Er-Doped LiNi0.5Mn1.5O4 Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
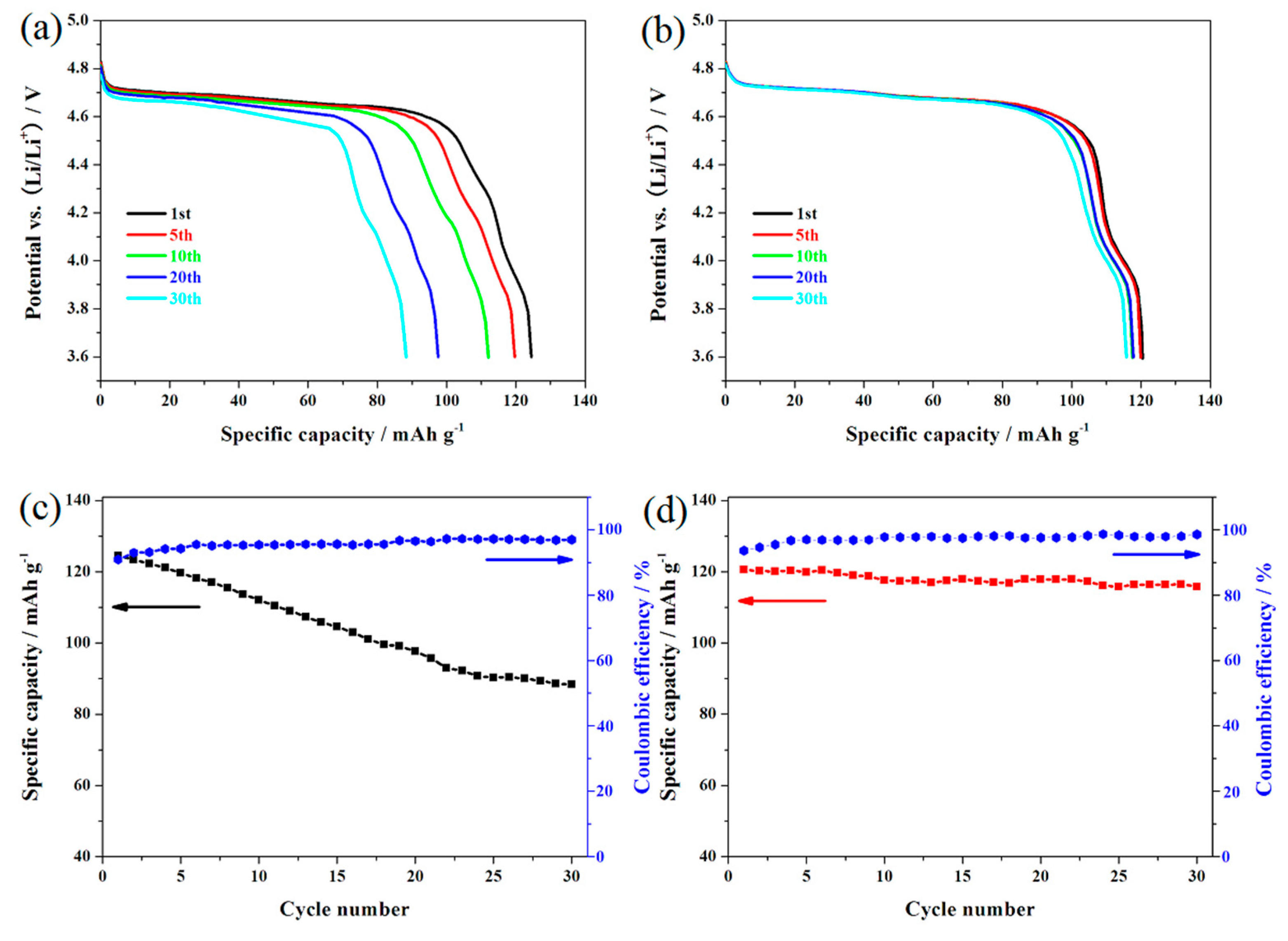
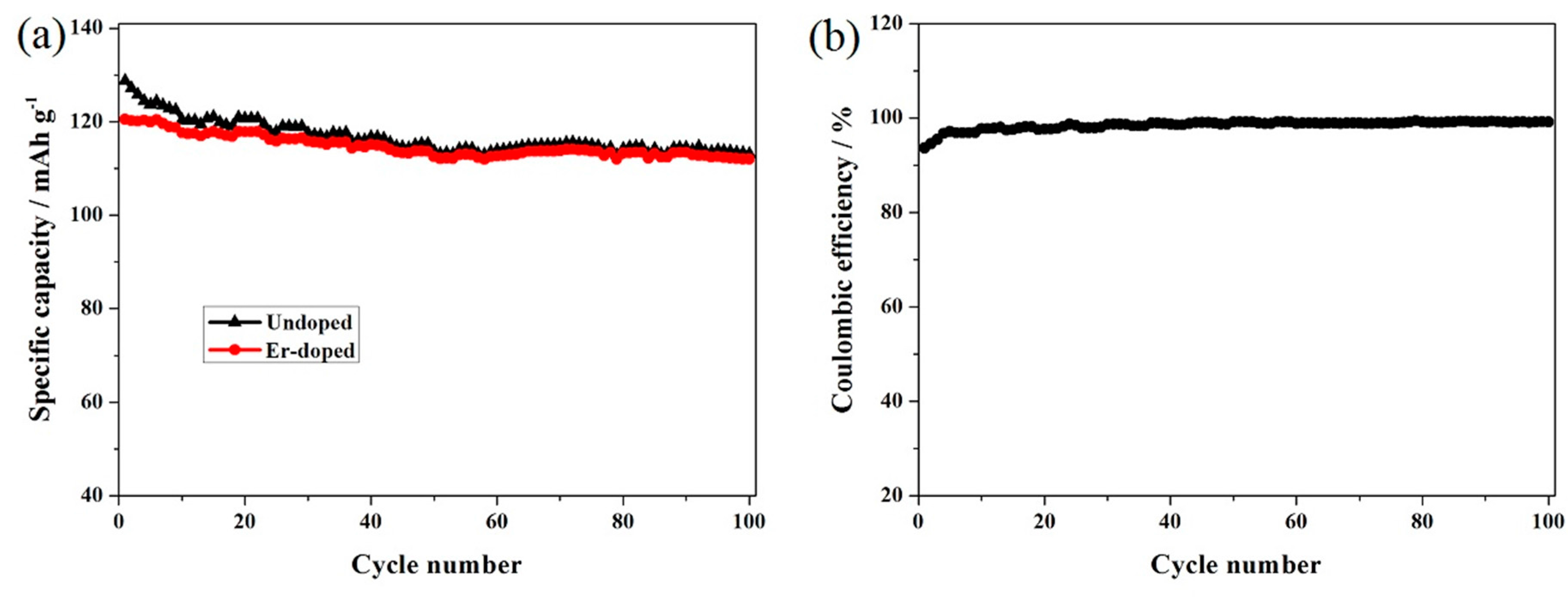
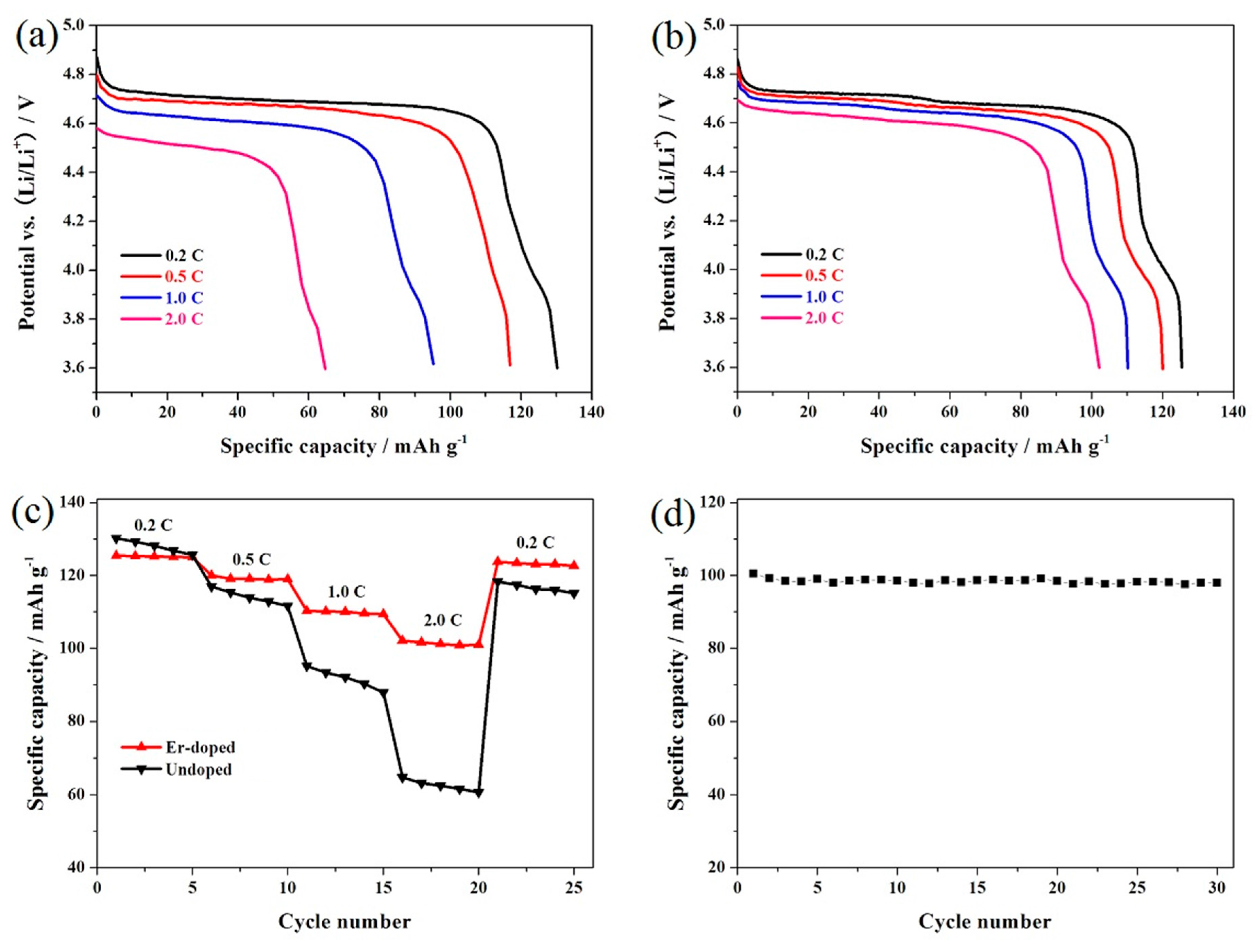
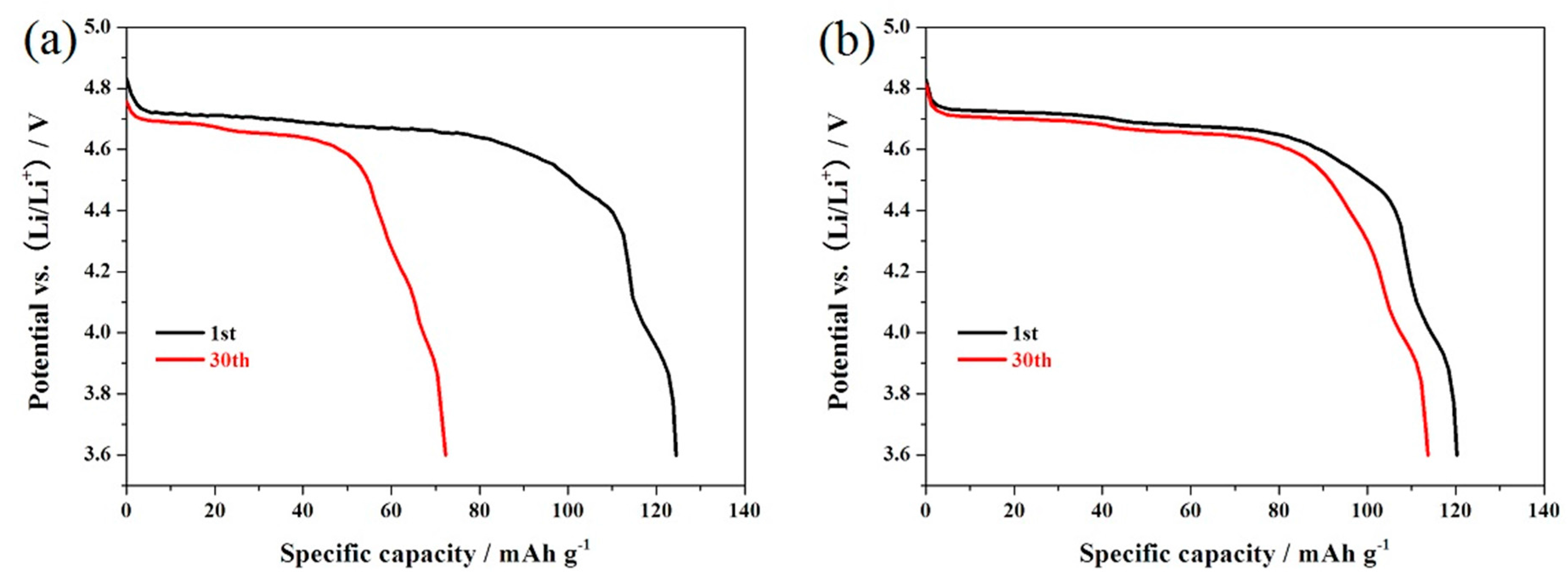
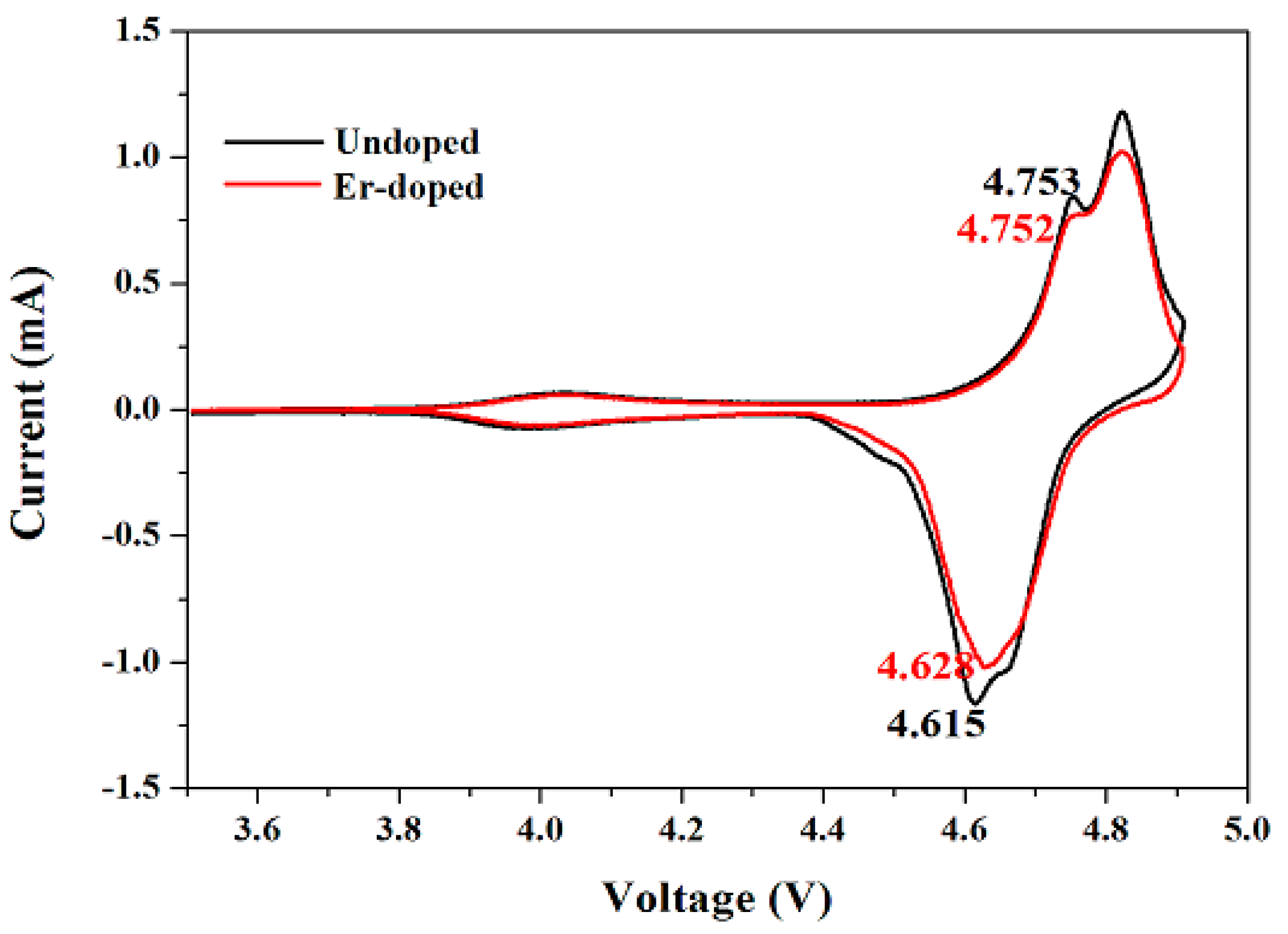
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Cheng, J.; Li, X.; Wang, Z.; Guo, H. Hydrothermal synthesis of LiNi0.5Mn1.5O4 sphere and its performance as high-voltage cathode material for lithium ion batteries. Ceram. Int. 2016, 42, 3715–3719. [Google Scholar] [CrossRef]
- Sulochana, A.; Thirunakaran, R.; Sivashanmugam, A.; Gopukumar, S.; Yamkiet, J. Sol-gel synthesis of 5 V LiCuxMn2−xO4 as a cathode material for lithium rechargeable batteries. J. Electrochem. Soc. 2008, 155, A206–A210. [Google Scholar] [CrossRef]
- Wei, Y.J.; Yan, L.Y.; Wang, C.Z.; Xu, X.G.; Wu, F.; Chen, G. Effect of Ni doping on [MnO6] octahedron in LiMn2O4. J. Phys. Chem. B 2004, 108, 18547–18551. [Google Scholar] [CrossRef]
- Shigemura, H.; Sakaebe, H.; Kageyama, H.; Kobayashi, H.; West, A.R.; Kanno, R.; Morimoto, S.; Nasu, S.; Tabuchi, M. Structure and electrochemical properties of LiFexMn2−xO4 (0 ≤ x ≤ 0.5) spinel as 5 V electrode material for lithium batteries. J. Electrochem. Soc. 2001, 148, A730–A736. [Google Scholar] [CrossRef]
- Mandal, S.; Rojas, R.M.; Amarilla, J.M.; Calle, P.; Kosova, N.V.; Anufrienko, V.F.; Rojo, J.M. High temperature co-doped LiMn2O4-based spinels. structural, electrical, and electrochemical characterization. Chem. Mater. 2002, 14, 1598–1605. [Google Scholar] [CrossRef]
- Sigala, C.; Guyomard, D.; Verbaere, A.; Piffard, Y.; Tournoux, M. Positive electrode materials with high operating voltage for lithium batteries: LiCryMn2−yO4 (0 ≤ y ≤1). Solid State Ionics 1995, 81, 167–170. [Google Scholar] [CrossRef]
- Kanamura, K.; Hoshikawa, W.; Umegaki, T. Electrochemical characteristics of LiNi0.5Mn1.5O4 cathodes with Ti or Al current collectors. J. Electrochem. Soc. 2002, 149, A339–A345. [Google Scholar] [CrossRef]
- Yi, T.F.; Zhu, Y.R.; Zhu, R.S. Density functional theory study of lithium intercalation for 5 V LiNi0.5Mn1.5O4 cathode materials. Solid State Ionics 2008, 179, 2132–2136. [Google Scholar] [CrossRef]
- Bae, S.Y.; Shin, W.K.; Kim, D.W. Protective organic additives for high voltage LiNi0.5Mn1.5O4 cathode materials. Electrochim. Acta 2014, 125, 497–502. [Google Scholar] [CrossRef]
- Idemoto, Y.; Narai, H.; Koura, N. Crystal structure and cathode performance dependence on oxygen content of LiMn1.5Ni0.5O4 as a cathode material for secondary lithium batteries. J. Power Sources 2003, 119, 125–129. [Google Scholar] [CrossRef]
- Kunduraci, M.; Amatucci, G.G. Synthesis and characterization of nanostructured 4.7 V LixMn1.5Ni0.5O4 spinels for high-power lithium-ion batteries. J. Electrochem. Soc. 2006, 153, A1345–A1352. [Google Scholar] [CrossRef]
- Kunduraci, M.; Al-Sharab, J.F.; Amatucci, G.G. High-power nanostructured LiMn2−xNixO4 high-voltage lithium-ion battery electrode materials: Electrochemical impact of electronic conductivity and morphology. Chem. Mater. 2006, 18, 3585–3592. [Google Scholar] [CrossRef]
- Ma, X.H.; Kang, B.; Ceder, G. High rate micron-sized ordered LiNi0.5Mn1.5O4. J. Electrochem. Soc. 2010, 157, A925–A931. [Google Scholar] [CrossRef]
- Yoon, T.; Park, S.; Mun, J.; Ji, H.R.; Choi, W. Failure mechanisms of LiNi0.5Mn1.5O4 electrode at elevated temperature. J. Power Sources 2012, 215, 312–316. [Google Scholar] [CrossRef]
- Jin, Y.C.; Lin, C.Y.; Duh, J.G. Improving rate capability of high potential LiNi0.5Mn1.5O4−x cathode materials via increasing oxygen non-stoichiometries. Electrochim. Acta 2012, 69, 45–50. [Google Scholar] [CrossRef]
- Xu, W.; Chen, X.; Ding, F.; Xiao, J.; Wang, D.Y.; Pan, A.Q.; Zheng, J.M.; Li, X.H.S.; Padmaperuma, A.B.; Zhang, J.G. Reinvestigation on the state-of-the-art nonaqueous carbonate electrolytes for 5 V Li-ion battery applications. J. Power Sources 2012, 213, 304–316. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, X.Q.; Cheng, C.; Li, Q.; Zhang, Z.; Wu, Y.; Chen, B.; Xiong, W. Synthesis and electrochemical characterizations of spinel LiMn1.94MO4 (M = Mn0.06, Mg0.06, Si0.06, (Mg0.03Si0.03)) compounds as cathode materials for lithium-ion batteries. J. Power Sources 2015, 282, 118–128. [Google Scholar] [CrossRef]
- Aklalouch, M.; Amarilla, J.M.; Rojas, R.M.; Saadoune, I.; Rojo, J.M. Chromium doping as a new approach to improve the cycling performance at high temperature of 5 V LiNi0.5Mn1.5O4-based positive electrode. J. Power Sources 2008, 185, 501–511. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhang, K.L. The synthesis and cycling behavior of LiErxMn2−xO4 for lithium-ion batteries. Mater. Lett. 2004, 58, 3049–3051. [Google Scholar] [CrossRef]
- Göktepe, H.; Sahan, H.; Ülgen, A.; Patat, S. Synthesis and electrochemical properties of carbon-mixed LiEr0.02Fe0.98PO4 cathode material for lithium-ion batteries. J. Mater. Sci. Technol. 2011, 27, 861–864. [Google Scholar] [CrossRef]
- Xie, L.L.; Xu, Y.D.; Zhang, J.J.; Zhang, C.; Cao, X.; Qu, L. Rheological phase synthesis of Er-doped LiV3O8 as electroactive material for a cathode of secondary lithium storage. Electron. Mater. Lett. 2013, 9, 549–553. [Google Scholar] [CrossRef]
- Dong, Y.; Young, B.T.; Zhang, Y.; Yoon, T.; Heskett, D.R.; Hu, Y.; Lucht, B.L. Effect of lithium borate additives on cathode film formation in LiNi0.5Mn1.5O4/Li cells. ACS Appl. Mater. Interfaces 2017, 9, 20467–20475. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.T.; Myung, S.T.; Cho, T.H.; Son, J.T. Lattice parameter as a measure of electrochemical properties of LiMn2O4. J. Power Sources 2001, 97, 454–457. [Google Scholar] [CrossRef]
- Feng, F.; Liang, C.; Fang, H.; Yang, B.; Ma, W.; Dai, Y. Chloride-promoted formation of octahedral LiNi0.5Mn1.5O4 crystal with greatly enhanced electrochemical performance. Ceram. Int. 2016, 42, 9038–9045. [Google Scholar] [CrossRef]
- Mo, M.; Hui, K.; Hong, X.; Guo, J.; Ye, C.; Li, A.; Hu, N.; Huang, Z.; Jiang, J.; Liang, J.; et al. Improved cycling and rate performance of Sm-doped LiNi0.5Mn1.5O4 cathode materials for 5 V lithium ion batteries. Appl. Surf. Sci. 2014, 290, 412–418. [Google Scholar] [CrossRef]
- Arunkumar, T.A.; Manthiram, A. Influence of chromium doping on the electrochemical performance of the 5 V spinel cathode LiMn1.5Ni0.5O4. Electrochim. Acta 2005, 50, 5568–5572. [Google Scholar] [CrossRef]
- Wu, H.M.; Tu, J.P.; Yuan, Y.F.; Li, Y.; Zhao, X.B.; Cao, G.S. Electrochemical and ex situ XRD studies of a Li Mn1.5Ni0.5O4 high-voltage cathode material. Electrochim. Acta 2005, 50, 4104–4108. [Google Scholar] [CrossRef]
- Wen, J.W.; Zhang, D.W.; Zhang, Y.; Sun, X.; Cheng, B.; Ding, C.X.; Yu, Y.; Chen, C.H. One-step synthesis and effect of heat-treatment on the structure and electrochemical properties of LiNi0.5Mn1.5O4 cathode material for lithium-ion batteries. Electrochim. Acta 2014, 133, 515–521. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Tu, W.; Wang, Z.; Xi, M.; Xing, L.; Li, W. Improving cyclic stability of lithium nickel manganese oxide cathode for high voltage lithium ion battery by modifying electrode/electrolyte interface with electrolyte additive. Electrochim. Acta 2014, 147, 636–642. [Google Scholar] [CrossRef]
- Yi, T.F.; Xie, Y.; Zhu, Y.R.; Zhu, R.S.; Ye, M.F. High rate micron-sized niobium-doped LiMn1.5Ni0.5O4 as ultra high power positive-electrode material for lithium-ion batteries. J. Power Sources 2012, 211, 59–65. [Google Scholar] [CrossRef]
- Ding, Y.L.; Xie, J.; Cao, G.S.; Zhu, T.J.; Yu, H.M.; Zhao, X.B. Single-crystalline LiMn2O4 nanotubes synthesized via template-engaged reaction as cathodes for high-power lithium ion batteries. Adv. Funct. Mater. 2011, 21, 348–355. [Google Scholar] [CrossRef]
- Xiong, L.L.; Xu, Y.L.; Tao, T.; Goodenough, J.B. Synthesis and electrochemical characterization of multi-cations doped spinel LiMn2O4 used for lithium ion batteries. J. Power Sources 2012, 199, 214–219. [Google Scholar] [CrossRef]
- Kunduraci, M.; Amatucci, G.G. Effect of oxygen non-stoichiometry and temperature on cation ordering in LiMn2−xNixO4 (0.50 ≥ x ≥ 0.36) spinels. J. Power Sources 2007, 165, 359–367. [Google Scholar] [CrossRef]
- Markovsky, B.; Talyossef, Y.; Salitra, G.; Aurbach, D.; Kim, H.J.; Choi, S. Cycling and storage performance at elevated temperatures of LiNi0.5Mn1.5O4 positive electrodes for advanced 5 V Li-ion batteries. Electrochem. Commun. 2004, 6, 821–826. [Google Scholar] [CrossRef]
- Zhong, Q.; Bonakdarpour, A.; Zhang, M.; Gao, Y.; Dahn, J.R. Synthesis and electrochemistry of LiNixMn2−xO4. J. Eletrochem. Soc. 1997, 144, 205–213. [Google Scholar] [CrossRef]
- Feng, C.Q.; Li, H.; Zhang, C.F.; Guo, Z.P. Synthesis and electrochemical properties of non-stoichiometric Li-Mn-spinel (Li1.02MxMn1.95O4−yFy) for lithium ion battery application. Electrochim. Acta 2012, 61, 87–93. [Google Scholar] [CrossRef]
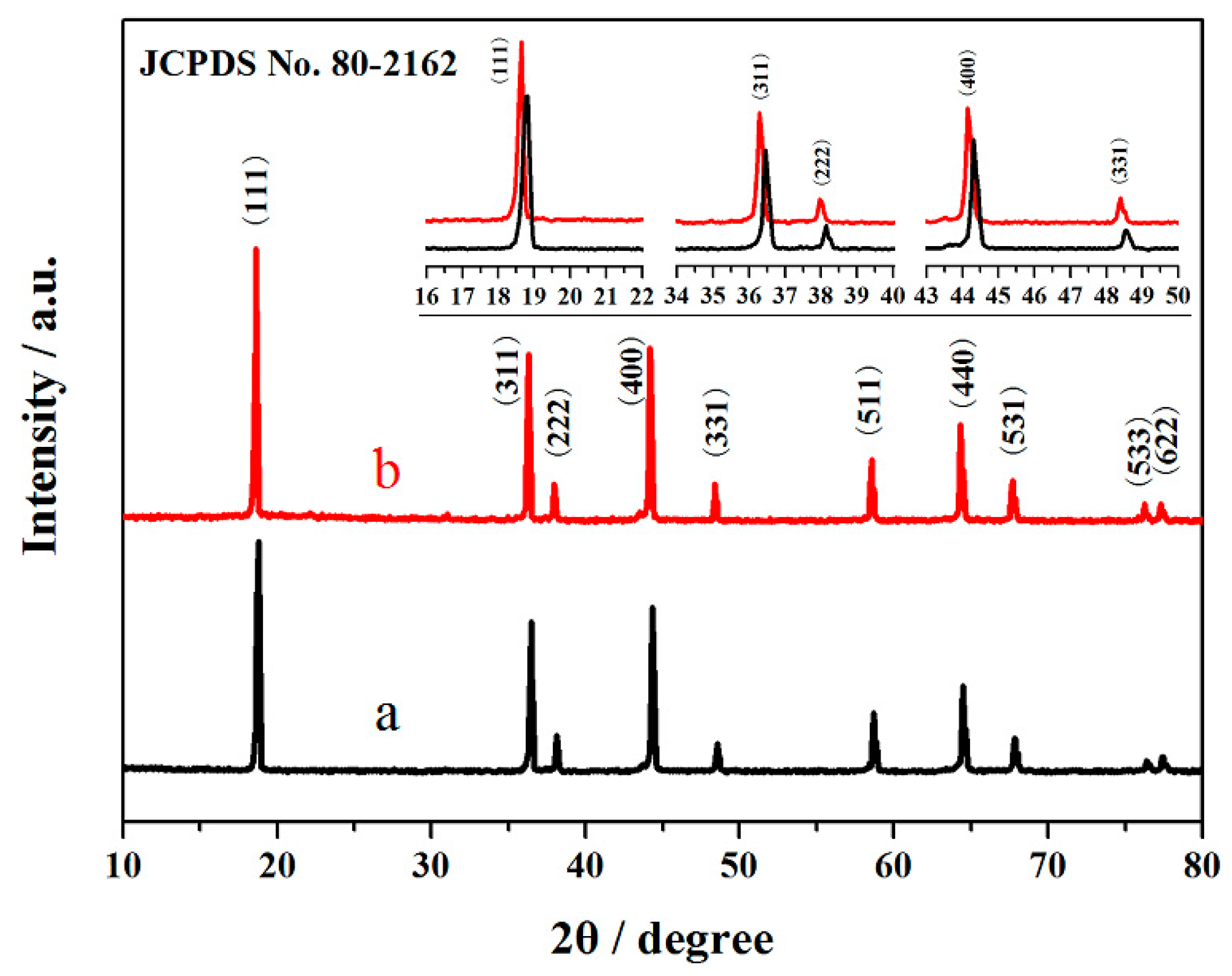
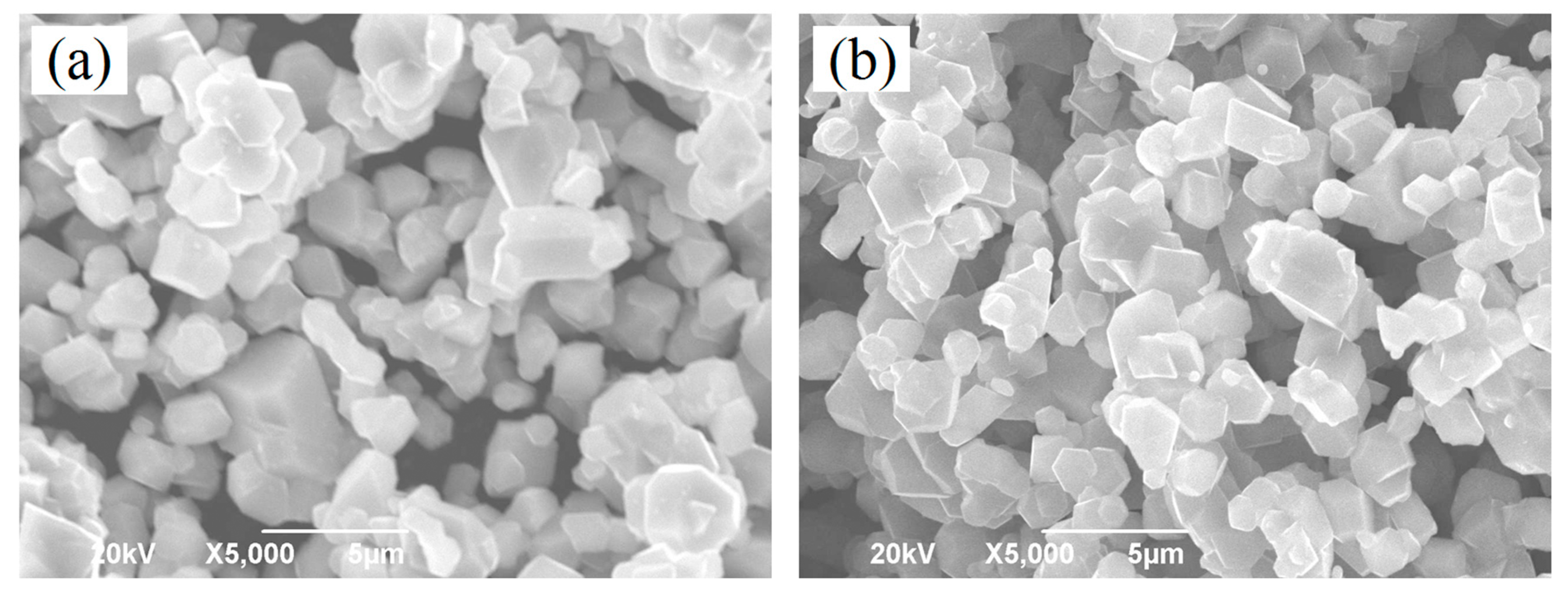
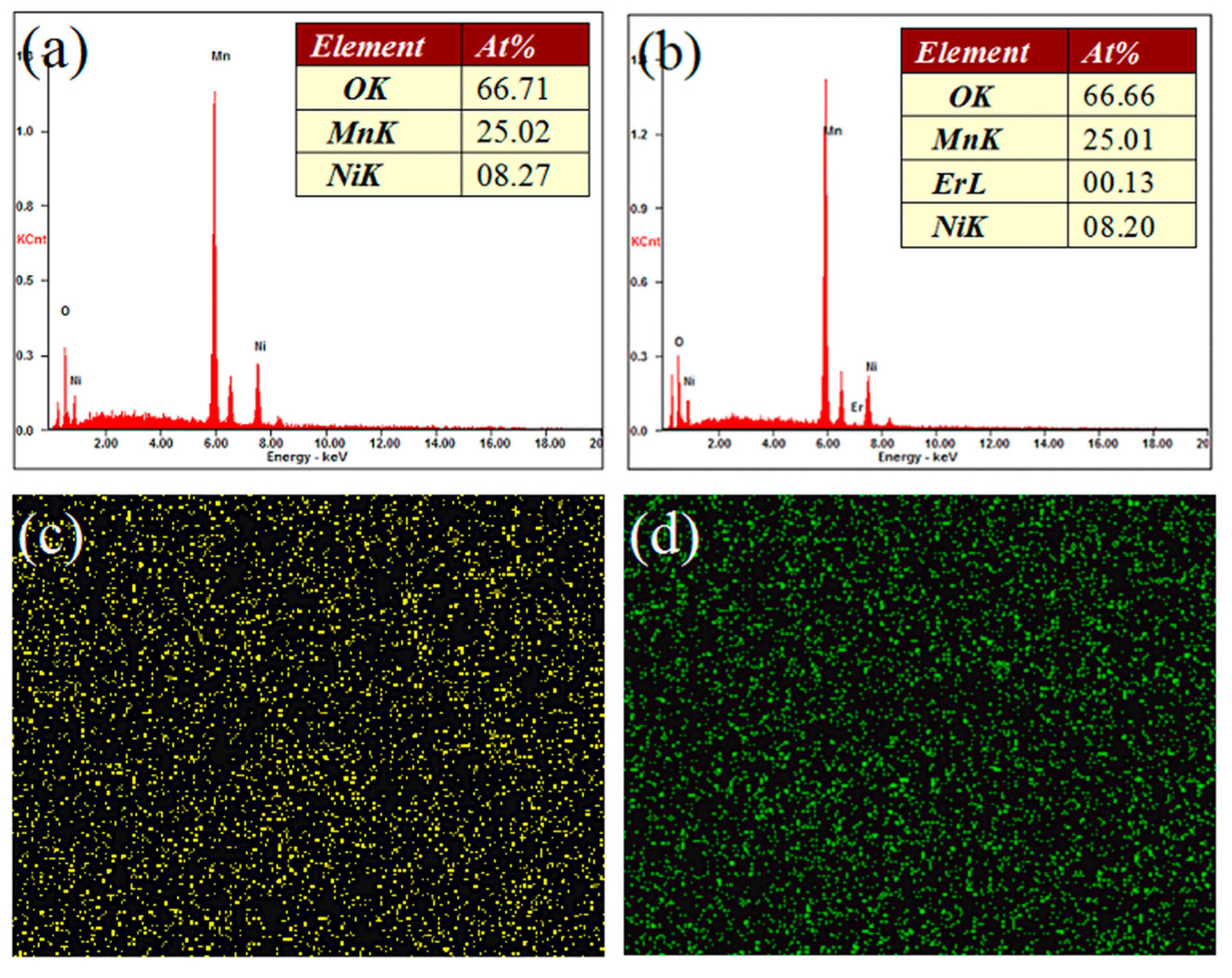
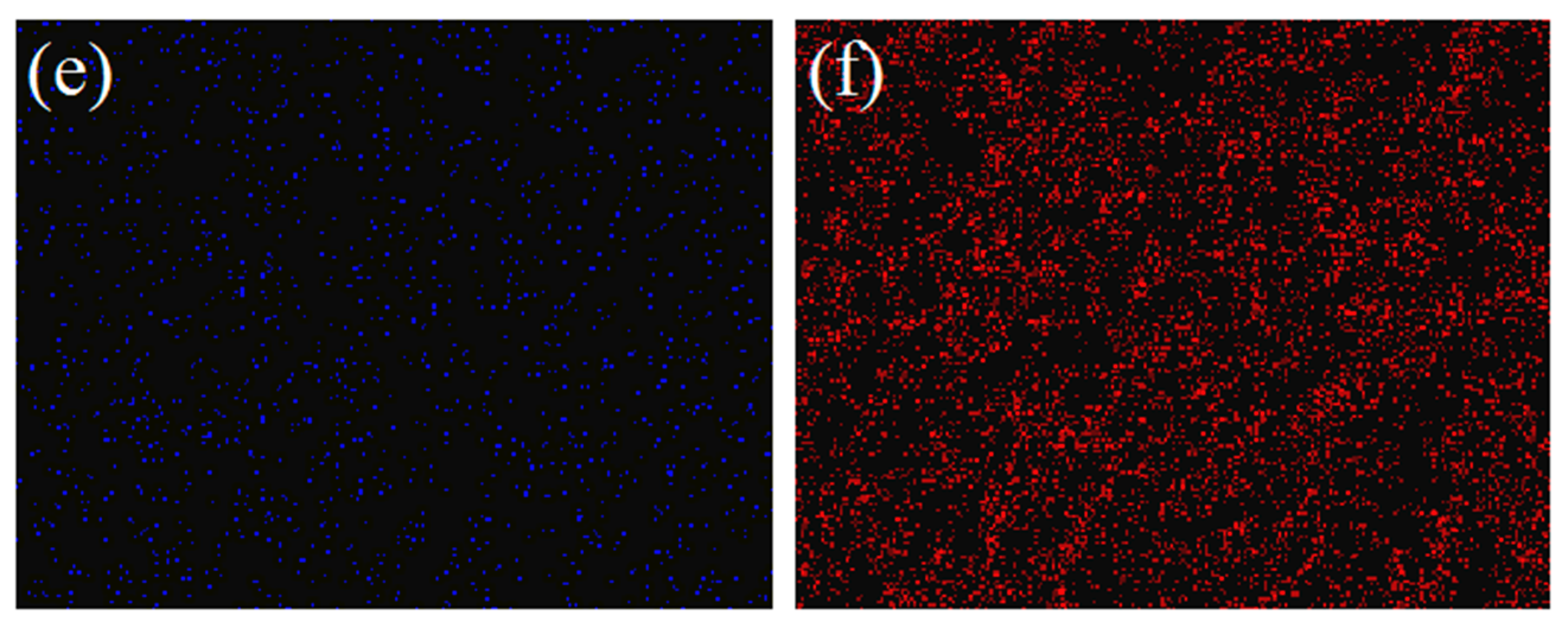


| Sample | Re (Ω) | Rct (Ω) |
|---|---|---|
| LiNi0.5Mn1.5O4 | 4.01 | 377.13 |
| Er-doped LiNi0.5Mn1.5O4 | 3.04 | 210.81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhao, H.; Tan, M.; Hu, Y.; Shu, X.; Zhang, M.; Chen, B.; Liu, X. Er-Doped LiNi0.5Mn1.5O4 Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries. Materials 2017, 10, 859. https://doi.org/10.3390/ma10080859
Liu S, Zhao H, Tan M, Hu Y, Shu X, Zhang M, Chen B, Liu X. Er-Doped LiNi0.5Mn1.5O4 Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries. Materials. 2017; 10(8):859. https://doi.org/10.3390/ma10080859
Chicago/Turabian StyleLiu, Shanshan, Hongyuan Zhao, Ming Tan, Youzuo Hu, Xiaohui Shu, Meiling Zhang, Bing Chen, and Xingquan Liu. 2017. "Er-Doped LiNi0.5Mn1.5O4 Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries" Materials 10, no. 8: 859. https://doi.org/10.3390/ma10080859




