A Review on Disorder-Driven Metal–Insulator Transition in Crystalline Vacancy-Rich GeSbTe Phase-Change Materials
Abstract
:1. Introduction
2. Discussions
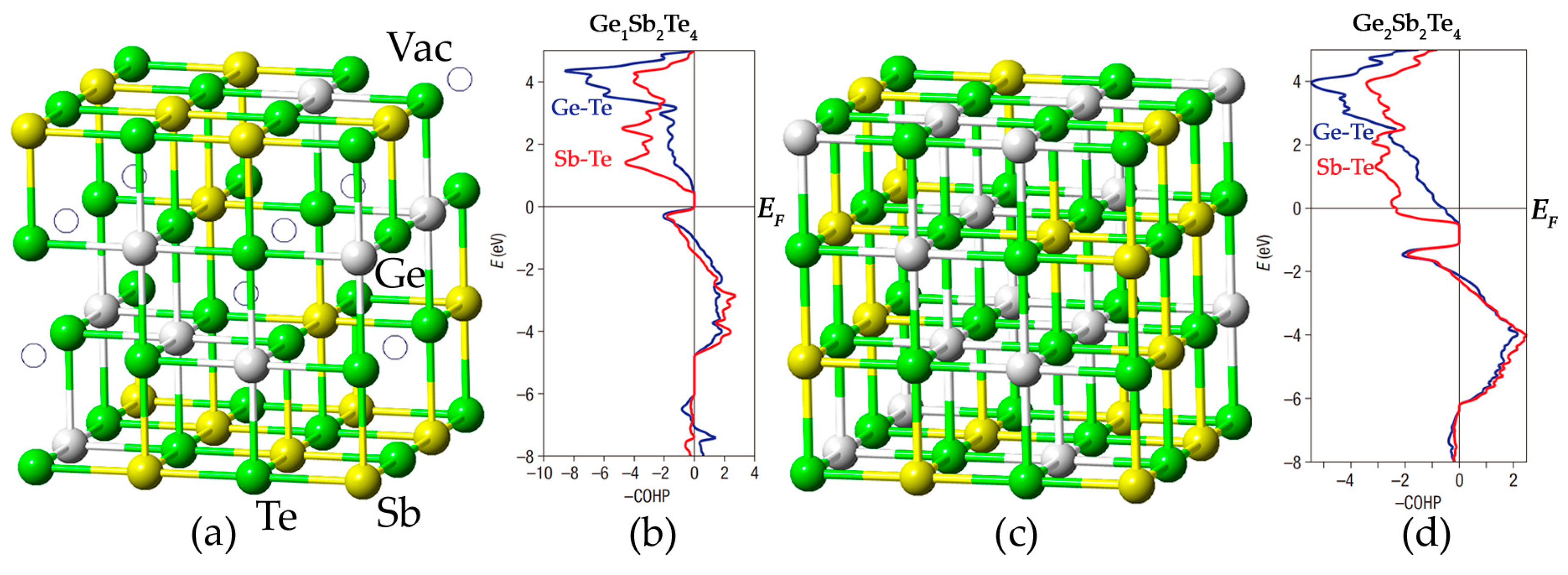
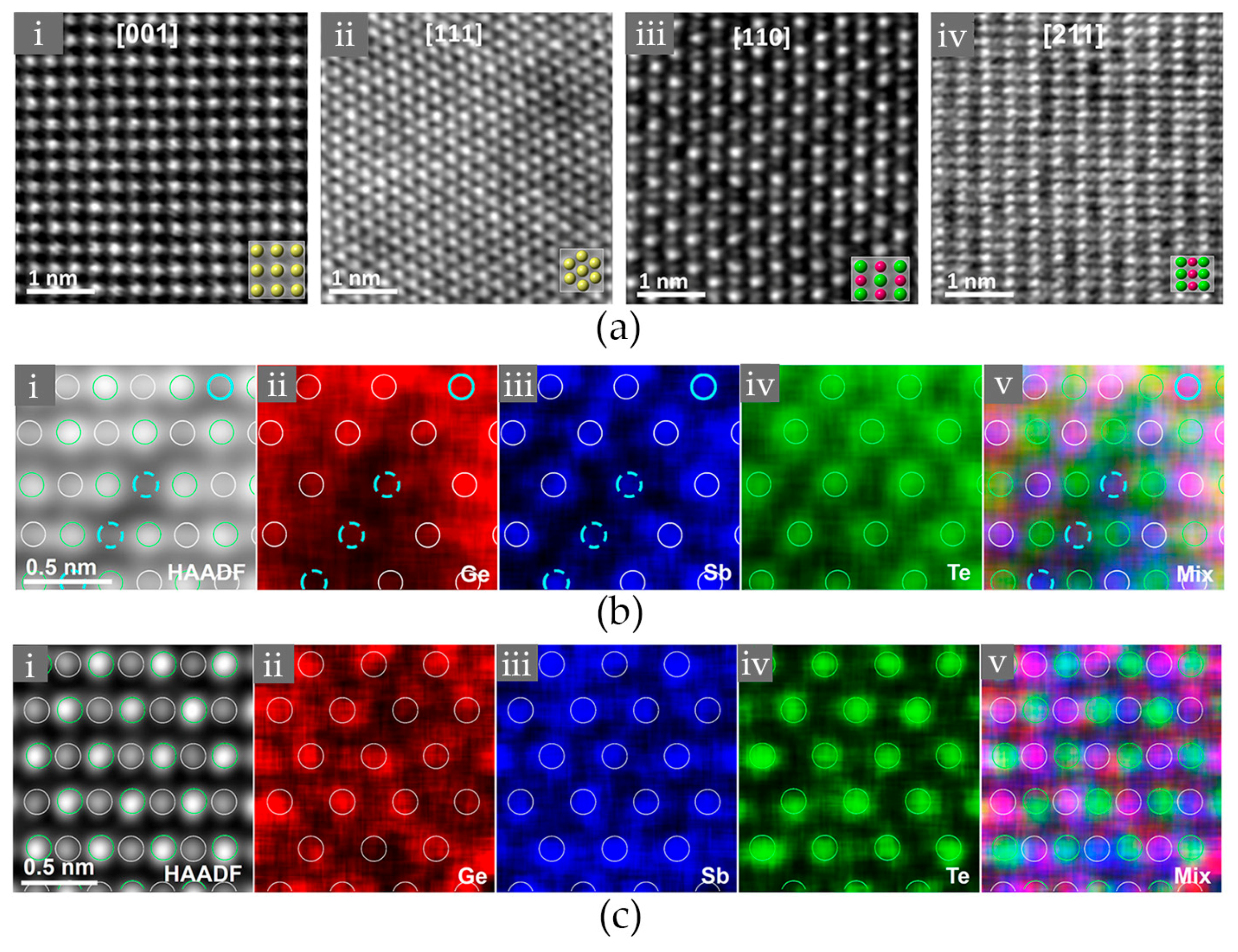
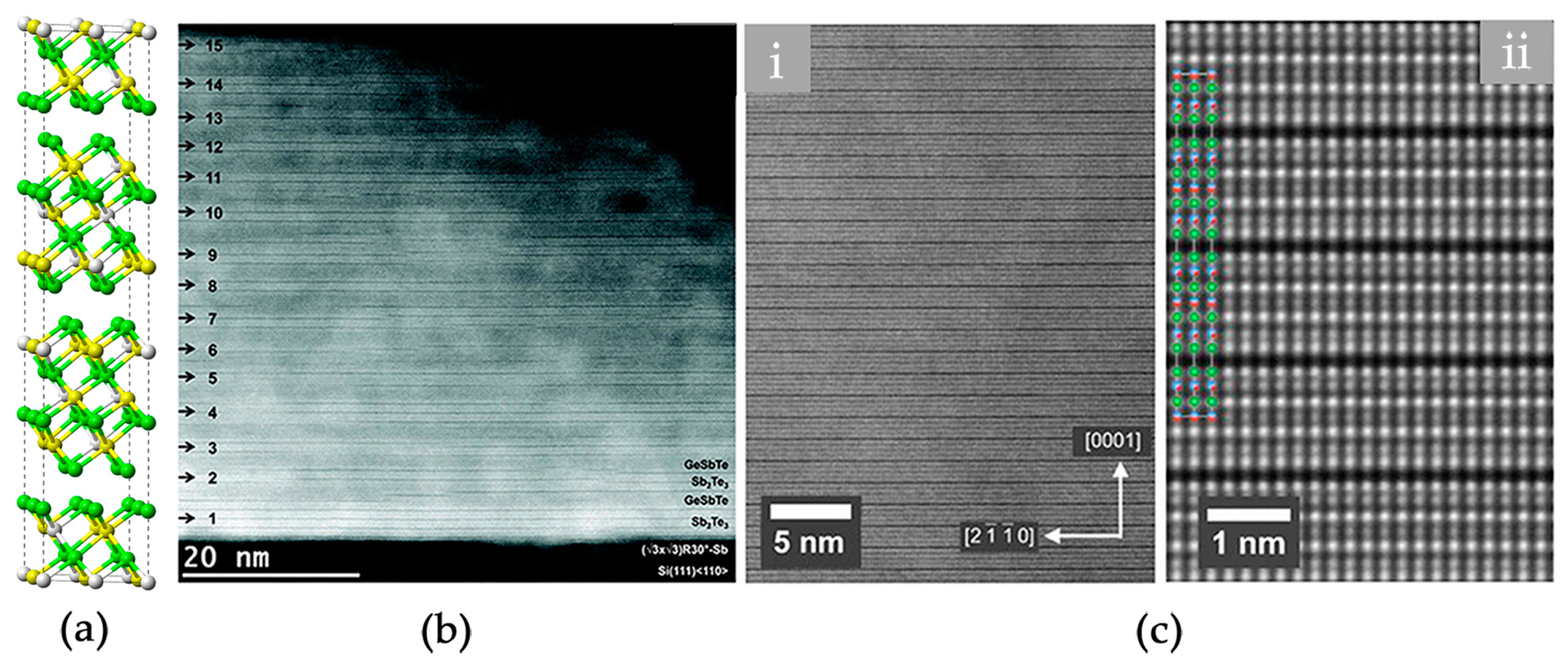
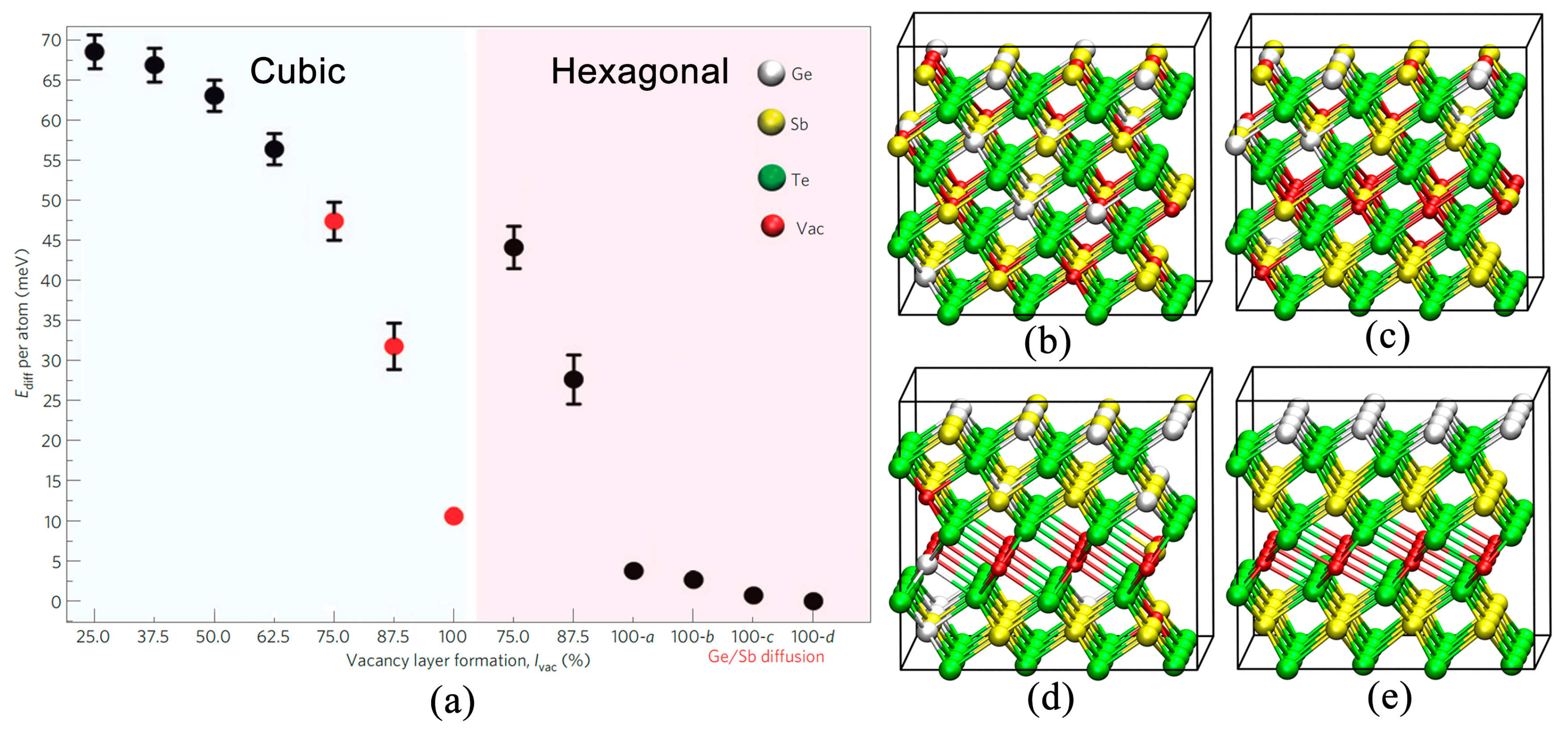
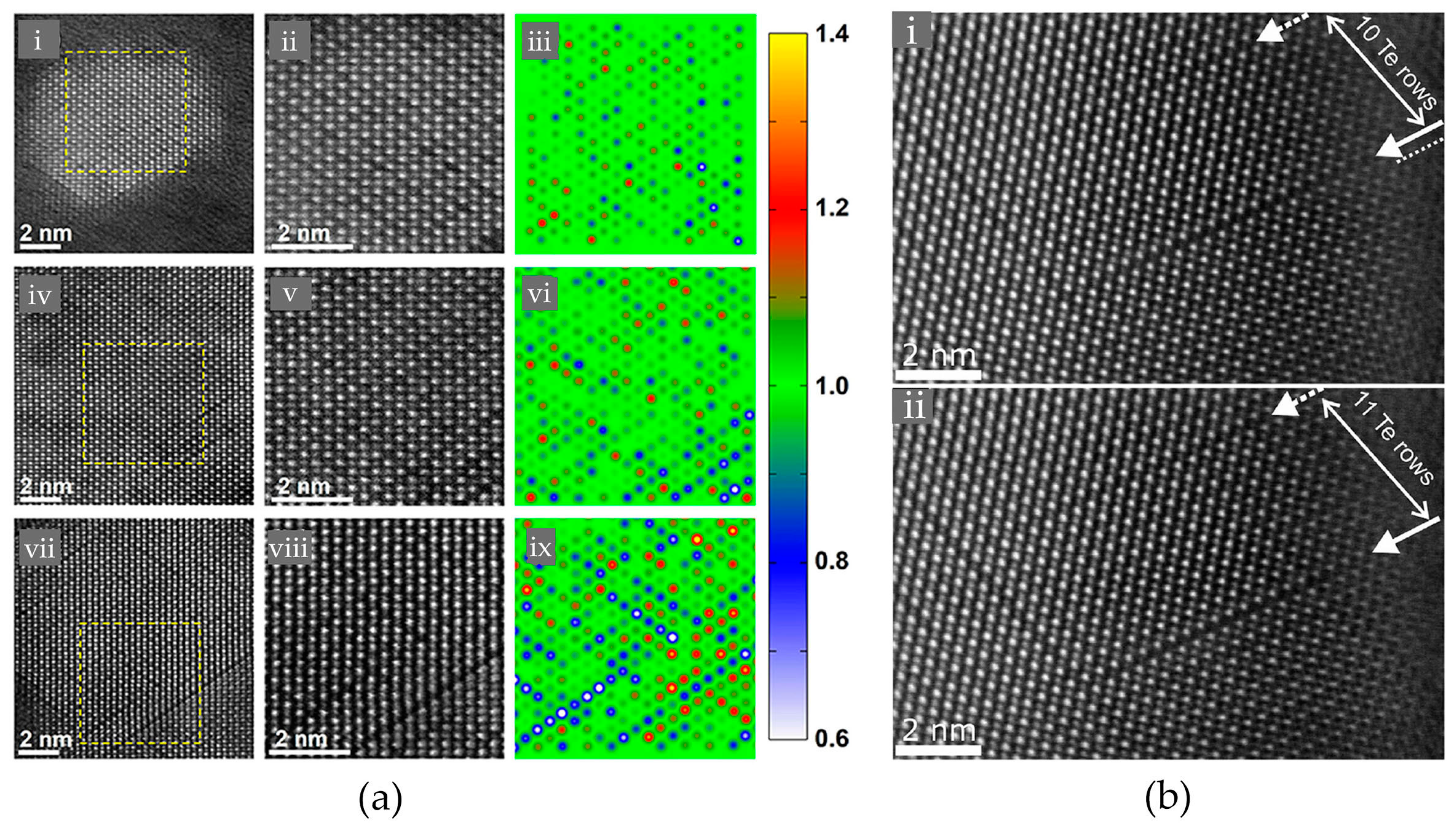
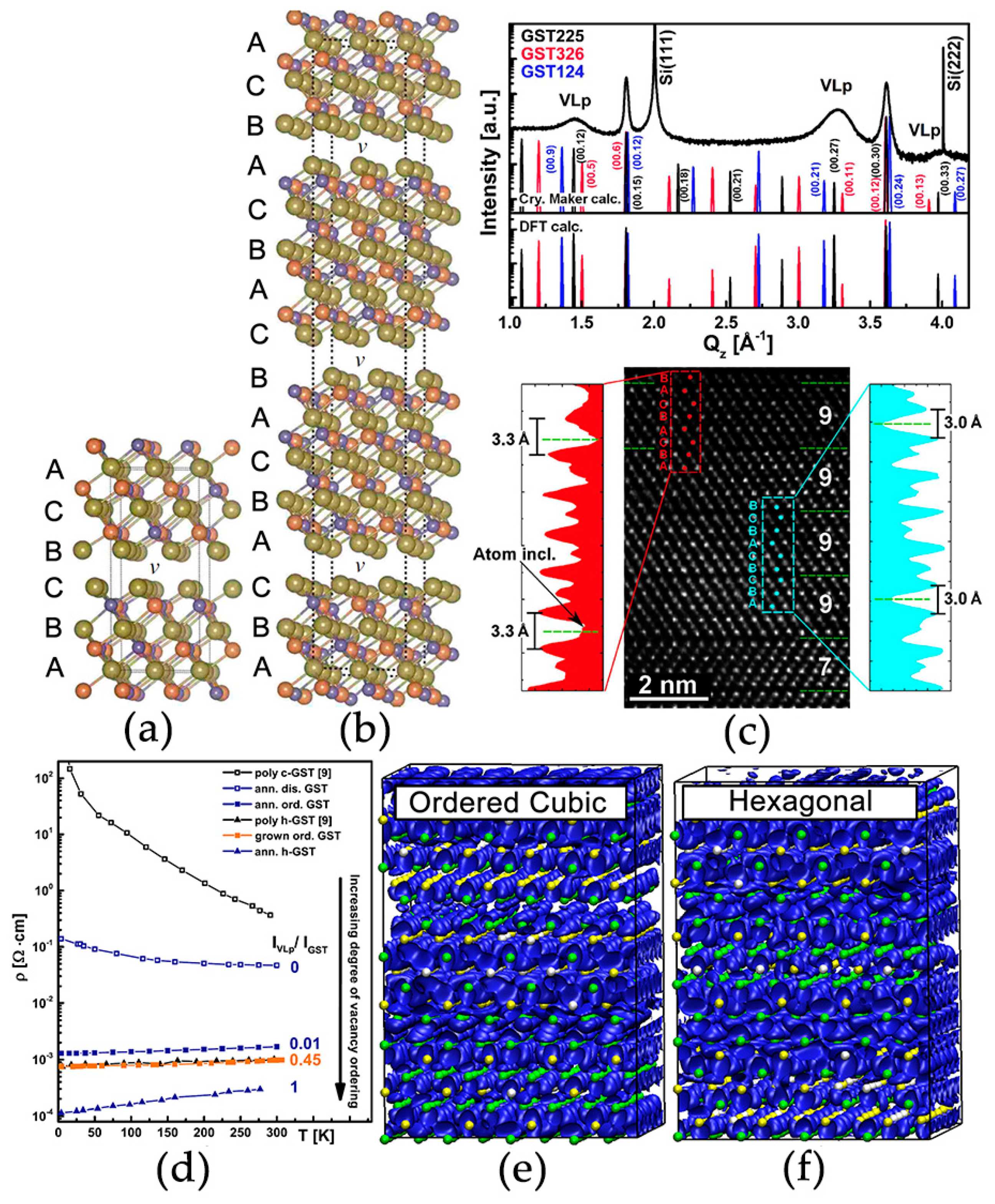
2.1. Atomic Disorder in GST Crystals
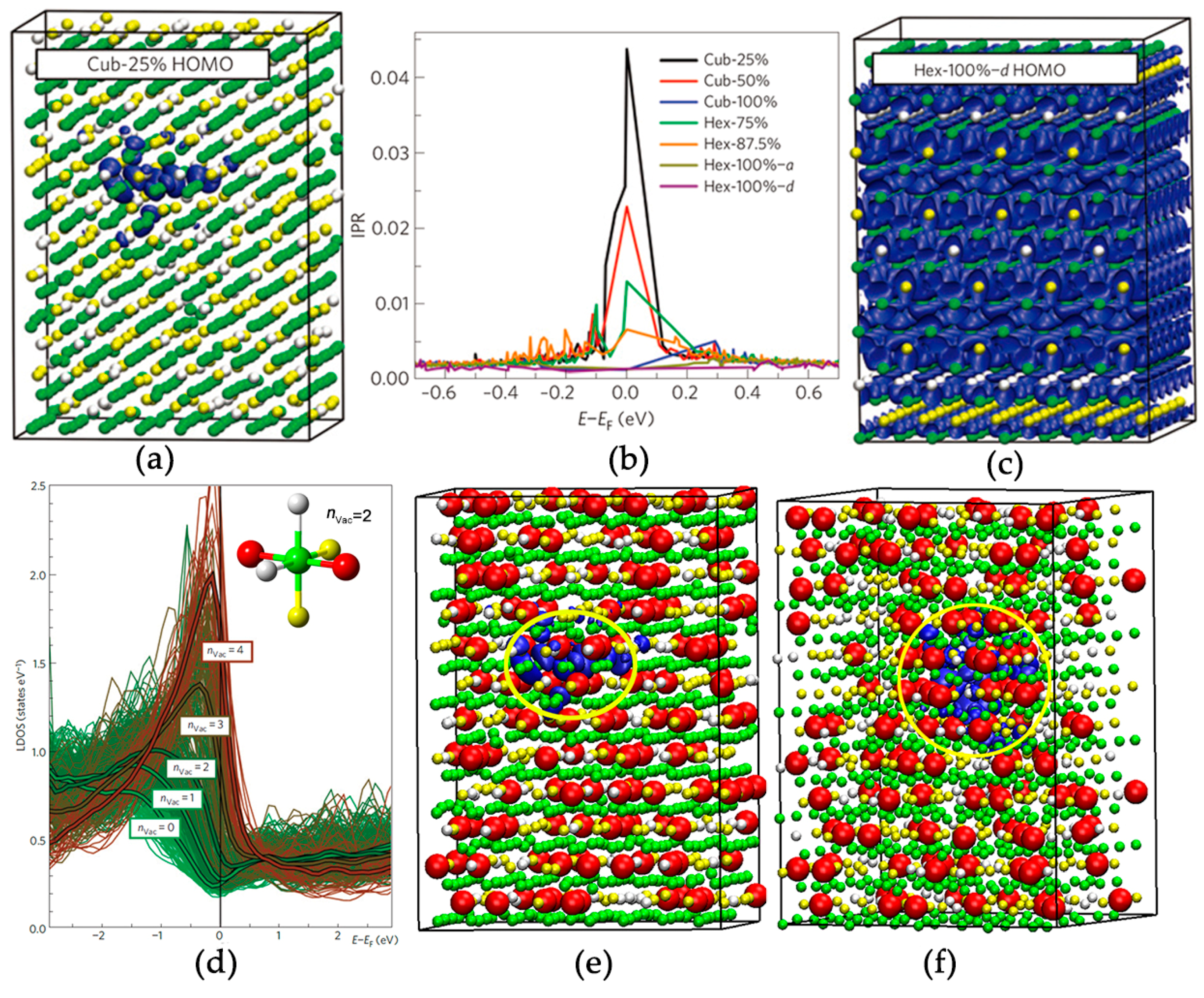
2.2. Disorder-Induced Electron Localization
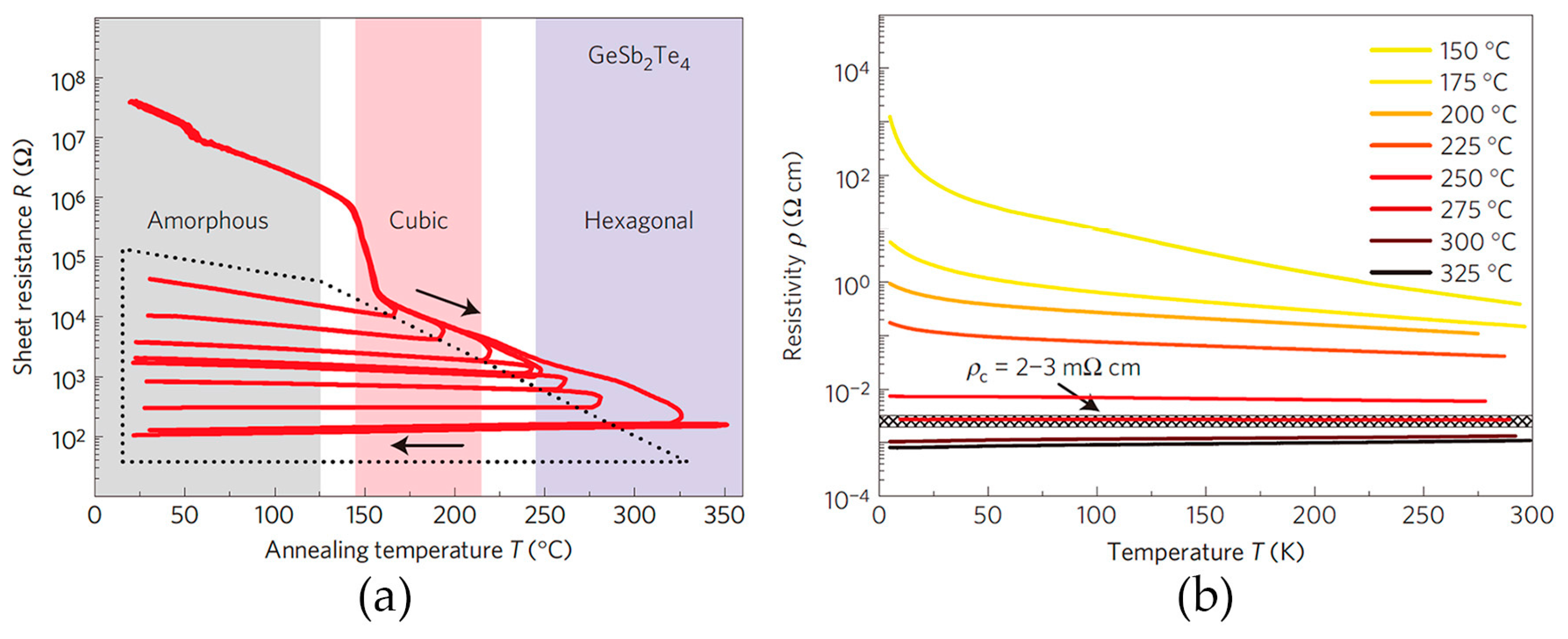
2.3. Disorder-Driven Metal–Insulator Transition
2.4. Metal–Insulator Transition without Cub–Hex Structural Transition
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Brooks Cole: Boston, MA, USA, 1976. [Google Scholar]
- De Boer, J.H.; Verwey, E.J.W. Semi-conductors with partially and with completely filled 3d-lattice bands. Proc. Phys. Soc. 1937, 49, 59. [Google Scholar] [CrossRef]
- Mott, N.F.; Peierls, R. Discussion of the paper by de Boer and Verwey. Proc. Phys. Soc. 1937, 49, 72. [Google Scholar] [CrossRef]
- Mott, N.F. Metal-Insulator Transition. Rev. Mod. Phys. 1968, 40, 677–683. [Google Scholar] [CrossRef]
- Mott, N.F. Review lecture: Metal-Insulator Transitions. Proc. R. Soc. Lond. A 1982, 382, 1–24. [Google Scholar] [CrossRef]
- Anderson, P.W. Absence of diffusion in certain random lattices. Phys. Rev. 1958, 109, 1492–1505. [Google Scholar] [CrossRef]
- Abrahams, E.; Anderson, P.W.; Licciardello, D.C.; Ramakrishnan, T.V. Scaling theory of localization: Absence of quantum diffusion in two dimensions. Phys. Rev. Lett. 1979, 42, 673–676. [Google Scholar] [CrossRef]
- Abrahams, E. (Ed.) 50 Years of Anderson Localization; World Scientific Publishing Company: Singapore, 2010. [Google Scholar]
- Rosenbaum, T.F.; Andres, K.; Thomas, G.A.; Bhatt, R.N. Sharp insulator transition in a random solid. Phys. Rev. Lett. 1980, 45, 1723–1726. [Google Scholar] [CrossRef]
- Rosenbaum, T.F.; Milligan, R.; Paalanen, M.; Thomas, G.A.; Bhatt, R.N.; Lin, W. Metal-insulator transition in a doped semiconductor. Phys. Rev. B 1983, 27, 7509–7523. [Google Scholar] [CrossRef]
- Shklovskii, B.I.; Efros, A.L. Electronic Properties of Doped Semiconductors; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Siegrist, T.; Jost, P.; Volker, H.; Woda, M.; Merkelbach, P.; Schlockermann, C.; Wuttig, M. Disorder-induced localization in crystalline phase-change materials. Nat. Mater. 2011, 10, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Volker, H.; Jost, P.; Wuttig, M. Low-Temperature Transport in Crystalline Ge1Sb2Te4. Adv. Funct. Mater. 2015, 25, 6390–6398. [Google Scholar] [CrossRef]
- Jost, P.; Volker, H.; Poitz, A.; Poltorak, C.; Zalden, P.; Schaefer, T.; Lange, F.; Schmidt, R.M.; Hollaender, B.; Wirtssohn, M.R.; et al. Disorder-Induced Localization in Crystalline Pseudo-Binary GeTe–Sb2Te3 Alloys between Ge3Sb2Te6 and GeTe. Adv. Funct. Mater. 2015, 25, 6399–6406. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Thiess, A.; Zalden, P.; Zeller, R.; Dederichs, P.H.; Raty, J.Y.; Wuttig, M.; Blügel, S.; Mazzarello, R. Role of vacancies in metal-insulator transitions of crystalline phase-change materials. Nat. Mater. 2012, 11, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wuttig, M.; Mazzarello, R. Effects of stoichiometry on the transport properties of crystalline phase-change materials. Sci. Rep. 2015, 5, 13496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Ab Initio Investigation of Phase Change Materials: Structural, Electronic and Kinetic Properties. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2014. [Google Scholar]
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.P.; Raoux, S.; Kim, S.B.; Liang, J.; Reifenberg, J.P.; Rajendran, B.; Asheghi, M.; Goodson, K.E. Phase Change Memory. Proc. IEEE 2010, 98, 2201–2227. [Google Scholar] [CrossRef]
- Raoux, S.; Welnic, W.; Ielmini, D. Phase change materials and their application to nonvolatile memories. Chem. Rev. 2010, 110, 240–267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, J.; Guo, Z.; Sun, Z. An overview of materials issues in resistive random access memory. J. Materiomics 2015, 1, 285–295. [Google Scholar] [CrossRef]
- Yamada, N.; Kojima, R.; Hisada, K.; Mihara, T.; Tsuchino, A.; Fujinoki, N.; Birukawa, M.; Matsunaga, T.; Yasuda, N.; Fukuyama, Y.; et al. Phase-Change Nanodot Material for an Optical Memory. Adv. Opt. Mater. 2013, 1, 820–826. [Google Scholar] [CrossRef]
- Ríos, C.; Stegmaier, M.; Hosseini, P.; Wang, D.; Scherer, T.; Wright, C.D.; Bhaskaran, H.; Pernice, W.H.P. Integrated all-photonic non-volatile multi-level memory. Nat. Photonics 2015, 9, 725–732. [Google Scholar] [CrossRef]
- Wright, C.D.; Liu, Y.; Kohary, K.I.; Aziz, M.M.; Hicken, R.J. Arithmetic and biologically-inspired computing using phase-change materials. Adv. Mater. 2011, 23, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.D.; Wang, L.; Aziz, M.M.; Diosdado, J.A.V.; Ashwin, P. Phase-change processors, memristors and memflectors. Phys. Status Solidi B 2012, 249, 1978–1984. [Google Scholar] [CrossRef]
- Shamoto, S. Large displacement of germanium atoms in crystalline Ge2Sb2Te5. Appl. Phys. Lett. 2005, 86, 081904. [Google Scholar] [CrossRef]
- Tuma, T.; Pantazi, A.; Le Gallo, M.; Sebastian, A.; Eleftheriou, E. Stochastic phase-change neurons. Nat. Nanotechnol. 2016, 11, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, Y.; Xu, L.; Zhang, J.; Xu, X.; Sun, H.; Miao, X. Ultrafast Synaptic Events in a Chalcogenide. Memristor 2013, 3, 1619. [Google Scholar]
- Song, W.-D.; Shi, L.-P.; Miao, X.-S.; Chong, C.-T. Synthesis and Characteristics of a Phase-Change Magnetic Material. Adv. Mater. 2008, 20, 2394–2397. [Google Scholar] [CrossRef]
- Zhang, W.; Ronneberger, I.; Li, Y.; Mazzarello, R. Magnetic properties of crystalline and amorphous phase-change materials doped with 3d impurities. Adv. Mater. 2012, 24, 4387–4391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mazzarello, R. Magnetic contrast in phase-change materials doped with Fe impurities. Adv. Mater. 2012, 24, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Miao, X.S.; Tong, F.; Luo, W.; Xia, Z.C. Ferromagnetism and electronic transport in epitaxial Ge1 − xFexTe thin film grown by pulsed laser deposition. Appl. Phys. Lett. 2013, 102, 102402. [Google Scholar] [CrossRef]
- Zhang, W.; Ronneberger, I.; Li, Y.; Mazzarello, R. Ab initio investigation of crystalline and amorphous GeTe doped with magnetic impurities. Sci. Adv. Mater. 2014, 6, 1655–1658. [Google Scholar] [CrossRef]
- Hosseini, P.; Wright, C.D.; Bhaskaran, H. An optoelectronic framework enabled by low-dimensional phase-change films. Nature 2014, 511, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Deringer, V.L.; Dronskowski, R.; Mazzarello, R.; Ma, E.; Wuttig, M. Density functional theory guided advances in phase-change materials and memories. MRS Bull. 2015, 40, 856–865. [Google Scholar] [CrossRef]
- Deringer, V.L.; Dronskowski, R.; Wuttig, M. Microscopic Complexity in Phase-Change Materials and its Role for Applications. Adv. Funct. Mater. 2015, 25, 6343–6359. [Google Scholar] [CrossRef]
- Yamada, N.; Ohno, E.; Nishiuchi, K.; Akahira, N.; Takao, M. Rapid-phase transitions of GeTe-Sb2Te3 pseudobinary amorphous thin films for an optical disk memory. J. Appl. Phys. 1991, 69, 2849–2856. [Google Scholar] [CrossRef]
- Yamada, N.; Matsunaga, T. Structure of laser-crystallized Ge2Sb2+xTe5 sputtered thin films for use in optical memory. J. Appl. Phys. 2000, 88, 7020–7028. [Google Scholar] [CrossRef]
- Simpson, R.E.; Krbal, M.; Fons, P.; Kolobov, A.V.; Tominaga, J.; Uruga, T.; Tanida, H. Toward the ultimate limit of phase change in Ge2Sb2Te5. Nano Lett. 2010, 10, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Shportko, K.; Kremers, S.; Woda, M.; Lencer, D.; Robertson, J.; Wuttig, M. Resonant bonding in crystalline phase-change materials. Nat. Mater. 2008, 7, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Lencer, D.; Salinga, M.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Wuttig, M. A map for phase-change materials. Nat. Mater. 2008, 7, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, W.; Shen, Z.J.; Chen, Y.J.; Li, J.X.; Zhang, S.B.; Zhang, Z.; Wuttig, M.; Mazzarello, R.; Ma, E.; et al. Element-resolved atomic structure imaging of rocksalt Ge2Sb2Te5 phase-change material. Appl. Phys. Lett. 2016, 108, 191902. [Google Scholar] [CrossRef]
- Yamada, N. Erasable Phase-Change Optical Materials. MRS Bull. 1996, 21, 48–50. [Google Scholar] [CrossRef]
- Matsunaga, T.; Yamada, N. Structural investigation of GeSb2Te4: A high-speed phase-change material. Phys. Rev. B 2004, 69, 104111. [Google Scholar] [CrossRef]
- Wuttig, M.; Lusebrink, D.; Wamwangi, D.; Welnic, W.; Gillessen, M.; Dronskowski, R. The role of vacancies and local distortions in the design of new phase-change materials. Nat. Mater. 2007, 6, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Martin, R.M. Electronic Structure: Basic Theory and Practical Methods; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Dronskowski, R.; Blöchl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- El-Mellouhi, F.; Mousseau, N.; Ordejon, P. Sampling the diffusion paths of a neutral vacancy in silicon with quantum mechanical calculations. Phys. Rev. B 2004, 70, 205202. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Maintz, S.; Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Deringer, V.L.; Zhang, W.; Rausch, P.; Mazzarello, R.; Wuttig, M.; Dronskowski, R. A chemical link between Ge-Sb-Te and In-Sb-Te phase-change materials. J. Mater. Chem. C 2015, 3, 9519–9523. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhang, W.; Wang, L.Y.; Zhuang, Z.; Ma, E.; Li, J.; Shan, Z.W. In situ TEM study of deformation-induced crystalline-to-amorphous transition in silicon. NPG Asia Mater. 2016, 8, e291. [Google Scholar] [CrossRef]
- Waghmare, U.V.; Spaldin, N.A.; Kandpal, H.C.; Seshadri, R. First-principles indicators of metallicity and cation off-centricity in the IV-VI rocksalt chalcogenides of divalent Ge, Sn, and Pb. Phys. Rev. B 2003, 67, 125111. [Google Scholar] [CrossRef]
- Deringer, V.L.; Zhang, W.; Lumeij, M.; Maintz, S.; Wuttig, M.; Mazzarello, R.; Dronskowski, R. Bonding nature of local structural motifs in amorphous GeTe. Angew. Chem. Int. Ed. 2014, 53, 10817–10820. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, R.P.; Deringer, V.L.; Simon, R.E.; Hermann, R.P.; Dronskowski, R. A density-functional study on the electronic and vibrational properties of layered antimony telluride. J. Phys. Condens. Matter 2015, 27, 085402. [Google Scholar] [CrossRef] [PubMed]
- Ross, U.; Lotnyk, A.; Thelander, E.; Rauschenbach, B. Direct imaging of crystal structure and defects in metastable Ge2Sb2Te5 by quantitative aberration-corrected scanning transmission electron microscopy. Appl. Phys. Lett. 2014, 104, 121904. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Sagendorf, J.; West, D.; Kou, X.; Wei, X.; He, L.; Wang, K.L.; Zhang, S.; Zhang, Z. Direct atom-by-atom chemical identification of nanostructures and defects of topological insulators. Nano Lett. 2013, 13, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Song, Z.; Cheng, Y.; Liu, X.; Xia, M.; Li, W.; Ding, K.; Feng, X.; Zhu, M.; Feng, S. Direct observation of titanium-centered octahedra in titanium-antimony-tellurium phase-change material. Nat. Commun. 2015, 6, 10040. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xia, M.; Cheng, Y.; Rao, F.; Ding, K.; Liu, W.; Jia, Y.; Song, Z.; Feng, S. Direct observation of metastable face-centered cubic Sb2Te3 crystal. Nano Res. 2016, 9, 3453–3462. [Google Scholar] [CrossRef]
- Pennycook, S.J.; Nellist, P.D. Scanning Transmission Electron Microscopy Imaging and Analysis; Springer: New York, NY, USA, 2011. [Google Scholar]
- Kolobov, A.V.; Fons, P.; Frenkel, A.I.; Ankudinov, A.L.; Tominaga, J.; Uruga, T. Understanding the phase-change mechanism of rewritable optical media. Nat. Mater. 2004, 3, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Wełnic, W.; Pamungkas, A.; Detemple, R.; Steimer, C.; Blügel, S.; Wuttig, M. Unravelling the interplay of local structure and physical properties in phase-change materials. Nat. Mater. 2005, 5, 56–62. [Google Scholar] [CrossRef]
- Momand, J.; Wang, R.; Boschker, J.E.; Verheijen, M.A.; Calarco, R.; Kooi, B.J. Interface formation of two- and three-dimensionally bonded materials in the case of GeTe-Sb2Te3 superlattices. Nanoscale 2015, 7, 19136–19143. [Google Scholar] [CrossRef] [PubMed]
- Hardtdegen, H.; Rieß, S.; Schuck, M.; Keller, K.; Jost, P.; Du, H.; Bornhöfft, M.; Schwedt, A.; Mussler, G.; Ahe, M.v.d.; et al. A model structure for interfacial phase change memories: Epitaxial trigonal Ge1Sb2Te4. J. Alloys Compd. 2016, 679, 285–292. [Google Scholar] [CrossRef]
- Kooi, B.J.; De Hosson, J.T.M. Electron diffraction and high-resolution transmission electron microscopy of the high temperature crystal structures of GexSb2Te3 + x (x = 1,2,3) phase change material. J. Appl. Phys. 2002, 92, 3584–3590. [Google Scholar] [CrossRef]
- Kooi, B.J.; Groot, W.M.G.; De Hosson, J.T.M. In situ transmission electron microscopy study of the crystallization of Ge2Sb2Te5. J. Appl. Phys. 2004, 95, 924–932. [Google Scholar] [CrossRef]
- Sun, Z.M.; Zhou, J.; Ahuja, R. Unique Melting Behavior in Phase-Change Materials for Rewritable Data Storage. Phys. Rev. Lett. 2007, 98, 055505. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Walsh, A.; Lee, H. Insights into the structure of the stable and metastable (GeTe)m(Sb2Te3)n compounds. Phys. Rev. B 2008, 78, 224111. [Google Scholar] [CrossRef]
- Momand, J.; Lange, F.R.L.; Wang, R.; Boschker, J.E.; Verheijen, M.A.; Calarco, R.; Wuttig, M.; Kooi, B.J. Atomic stacking and van-der-Waals bonding in GeTe–Sb2Te3 superlattices. J. Mater. Res. 2016, 31, 3115–3124. [Google Scholar] [CrossRef]
- Thelander, E.; Gerlach, J.W.; Ross, U.; Lotnyk, A.; Rauschenbach, B. Low temperature epitaxy of Ge-Sb-Te films on BaF2 (111) by pulsed laser deposition. Appl. Phys. Lett. 2014, 105, 221908. [Google Scholar] [CrossRef]
- Ross, U.; Lotnyk, A.; Thelander, E.; Rauschenbach, B. Microstructure evolution in pulsed laser deposited epitaxial Ge-Sb-Te chalcogenide thin films. J. Alloys Compd. 2016, 676, 582–590. [Google Scholar] [CrossRef]
- Caravati, S.; Bernasconi, M.; Kühne, T.D.; Krack, M.; Parrinello, M. First principles study of crystalline and amorphous Ge2Sb2Te5 and the effects of stoichiometric defects. J. Phys. Condens. Matter 2009, 21, 255501. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Pan, Y.; Zhou, J.; Sa, B.; Ahuja, R. Origin of p-type conductivity in layered nGeTe·mSb2Te3 chalcogenide semiconductors. Phys. Rev. B 2011, 83, 113201. [Google Scholar] [CrossRef]
- Edwards, A.; Pineda, A.; Schultz, P.; Martin, M.; Thompson, A.; Hjalmarson, H.; Umrigar, C. Electronic structure of intrinsic defects in crystalline germanium telluride. Phys. Rev. B 2006, 73, 045210. [Google Scholar] [CrossRef]
- Chen, C.; Jost, P.; Volker, H.; Kaminski, M.; Wirtssohn, M.; Engelmann, U.; Krüger, K.; Schlich, F.; Schlockermann, C.; Lobo, R.P.S.M.; et al. Dielectric properties of amorphous phase-change materials. Phys. Rev. B 2017, 95, 094111. [Google Scholar] [CrossRef]
- Lee, T.H.; Elliott, S.R. Ab Initio Computer Simulation of the Early Stages of Crystallization: Application to Ge2Sb2Te5 Phase-Change Materials. Phys. Rev. Lett. 2011, 107, 145702. [Google Scholar] [CrossRef] [PubMed]
- Skelton, J.M.; Loke, D.; Lee, T.H.; Elliott, S.R. Structural insights into the formation and evolution of amorphous phase-change materials. Phys. Status Solidi B 2013, 250, 968–975. [Google Scholar] [CrossRef]
- Kalikka, J.; Akola, J.; Larrucea, J.; Jones, R.O. Nucleus-driven crystallization of amorphous Ge2Sb2Te5: A density functional study. Phys. Rev. B 2012, 86, 144113. [Google Scholar] [CrossRef]
- Kalikka, J.; Akola, J.; Jones, R.O. Simulation of crystallization in Ge2Sb2Te5: A memory effect in the canonical phase-change material. Phys. Rev. B 2014, 90, 184109. [Google Scholar] [CrossRef]
- Kalikka, J.; Akola, J.; Jones, R.O. Crystallization processes in the phase change material Ge2Sb2Te5: Unbiased density functional/molecular dynamics simulations. Phys. Rev. B 2016, 94, 134105. [Google Scholar] [CrossRef]
- Ronneberger, I.; Zhang, W.; Eshet, H.; Mazzarello, R. Crystallization properties of the Ge2Sb2Te5 phase-change compound from advanced simulations. Adv. Funct. Mater. 2015, 25, 6407–6413. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Mazzarello, R.; Wuttig, M. Disorder Control in Crystalline GeSb2Te4 using High Pressure. Adv. Sci. 2015, 2, 1500117. [Google Scholar] [CrossRef] [PubMed]
- Pauly, C.; Liebmann, M.; Giussani, A.; Kellner, J.; Just, S.; Sánchez-Barriga, J.; Rienks, E.; Rader, O.; Calarco, R.; Bihlmayer, G.; et al. Evidence for topological band inversion of the phase change material Ge2Sb2Te5. Appl. Phys. Lett. 2013, 103, 243109. [Google Scholar] [CrossRef] [Green Version]
- Sa, B.; Zhou, J.; Sun, Z.; Tominaga, J.; Ahuja, R. Topological insulating in GeTe/Sb2Te3 phase-change superlattice. Phys. Rev. Lett. 2012, 109, 096802. [Google Scholar] [CrossRef] [PubMed]
- Sa, B.; Zhou, J.; Song, Z.; Sun, Z.; Ahuja, R. Pressure-induced topological insulating behavior in the ternary chalcogenide Ge2Sb2Te5. Phys. Rev. B 2011, 84, 085130. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Jhi, S.-H. Prediction of topological insulating behavior in crystalline Ge-Sb-Te. Phys. Rev. B 2010, 82, 201312. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, K.S.; Jhi, S.H. Topological Phase Transition in the Interaction of Surface Dirac Fermions in Heterostructures. Phys. Rev. Lett. 2012, 109, 146601. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.; Awano, H.; Tominaga, J.; Kolobov, A.V.; Fons, P.; Saito, Y.; Makino, K.; Nakano, T.; Hase, M.; Takagaki, Y.; et al. Mirror-symmetric Magneto-optical Kerr Rotation using Visible Light in [(GeTe)2(Sb2Te3)1]n Topological Superlattices. Sci. Rep. 2014, 4, 5727. [Google Scholar] [CrossRef] [PubMed]
- Lotnyk, A.; Bernütz, S.; Sun, X.; Ross, U.; Ehrhardt, M.; Rauschenbach, B. Real-space imaging of atomic arrangement and vacancy layers ordering in laser crystallised Ge2Sb2Te5 phase change thin films. Acta Mater. 2016, 105, 1–8. [Google Scholar] [CrossRef]
- Bragaglia, V.; Arciprete, F.; Zhang, W.; Mio, A.M.; Zallo, E.; Perumal, K.; Giussani, A.; Cecchi, S.; Boschker, J.E.; Riechert, H.; et al. Metal-Insulator Transition Driven by Vacancy Ordering in GeSbTe Phase Change Materials. Sci. Rep. 2016, 6, 23843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, X.P.; Shen, Z.J.; Li, X.B.; Wang, C.S.; Chen, Y.J.; Li, J.X.; Zhang, J.X.; Zhang, Z.; Zhang, S.B.; et al. Vacancy Structures and Melting Behavior in Rock-Salt GeSbTe. Sci. Rep. 2016, 6, 25453. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, I.; Lotnyk, A.; Gerlach, J.W.; Schumacher, P.; Rauschenbach, B. Epitaxial formation of cubic and trigonal Ge-Sb-Te thin films with heterogeneous vacancy structures. Mater. Des. 2017, 115, 138–146. [Google Scholar] [CrossRef]
- Park, Y.J.; Cho, J.Y.; Jeong, M.W.; Na, S.; Joo, Y.C. New pathway for the formation of metallic cubic phase Ge-Sb-Te compounds induced by an electric current. Sci. Rep. 2016, 6, 21466. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Chung, H.S.; Lo, Y.C.; Qi, L.; Li, J.; Lu, Y.; Johnson, A.T.; Jung, Y.; Nukala, P.; Agarwal, R. Electrical wind force-driven and dislocation-templated amorphization in phase-change nanowires. Science 2012, 336, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Nukala, P.; Agarwal, R.; Qian, X.; Jang, M.H.; Dhara, S.; Kumar, K.; Johnson, A.T.; Li, J.; Agarwal, R. Direct observation of metal-insulator transition in single-crystalline germanium telluride nanowire memory devices prior to amorphization. Nano Lett. 2014, 14, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, A.; Lacaita, A.L.; Pellizzer, F.; Kostylev, S.A.; Benvenuti, A.; Bez, R. Low-field amorphous state resistance and threshold voltage drift in chalcogenide materials. IEEE Trans. Electron Devices 2004, 51, 714–719. [Google Scholar] [CrossRef]
- Ielmini, D.; Lacaita, A.L.; Mantegazza, D. Recovery and Drift Dynamics of Resistance and Threshold Voltages in Phase-Change Memories. IEEE Trans. Electron Devices 2007, 54, 308–315. [Google Scholar] [CrossRef]
- Fantini, P.; Brazzelli, S.; Cazzini, E.; Mani, A. Band gap widening with time induced by structural relaxation in amorphous Ge2Sb2Te5 films. Appl. Phys. Lett. 2012, 100, 013505. [Google Scholar] [CrossRef]
- Fantini, P.; Ferro, M.; Calderoni, A.; Brazzelli, S. Disorder enhancement due to structural relaxation in amorphous Ge2Sb2Te5. Appl. Phys. Lett. 2012, 100, 213506. [Google Scholar] [CrossRef]
- Mitrofanov, K.V.; Kolobov, A.V.; Fons, P.; Wang, X.; Tominaga, J.; Tamenori, Y.; Uruga, T.; Ciocchini, N.; Ielmini, D. Ge L3-edge X-ray absorption near-edge structure study of structural changes accompanying conductivity drift in the amorphous phase of Ge2Sb2Te5. J. Appl. Phys. 2014, 115, 173501. [Google Scholar] [CrossRef]
- Raty, J.-Y.; Zhang, W.; Luckas, J.; Chen, C.; Bichara, C.; Mazzarello, R.; Wuttig, M. Aging mechanism of amorphous phase change materials. Nat. Commun. 2015, 6, 7467. [Google Scholar] [CrossRef] [PubMed]
- Polking, M.J.; Zheng, H.; Ramesh, R.; Alivisatos, A.P. Controlled synthesis and size-dependent polarization domain structure of colloidal germanium telluride nanocrystals. J. Am. Chem. Soc. 2011, 133, 2044–2047. [Google Scholar] [CrossRef] [PubMed]
- Giusca, C.E.; Stolojan, V.; Sloan, J.; Borrnert, F.; Shiozawa, H.; Sader, K.; Rummeli, M.H.; Buchner, B.; Silva, S.R. Confined crystals of the smallest phase-change material. Nano Lett. 2013, 13, 4020–4027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Cheng, Y.Q.; Wang, L.; Sheng, H.W.; Meng, Y.; Yang, W.G.; Han, X.D.; Ma, E. Pressure tunes electrical resistivity by four orders of magnitude in amorphous Ge2Sb2Te5 phase-change memory alloy. Proc. Natl. Acad. Sci. USA 2012, 109, E1055–E1062. [Google Scholar] [CrossRef] [PubMed]
- Caravati, S.; Bernasconi, M.; Kühne, T.; Krack, M.; Parrinello, M. Unravelling the Mechanism of Pressure Induced Amorphization of Phase Change Materials. Phys. Rev. Lett. 2009, 102, 205502. [Google Scholar] [CrossRef] [PubMed]
- Caravati, S.; Sosso, G.C.; Bernasconi, M.; Parrinello, M. Density functional simulations of hexagonal Ge2Sb2Te5 at high pressure. Phys. Rev. B 2013, 87, 094117. [Google Scholar] [CrossRef]
- Krbal, M.; Kolobov, A.; Haines, J.; Fons, P.; Levelut, C.; Le Parc, R.; Hanfland, M.; Tominaga, J.; Pradel, A.; Ribes, M. Initial Structure Memory of Pressure-Induced Changes in the Phase-Change Memory Alloy Ge2Sb2Te5. Phys. Rev. Lett. 2009, 103, 115502. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhou, J.; Pan, Y.; Song, Z.; Mao, H.K.; Ahuja, R. Pressure-induced reversible amorphization and an amorphous-amorphous transition in Ge2Sb2Te5 phase-change memory material. Proc. Natl. Acad. Sci. USA 2011, 108, 10410–10414. [Google Scholar] [CrossRef] [PubMed]
- Kalikka, J.; Zhou, X.; Dilcher, E.; Wall, S.; Li, J.; Simpson, R.E. Strain-engineered diffusive atomic switching in two-dimensional crystals. Nat. Commun. 2016, 7, 11983. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Kalikka, J.; Ji, X.L.; Wu, L.C.; Song, Z.T.; Simpson, R.E. Phase-Change Memory Materials by Design: A Strain Engineering Approach. Adv. Mater. 2016, 28, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Privitera, S.M.S.; Mio, A.M.; Smecca, E.; Alberti, A.; Zhang, W.; Mazzarello, R.; Benke, J.; Persch, C.; La Via, F.; Rimini, E. Structural and electronic transitions in Ge2Sb2Te5 induced by ion irradiation damage. Phys. Rev. B 2016, 94, 094103. [Google Scholar] [CrossRef]
- Simpson, R.E.; Fons, P.; Kolobov, A.V.; Fukaya, T.; Krbal, M.; Yagi, T.; Tominaga, J. Interfacial phase-change memory. Nat. Nanotechnol. 2011, 6, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, J.; Kolobov, A.V.; Fons, P.; Nakano, T.; Murakami, S. Ferroelectric Order Control of the Dirac-Semimetal Phase in GeTe-Sb2Te3 Superlattices. Adv. Mater. Interfaces 2014, 1, 1300027. [Google Scholar] [CrossRef]
- Loke, D.; Lee, T.H.; Wang, W.J.; Shi, L.P.; Zhao, R.; Yeo, Y.C.; Chong, T.C.; Elliott, S.R. Breaking the speed limits of phase-change memory. Science 2012, 336, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xia, M.; Rao, F.; Li, X.; Wu, L.; Ji, X.; Lv, S.; Song, Z.; Feng, S.; Sun, H.; et al. One order of magnitude faster phase change at reduced power in Ti-Sb-Te. Nat. Commun. 2014, 5, 4086. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhu, M.; Wang, Y.; Song, Z.; Rao, F.; Wu, L.; Cheng, Y.; Song, S. Ti–Sb–Te Alloy: A Candidate for Fast and Long-Life Phase-Change Memory. ACS Appl. Mater. Interface 2015, 7, 7627–7634. [Google Scholar] [CrossRef] [PubMed]
- Kolobov, A.V.; Krbal, M.; Fons, P.; Tominaga, J.; Uruga, T. Distortion-triggered loss of long-range order in solids with bonding energy hierarchy. Nat. Chem. 2011, 3, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanov, K.V.; Fons, P.; Makino, K.; Terashima, R.; Shimada, T.; Kolobov, A.V.; Tominaga, J.; Bragaglia, V.; Giussani, A.; Calarco, R.; et al. Sub-nanometre resolution of atomic motion during electronic excitation in phase-change materials. Sci. Rep. 2016, 6, 20633. [Google Scholar] [CrossRef] [PubMed]
- Bragaglia, V.; Jenichen, B.; Giussani, A.; Perumal, K.; Riechert, H.; Calarco, R. Structural change upon annealing of amorphous GeSbTe grown on Si(111). J. Appl. Phys. 2014, 116, 054913. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W.; Momand, J.; Ronneberger, I.; Boschker, J.E.; Mazzarello, R.; Kooi, B.J.; Riechert, H.; Wuttig, M.; Calarco, R. Formation of resonant bonding during growth of ultrathin GeTe films. NPG Asia Mater. 2017, 9, e396. [Google Scholar] [CrossRef]
- Momand, J.; Wang, R.; Boschker, J.E.; Verheijen, M.A.; Calarco, R.; Kooi, B.J. Dynamic reconfiguration of van der Waals gaps within GeTe-Sb2Te3 based superlattices. Nanoscale 2017, 9, 8774–8780. [Google Scholar] [CrossRef] [PubMed]
- Breznay, N.P.; Volker, H.; Palevski, A.; Mazzarello, R.; Kapitulnik, A.; Wuttig, M. Weak antilocalization and disorder-enhanced electron interactions in annealed films of the phase-change compound GeSb2Te4. Phys. Rev. B 2012, 86, 205302. [Google Scholar] [CrossRef]
- Liao, J.; Ou, Y.; Feng, X.; Yang, S.; Lin, C.; Yang, W.; Wu, K.; He, K.; Ma, X.; Xue, Q.K.; et al. Observation of Anderson localization in ultrathin films of three-dimensional topological insulators. Phys. Rev. Lett. 2015, 114, 216601. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.P.; Gu, Y.Q.; Chen, X.; Wang, X.B.; Jin, S.F.; Zhao, L.L.; Zhang, W.; Chen, X.L. Anderson localization of electrons in single crystals: LixFe7Se8. Sci. Adv. 2016, 2, e1501283. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-J.; Xu, Y.-Z.; Mazzarello, R.; Wuttig, M.; Zhang, W. A Review on Disorder-Driven Metal–Insulator Transition in Crystalline Vacancy-Rich GeSbTe Phase-Change Materials. Materials 2017, 10, 862. https://doi.org/10.3390/ma10080862
Wang J-J, Xu Y-Z, Mazzarello R, Wuttig M, Zhang W. A Review on Disorder-Driven Metal–Insulator Transition in Crystalline Vacancy-Rich GeSbTe Phase-Change Materials. Materials. 2017; 10(8):862. https://doi.org/10.3390/ma10080862
Chicago/Turabian StyleWang, Jiang-Jing, Ya-Zhi Xu, Riccardo Mazzarello, Matthias Wuttig, and Wei Zhang. 2017. "A Review on Disorder-Driven Metal–Insulator Transition in Crystalline Vacancy-Rich GeSbTe Phase-Change Materials" Materials 10, no. 8: 862. https://doi.org/10.3390/ma10080862



