and dosing strategy. Int. J. Nanomed. 2012, 7, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Mayer, L.; Weinreich, D.; Gola, D.; Pavel, N.; McLaughlin, R.E.; Tamakin, L. Colloidal gold: A novel nanoparticle vector for tumour directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Murkherjee, P. Biological properties of ‘naked’ metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Virkutyte, J.; Varma, R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Huang, X.; Jian, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the minimum temperature required for selective photothermal destruction of cancer cells with the use of immune-targeted gold nanoparticles. Photochem. Photobiol. 2006, 82, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Asha Rani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Duan, S.S.; Ouyang, Y.S.; Chen, Y.B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Poinern, G.E.J.; Fawcett, D. Biogenic synthesis of silver nanoparticles via indigenous Anigozanthos manglesii (red and green kangaroo paw) leaf extract and its potential antibacterial activity. Int. J. Res. Med. Sci. 2016, 4, 3427–3432. [Google Scholar] [CrossRef]
- Shah, M.; Poinern, G.E.J.; Fawcett, D. Biosynthesis of silver nanoparticles using indigenous Xanthorrhoea glauca leaf extract and their antibacterial activity against Escherichia coli and Staphylococcus epidermis. Int. J. Res. Med. Sci. 2016, 4, 2886–2892. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F.; Catalno, M.; Taurino, A.; Licciull, A.; Maffezzoli, A.; Sannino, A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.; Watt, J.D.; Tilley, R.D. Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale 2010, 2, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, M.; Wu, B.; Kuga, S.; Endo, T.; Huang, Y. Platinum nanoparticles using wood nanomaterials: Eco-friendly synthesis, shape control and catalytic activity for p-nitrophenol reduction. Green Chem. 2011, 13, 283–287. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; d’Alessandro, N. One pot synthesis of lignin-stabilized platinum and palladium nanoparticles and their catalytic behaviours in oxidation and reduction reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; Ei-Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Azizi, S.; Tahir, P.M.; Chartrand, M.S.; Yeap, S.K. Cytotoxic Effects of Biosynthesized Zinc Oxide Nanoparticles on Murine Cell Lines. Evid. Based Complement. Altern. Med. 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Soares, N.F.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, F.; Liu, A.; Wang, L.; Wang, J.C.; Ren, L.; Liu, W.; Tu, Q.; Li, L.; Wang, J. Cancer stem cell labeling using poly (l-lysine)-modified iron oxide nanoparticles. Biomaterials 2012, 33, 3719–3732. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Ulrih, N.P. Multifunctional superparamagnetic iron oxide nanoparticles: Promising tools in cancer theranostics. Cancer Lett. 2012, 336, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.J.I.; Sethuraman, M. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012, 47, 1351–1357. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S. Green Synthesis of Silver Nanoparticles Using Extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Malhotra, A.; Sharma, N.; Kumar, N.N.; Dolma, K.; Sharma, D.; Nandanwar, H.S.; Choudhury, A.R. Multi-analytical approach to understand bio-mineralization of gold using rice bran: A novel and economical route. RSC Adv. 2014, 4, 39484–39490. [Google Scholar]
- Akhtar, M.S.; Panwar, J.; Yun, Y.S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Lahtinen, M.; Sillanpaa, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Ghodake, G.; Deshpande, N.; Lee, Y.; Jin, E. Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf. B Biointerfaces 2010, 75, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Konwarha, R.; Gogoia, B.; Philip, R.; Laskarb, M.A.; Karaka, N. Biomimetic preparation of polymer- supported free radical scavenging, cytocompatible and antimicrobial ‘green’ silver nanoparticles using aqueous extract of Citrus sinensis peel. Colloids Surf. B Biointerfaces 2011, 84, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peeled extract mediated novel route for the synthesis of palladium nanoparticles. Mater. Lett. 2010, 64, 1951–1953. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Multhumary, J.; Srinivvasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J.; Chapman, P.; Le, X.; Fawcett, D. Green biosynthesis of gold nanometre scale plates using the leaf extracts from an indigenous Australian plant Eucalyptus macrocarpa. Gold Bull. 2013, 46, 165–173. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green Chemistry Based Benign Routes for Nanoparticle Synthesis. J. Nanopart. 2014, 2014, 302429. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Shah, M.; Thomsen, G.; Fawcett, D. Microscopy study of Xanthorrhoea glauca leaves and preliminary investigation into biogenic synthesis of silver nanoparticles. Int. J. Sci. 2016, 2, 58–62. [Google Scholar]
- Chiu, C.Y.; Ruan, L.; Huang, Y. Biomolecular specificity controlled nanomaterial synthesis. Chem. Soc. Rev. 2013, 42, 2512–2527. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J.; Shah, M.; Sharma, S.B.; Fawcett, D. Biogenic synthesis of gold and silver nanoparticles using the leaf extract from Eucalyptus macrocarpa. Int. J. Sci. 2015, 4, 27–33. [Google Scholar]
- Dwivedi, A.D.; Gopal, K. Plant-mediated biosynthesis of silver and gold nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Elsaesser, A.; Vyvyan Howard, C. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gautama, A.; van Veggel, F.C.J.M. Synthesis of nanoparticles, their biocompatibility, and toxicity behaviour for biomedical applications. J. Mater. Chem. B 2013, 1, 5186–5200. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Tallansky, M.E.; Kalinina, N.O. Green Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 6, 35–44. [Google Scholar]
- Seabra, A.B.; Dura, N. Nanotoxicology of metal oxide nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plants. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Asmathunisha, N.; Kathiresan, K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf. B Biointerfaces 2013, 103, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical applications. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Vasir, J.K.; Labhasetwar, V. Quantification of the force of nanoparticle–cell membrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials 2008, 29, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Rok Lee, Y. Synthesis of silver nanoparticles using Satsuma mandarin (Citrus unshiu) peel extract: A novel approach towards waste utilization. Mater. Lett. 2013, 109, 31–33. [Google Scholar] [CrossRef]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Sulb, S.L. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous Sorghum bran extracts. Langmuir 2010, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B.; Damle, C.; Ahmad, A.; Sastry, M. Bio-synthesis of gold and silver using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol. 2005, 5, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.R.; Stirk, W.A.; van Staden, J. Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S. Afr. J. Bot. 2013, 86, 1–4. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; Munoz, J.A.; Gonzaliez, F.; Ballester, A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013, 7, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Raeshkumar, S.; Kannan, C.; Amnadurai, G. Synthesis and characterisation of antimicrobial silver nanoparticles using marine brown seaweed Padina tetrastromatica. Drug Invent. Today 2012, 4, 511–513. [Google Scholar]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Catalytic activity of unsupported gold nanoparticles. Catal. Sci. Technol. 2013, 3, 58–69. [Google Scholar] [CrossRef]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Thajamanbi, M.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of gold nanoparticles using a freshwater green alga, Prasiola crispa. Mater. Lett. 2014, 116, 94–97. [Google Scholar] [CrossRef]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Thajamanbi, M.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C. Ag and antioxidant activity of biomatrix loaded nanoparticles. Bioprocess Biosyst. Eng. 2014, 37, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 1–11. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Phytosynthesis of gold nanoparticles using leaf extract of Coleus amboinicus Lour. Mater. Charact. 2010, 61, 1232–1238. [Google Scholar] [CrossRef]
- Ahmada, T.; Irfana, M.; Bhattacharjeea, S. Parametric Study on Gold Nanoparticle Synthesis Using Aqueous Elaise Guineensis (Oil palm) Leaf Extract: Effect of Precursor Concentration. Procedia Eng. 2016, 148, 1396–1401. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Palakshi Reddy, B.; Sarada, N.C.; Chidambaram, K.; Khadeer Pasha, S. Watermelon rind-mediated green synthesis of noble palladium nanoparticles: Catalytic application. Appl. Nanosci. 2015, 5, 223–228. [Google Scholar] [CrossRef]
- Lunge, S.; Singh, S.; Sinha, A. Magnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removal. J. Magnet. Mag. Mater. 2014, 356, 21–31. [Google Scholar] [CrossRef]
- Mahdavi, M.; Namvar, F.; Ahmad, M.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Khanehzaei, H.; Ahmad, M.B.; Shameli, K.; Ajdari, Z. Synthesis and Characterization of Cu@Cu2O Core Shell Nanoparticles Prepared in Seaweed Kappaphycus alvarezii Media. Int. J. Electrochem. Sci. 2014, 9, 8189–8198. [Google Scholar]
- Ganapathi Rao, K.; Ashok, C.H.; Venkateswara Rao, K.; Shilpa Chakra, C.H.; Akshaykranth, A. Eco-Friendly Synthesis of MgO Nanoparticles from Orange Fruit Waste. Int. J. Adv. Res. Phys. Sci. 2015, 2, 1–6. [Google Scholar]
- Yan, D.; Zhang, H.; Chen, L.; Zhu, G.; Wang, Z.; Xu, H.; Yu, A. Super-capacitive properties of Mn3O4 nanoparticles biosynthesized from banana peel extract. RSC Adv. 2014, 4, 23649–23652. [Google Scholar] [CrossRef]
- Mude, N.; Ingle, A.; Gade, A.; Rai, M. Synthesis of silver nanoparticles using Callus extract of Carica papaya—A First Report. J. Plant Biochem. Biotechnol. 2008, 18, 83–86. [Google Scholar] [CrossRef]
- Jain, D.; Daima, H.K.; Kachhwaha, S.; Kothari, S. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biostruct. 2009, 4, 557–563. [Google Scholar]
- Sriram, T.; Pandidurai, V. Synthesis of silver nanopar- ticles from leaf extract of Psidium guajava and its antibacterial activity against pathogens. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 146–152. [Google Scholar]
- Abubakar, A.S.; Salisu, I.B.; Chahal, S.; Pudake, R.N. Biosynthesis and characterization of silver nano particles using black carrot root extract. Int. J. Curr. Res. Rev. 2014, 6, 5–8. [Google Scholar]
- Ahamed, M.; Khan, M.; Siddiqui, M.K.J.; Al-Salhiu, M.A.; Alrokayan, S.A. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2011, 43, 1266–1271. [Google Scholar] [CrossRef]
- Ndikau, M.; Noah, N.M.; Andala, D.M.; Masika, E. Green Synthesis and Characterization of Silver Nanoparticles Using Citrullus lanatus Fruit Rind Extract. Int. J. Anal. Chem. 2017, 2017, 8108504. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Baek, K.H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar]
- Pattanayak, M.; Muralikrishnan, T.; Nayak, P.L. Green Synthesis of Gold Nanoparticles Using Daucus Carota (carrot) Aqueous Extract. World J. Nano Sci. Technol. 2014, 3, 52–58. [Google Scholar]
- Ghule, K.; Ghule, A.V.; Liu, J.Y.; Ling, Y.C. Microscale size triangular gold prisms synthesized using Bengal gram beans (Cicer arietinum L.) extract and HAuCl4.3H2O: A green biogenic approach. J. Nanosci. Nanotechnol. 2006, 6, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Blazquez, M.L.; Munoz, J.A.; Gonzaliez, F.; Garcia-Balboa, C.; Ballester, A. Biosynthesis of gold nanowires using sugar beet pulp. Process Biochem. 2011, 46, 1076–1082. [Google Scholar] [CrossRef]
- Ramkumara, V.S.; Pugazhendhib, A.; Gopalakrishnanb, K.; Sivagurunathanc, P.; Sarataled, G.D.; Dunge, T.N.B.; Kannapiranf, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mmola, M.; Roes-Hill, M.L.; Durrell, K.; Bolton, J.J.; Sibuyi, N.; Meyer, M.E.; Beukes, D.R.; Antunes, E. Enhanced Antimicrobial and Anticancer Activity of Silver and Gold Nanoparticles Synthesised Using Sargassum incisifolium Aqueous Extracts. Molecules 2016, 21, 1633. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Malarkodi, C.; Gananajobitha, G.; Paulkumar, K.; Vanaja, M.; Kannan, C.; Annadurai, G. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J. Nanostruct. Chem. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Arockiya Aarthi Rajathi, F.; Parthiban, C.; Ganesh Kumar, V.; Anantharaman, P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.L.; Dodson, J.R.; Budarin, V.L.; Clark, J.H.; Hunt, A.J. Direct synthesis of Pd nanoparticles on alginic acid and seaweed supports. Green Chem. 2015, 17, 2200–2207. [Google Scholar] [CrossRef]
- Francavilla, M.; Pineda, A.; Romero, A.A.; Colmenares, J.C.; Vargas, C.; Monteleone, M.; Lugue, R. Efficient and simple reactive milling preparation of photocatalytically active porous ZnO nanostructures using biomass derived polysaccharides. Green Chem. 2014, 16, 2876–2885. [Google Scholar] [CrossRef]
- Youns, M.; Hoheisel, J.D.; Efferth, T. Therapeutic and diagnostic applications of nanoparticles. Curr. Drug Targets 2011, 12, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Sanchez-Gaytan, B.L.; Qian, Z.X.; Park, S.J. Noble metal nanoparticles in DNA detection and delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Glestas, L.; Almelda, C.; Assuncao, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for bio-sensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Fortina, P.; Kricka, L.J.; Graves, D.J.; Park, J.; Hyslop, T.; Tam, F.; Halas, N.; Surrey, S.; Waldman, S.A. Applications of nanoparticles to diagnostics and therapeutics in colorectal cancer. Trends Biotechnol. 2007, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sache, L. Gold nanoparticles enhance DNA damage induced by anti-cancer drugs and radiation. Radiat. Res. 2009, 172, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.M.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar]
- Jacob, S.; Finub, J.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B Biointerfaces 2011, 91, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.; Rajkamal, P.; Cholan, S.; Kannadasan, N.; Sathishkumar, K.; Viruthagiri, G.; Sundaramanickam, A. Biosynthesis of silver nanoparticles from marine seaweed Sargassum wightii and their antimicrobial activity against some human pathogens. Appl. Nanosci. 2014, 4, 881–888. [Google Scholar] [CrossRef]
- Haider, A.; Kang, I.K. Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, 165257. [Google Scholar] [CrossRef]
- Petla, R.K.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K.; Satyanarayana, N. Soybean (Glycine Max) leaf extract based green synthesis of palladium nanoparticles. J. Bioma. Nanobiotechnol. 2012, 3, 14–19. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds-a critical review. Int. J. Antimicrob. Ag 2012, 5, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, G.; Jang, N.R.; Yun, J.H.; Song, J.Y.; Kim, B.S. Biological synthesis of copper nanoparticles using plant extract. Nanotechnology 2011, 1, 371–374. [Google Scholar]
- Nagarajan, S.; Kuppusamy, K.A. Extracellular synthesis of zinc oxide nanoparticle using seaweeds of Gulf of Mannar, India. J. Nanobiotechnol. 2013, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Baharara, J.; Mahdavi, M.; Amini, E.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int. J. Nanomed. 2014, 9, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticle | Size & Morphology | Food Source | Year | Reference |
|---|---|---|---|---|
| Ag | 5 to 35 nm, Spherical | Ananas comosus (Pineapple) | 2012 | [79] |
| Ag & Au | Ag: 16 nm, Spherical, Au: 11 nm, triangular | Tanacetum vulgare (tansy fruit) | 2010 | [84] |
| Ag | 3 to 12 nm, Spherical | Citrus sinensis (orange) peel | 2011 | [86] |
| Ag | 35 ± 2 nm @ 25 °C, Spherical 10 ± 1 nm @ 60 °C, Spherical | Citrus sinensis (orange) peel | 2011 | [89] |
| Ag | 5 to 20 nm, Spherical | Citrus unshiu (mandarin) peel | 2013 | [109] |
| Ag | 10 nm, Quasi-spherical | Sorghum spp. (bran) (aw) | 2010 | [110] |
| Ag & Au | Ag: 10 nm to 20 nm, Spherical Au: 15 nm to 25 nm, Spherical | Emblica officinalis (Indian Gooseberry) | 2005 | [111] |
| Ag | Large nanoclusters | Musa paradisiac (banana) peel | 2010 | [88] |
| Ag | 60 to 80 nm, Spherical | Carica papaya (pawpaw) | 2008 | [130] |
| Ag | 15 nm, Cubic | Carica papaya (pawpaw) | 2009 | [131] |
| Ag | 0.1 µm to 0.5 µm, Granular | Psidium guajava (guava) | 2014 | [132] |
| Ag | 4.32 nm to 17.65 nm, Spherical | Daucus carrota L. (Black Carrot) | 2014 | [133] |
| Ag | 4 nm to 22 nm, Spherical | Allium sativum (garlic clove) | 2011 | [134] |
| Ag | 17.96 ± 0.16 nm, Spherical | Citrullus lanatus rind | 2017 | [135] |
| Au | 20 to 140 nm, Spherical | Citrullus lanatus rind | 2015 | [136] |
| Au | 20 to 25 nm, Quasi-spherical | Grape skin, stalk and seed waste | 2014 | [80] |
| Au | 50 to 100 nm, Spherical | Rice bran (aw) | 2014 | [81] |
| Au | 200 to 500 nm, Triangular, hexagonal | Pyrus sp. (pear) | 2010 | [85] |
| Au | 6.03 ± 2.77 to 18.01 ± 3.67 nm, Spherical | Mangifera indica (mango) peel | 2014 | [49] |
| Au | 432.3 nm, Shape not specified | Daucus carota, subsp. Sativus (Carrot) | 2014 | [137] |
| Au | Micro-scale, Triangular | Cicer arietinum L. (Bean extract) | 2006 | [138] |
| Au | pH 9: 10 nm, Spherical, pH 10: 25 nm, Spherical, rods, pH 11: 15 nm diameter nanowires of varying length | Beta vulgaris (sugar beet pulp) | 2011 | [139] |
| Pd | 50 nm, Crystalline, irregular shape | Musa paradisiac (banana) peel | 2010 | [88] |
| Pd & Ag | 20 nm to 60 nm, Spherical | Various commercially available tea/coffee extracts | 2008 | [87] |
| Pd & Pt | 16 to 20 nm, Spherical | Lignin (aw) | 2012 | [71] |
| Pd | 96 nm, Spherical | Citrullus lanatus (watermelon) rind | 2015 | [124] |
| Fe3O4 | 5 to 25 nm, Cubes & Pyramids | Tea Waste | 2014 | [125] |
| MgO | 29 nm, Spherical | Citrus sinensis (orange) peel | 2015 | [128] |
| Mn3O4 | 20 nm to 50 nm, Spherical | Musa paradisiac (banana) peel | 2014 | [49] |
| Nanoparticle | Size & Shape | Marine Alga | Year | Reference |
|---|---|---|---|---|
| Ag | 3 to 44 nm, Spherical and Cubic | Codium capitatum | 2013 | [112] |
| Ag | 30 nm, Spherical | Spyrogira insignis | 2013 | [113] |
| Ag | 4 to 24 nm, Spherical | Enteromorpha compressa | 2017 | [140] |
| Ag Au | 20 nm, Spherical 5 to 260 nm, Triangles, Spheres and Hexagons | Sargassum incisifolium | 2016 | [141] |
| Au | 6 to 10 nm, Spherical & Triangular | Turbinaria conoides | 2013 | [142] |
| Au | 18.7 to 93.7 nm, Spherical | Stoechospermum marginatum | 2012 | [143] |
| Pd | 4 to 6 nm, Spherical | Laminaria digitata | 2015 | [144] |
| Cu2O, CuO | 5 to 45 nm, Spherical | Bifurcaria bifurcata | 2014 | [72] |
| Cu/Cu2O | 53 nm, Spherical | Kappaphycus alvarezii | 2014 | [127] |
| Fe3O4 | 18 ± 4 nm, Cubic | Sargassum muticum | 2013 | [126] |
| ZnO | 18 to 50 nm, Hexagonal | Gracilaria gracilis | 2014 | [145] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Production of High-Value Nanoparticles via Biogenic Processes Using Aquacultural and Horticultural Food Waste. Materials 2017, 10, 852. https://doi.org/10.3390/ma10080852
Ghosh PR, Fawcett D, Sharma SB, Poinern GEJ. Production of High-Value Nanoparticles via Biogenic Processes Using Aquacultural and Horticultural Food Waste. Materials. 2017; 10(8):852. https://doi.org/10.3390/ma10080852
Chicago/Turabian StyleGhosh, Purabi R., Derek Fawcett, Shashi B. Sharma, and Gerrard E. J. Poinern. 2017. "Production of High-Value Nanoparticles via Biogenic Processes Using Aquacultural and Horticultural Food Waste" Materials 10, no. 8: 852. https://doi.org/10.3390/ma10080852





