Preparation of Cationic MOFs with Mobile Anions by Anion Stripping to Remove 2,4-D from Water
Abstract
:1. Introduction
2. Results and Discussion
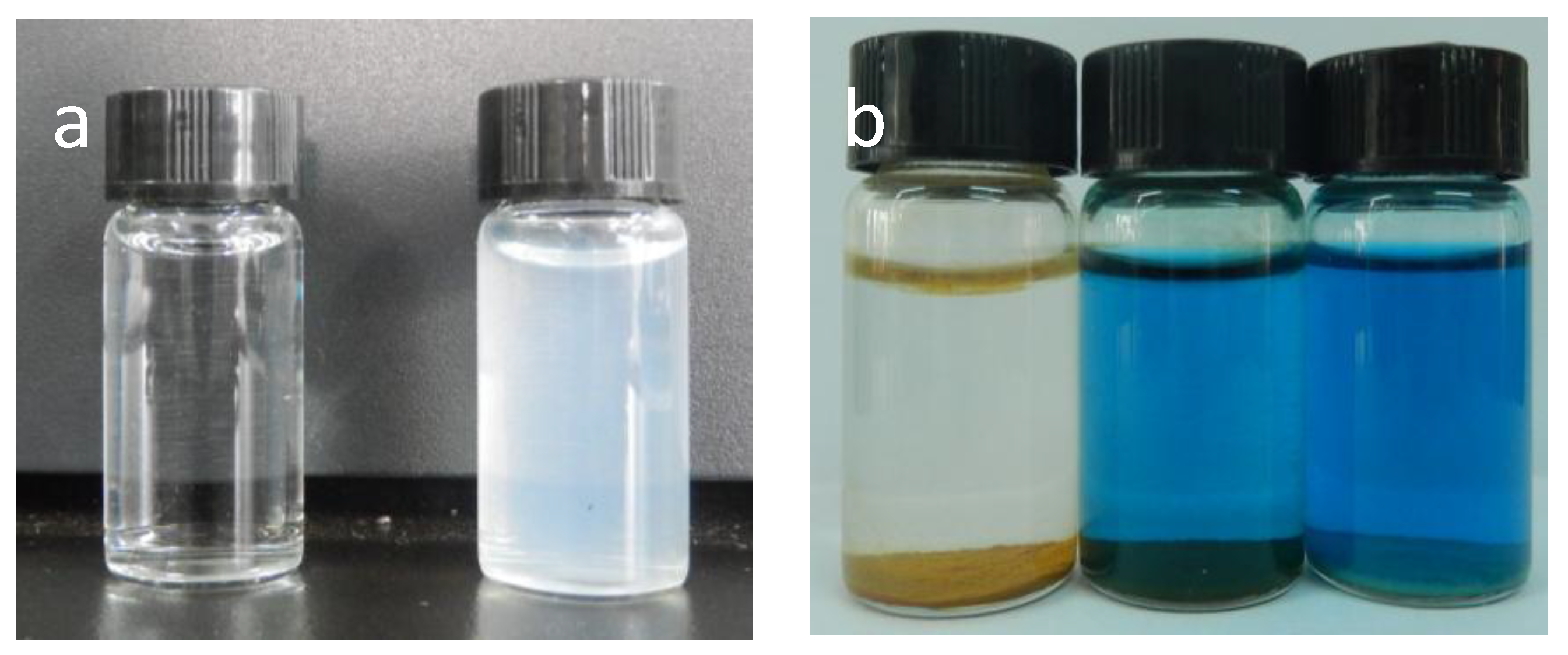
2.1. Characterization of As-Prepared Materials
2.2. Adsorptive Kinetics
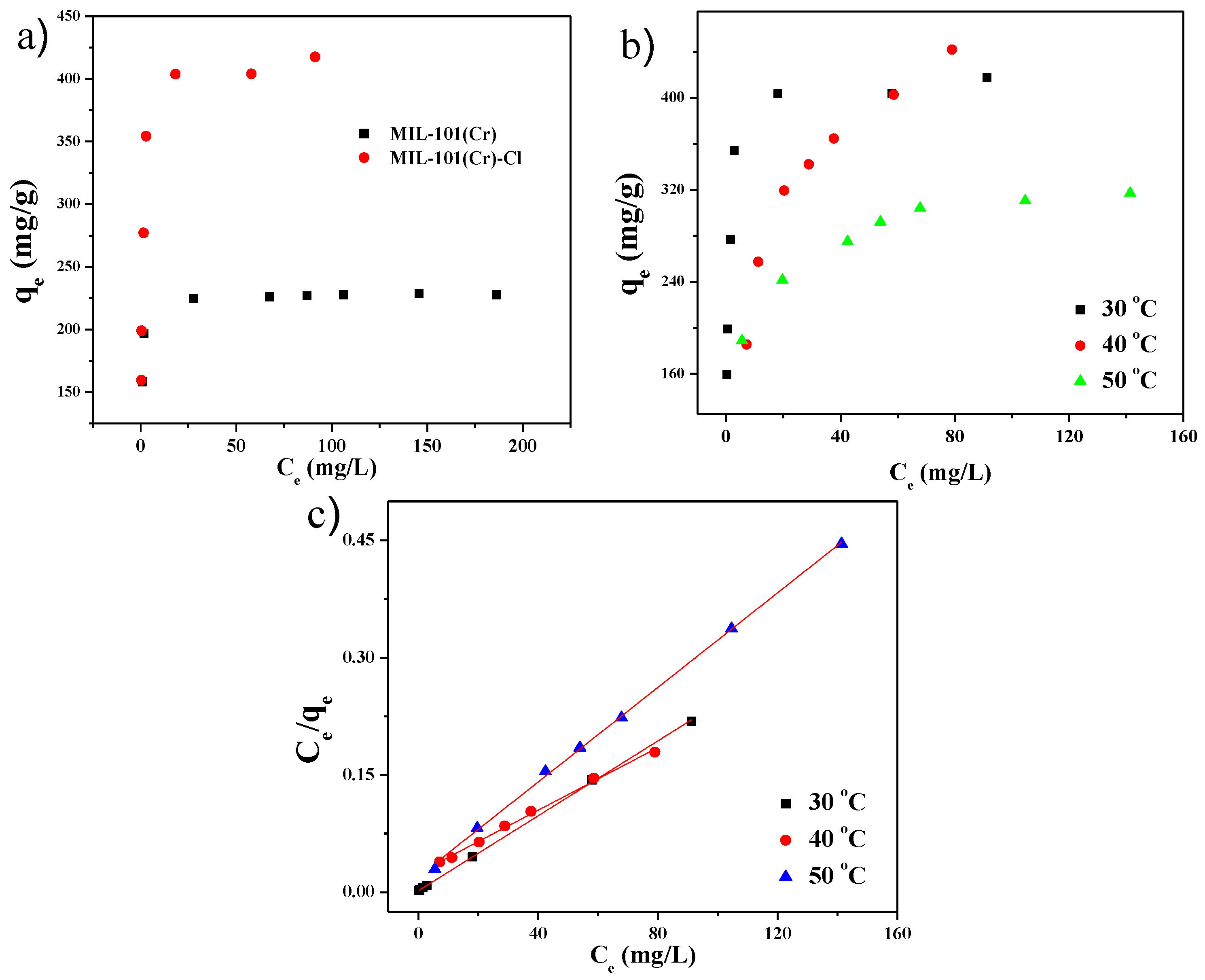
2.3. Adsorptive Isotherms
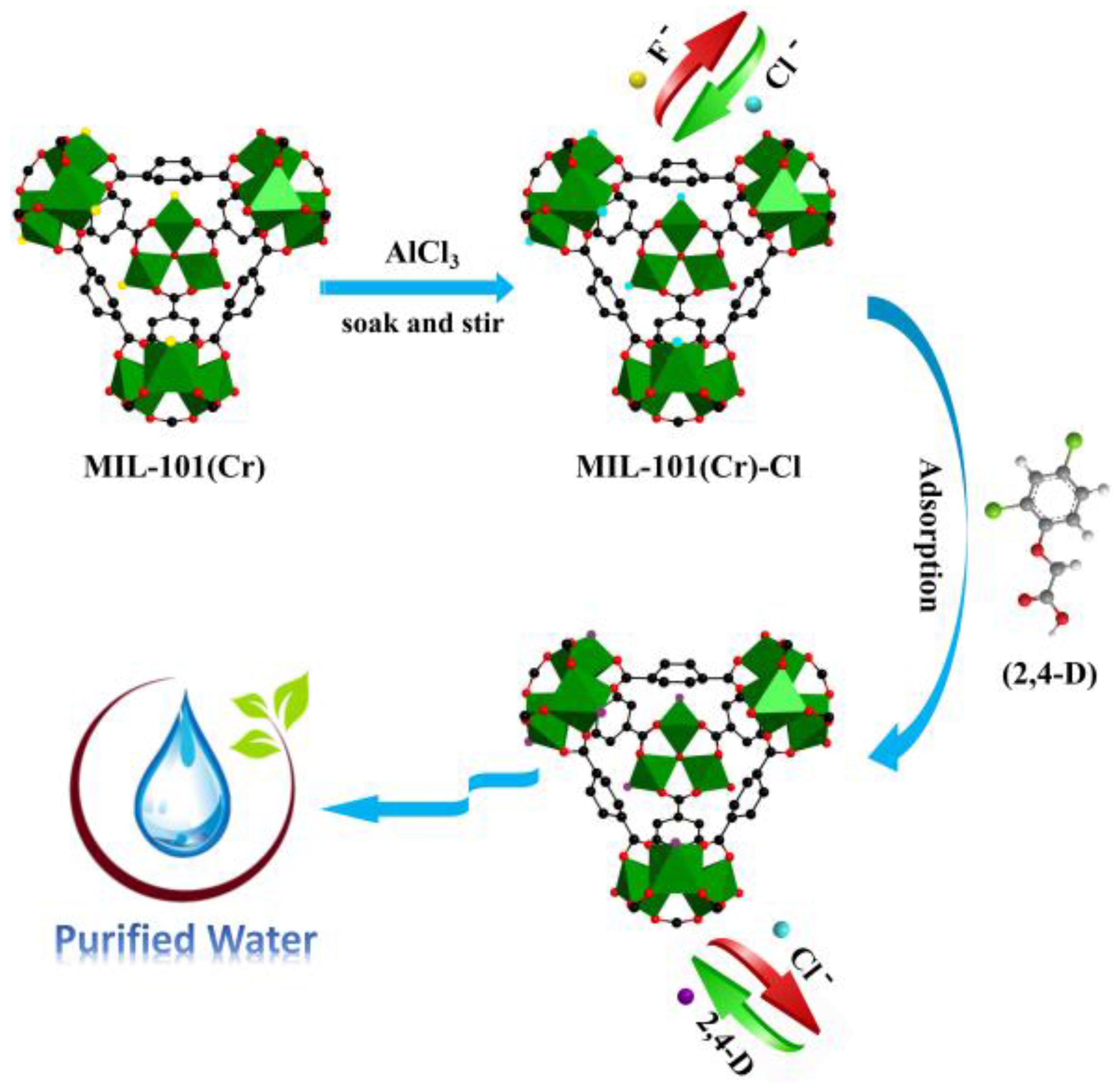
2.4. Adsorption Mechanism
2.5. Regeneration
3. Materials and Methods
3.1. Characterization of As-Prepared Materials
3.2. Apparatus
3.3. Synthesis of MIL-101(Cr)
3.4. Anion Stripping of MIL-101(Cr)
3.5. Adsorption Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, A.B.; Jibril, B.; Danwittayakulb, S.; Dutta, J. Microwave-enhanced degradation of phenol over Ni-loaded ZnO nanorods catalyst. Appl. Catal. B Environ. 2014, 156, 456–465. [Google Scholar] [CrossRef]
- Daneshvar, N.; Khataee, A.R.; Rasoulifard, M.H.; Pourhassan, M. Biodegradation of dye solution containing Malachite Green: Optimization of effective parameters using Taguchi method. J. Hazard. Mater. 2007, 143, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Cao, C.; Huang, P.; Song, W. A new ion exchange adsorption mechanism between carbonate groups and fluoride ions of basic aluminum carbonate nanospheres. RSC Adv. 2015, 5, 13256–13260. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, M.; Li, J.; Li, J.; Xu, J.; Wang, X. Porous magnetic carbon sheets from biomass as an adsorbent for the fast removal of organic pollutants from aqueous solution. J. Mater. Chem. A 2014, 2, 4391–4397. [Google Scholar] [CrossRef]
- Nakamura, H.; Okumura, M.; Machida, M. Monte Carlo simulation studies of cation selectivity in ion exchange of zeolites. RSC Adv. 2014, 4, 52757–52761. [Google Scholar] [CrossRef]
- Peng, F.; Luo, T.; Yuan, Y. Controllable synthesis of Mg-Fe layered double hydroxide nanoplates with specific Mg/Fe ratios and their effect on adsorption of As(V) from water. New J. Chem. 2014, 38, 4427–4433. [Google Scholar] [CrossRef]
- Moya, A.A. Theory of the formation of the electric double layer at the ion exchange membrane-solution interface. Phys. Chem. Chem. Phys. 2015, 17, 5207–5218. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Kumar, M.; Tripathi, B.P.; Shahi, V.K. Sol-gel derived poly (vinylalcohol)-3-(2-aminoethylamino) propyl trimethoxysilane: Cross-linked organic-inorganic hybrid beads for the removal of Pb(II) from aqueous solution. Chem. Eng. J. 2010, 162, 28–36. [Google Scholar] [CrossRef]
- Chen, J.-J.; Chen, Y.-T.; Raja, D.S.; Kang, Y.-H.; Tseng, P.-C. Metal-Organic Frameworks to Metal/Metal Oxide Embedded Carbon Matrix: Synthesis, Characterization and Gas Sorption Properties. Materials 2015, 8, 5336–5347. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Franco, A.M.M. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron-Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhan, Z.B.; Li, Z.; Di, L.B. Thermal Activation of CuBTC MOF for CO Oxidation: The Effect of Activation Atmosphere. Catalysts 2017, 7, 106. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, J.; Liu, M.; Liu, A.; Dou, Z.; Yang, Y. A low cytotoxic cationic metal-organic framework carrier for controllable drug release. J. Med. Chem. 2014, 57, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Dincă, M. Metal-organic frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.M.; Barbosa, A.D.S.; Pires, J.; Balula, S.S.; Cunha-Silva, L.; Freire, C. Novel composite material polyoxovanadate@MIL-101(Cr): A highly efficient electrocatalyst for ascorbic acid oxidation. ACS Appl. Mater. Interfaces 2013, 5, 13382–13390. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.R.; Xu, Z.X.; Zhang, J. Water-stable metal-organic frameworks for fast and high dichromate trapping via single-crystal-to-single-crystal ion exchange. Chem. Mater. 2014, 27, 205–210. [Google Scholar] [CrossRef]
- Tan, Y.X.; Zhang, Y.; He, Y.P.; Zheng, Y.J.; Zhang, J. Multifunctional anionic MOF material for dye enrichment and selective sorption of C2 hydrocarbons over methane via Ag+-Exchange. Inorg. Chem. 2014, 53, 12973–12976. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Khani, S.K.; Akhbari, K.; Morsali, A.; Retailleau, P. Achieve to easier opening of channels in anionic nanoporous Metal-organic framework by cation exchange process. Microporous Mesoporous Mater. 2014, 199, 93–98. [Google Scholar] [CrossRef]
- Nanthamathee, C.; Ling, S.; Slater, B.; Attfield, M.P. Contradistinct thermoresponsive behavior of isostructural MIL-53 type Metal-organic frameworks by modifying the framework inorganic anion. Chem. Mater. 2015, 27, 85–95. [Google Scholar] [CrossRef]
- Ding, L.; Lu, X.; Deng, H.; Zhang, X. Adsorptive removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solutions using MIEX resin. Ind. Eng. Chem. Res. 2012, 51, 11226–11235. [Google Scholar] [CrossRef]
- Bakhtiary, S.; Shirvani, M.; Shariatmadari, H. Adsorption-desorption behavior of 2,4-D on NCP-modified bentonite and zeolite: Implications for slow-release herbicide formulations. Chemosphere 2013, 90, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Njoku, V.O.; Foo, K.Y.; Hameed, B.H. Microwave-assisted preparation of pumpkin seed hull activated carbon and its application for the adsorptive removal of 2,4-dichlorophenoxyacetic acid. Chem. Eng. J. 2013, 215, 383–388. [Google Scholar] [CrossRef]
- Nejati, K.; Davary, S.; Saati, M. Study of 2,4-dichlorophenoxyacetic acid (2,4-D) removal by Cu-Fe-layered double hydroxide from aqueous solution. Appl. Surf. Sci. 2013, 280, 67–73. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, C.; Zhang, X.; Zhou, H.; Li, H.; Zhu, X.; Wang, Y. A novel molecularly imprinted material based on magnetic halloysite nanotubes for rapid enrichment of 2,4-dichlorophenoxyacetic acid in water. J. Hazard. Mater. 2014, 276, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, W.; Luo, S.; Luo, Y. White fungus-like mesoporous Bi2S3 ball/TiO2 heterojunction with high photocatalytic efficiency in purifying 2,4-dichlorophenoxyacetic acid/Cr(VI) contaminated water. Appl. Catal. B Environ. 2014, 156, 25–34. [Google Scholar] [CrossRef]
- Jung, B.K.; Hasan, Z.; Jhung, S.H. Adsorptive removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from water with a metal-organic framework. Chem. Eng. J. 2013, 234, 99–105. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Sharma, P.; Sharma, M.; Tomar, R. Surfactant modified tectosilicates and phyllosilicates for 2,4-D removal and slow release formulation. RSC Adv. 2014, 4, 4504–4514. [Google Scholar] [CrossRef]
- Hong, D.Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.-S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Mao, C.; Kudla, R.A.; Zuo, F.; Zhao, X.; Mueller, L.J.; Bu, X.; Feng, P. Anion stripping as a general method to create cationic porous framework with mobile anions. J. Am. Chem. Soc. 2014, 136, 7579–7582. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Yan, X. Metal-organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J. Mater. Chem. 2012, 22, 7449–7455. [Google Scholar] [CrossRef]
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Meng, Y.; Gao, F.; Jiao, S.; Ke, Y. Fast syntheses of MOFs using nanosized zeolite crystal seeds in situ generated from microsized zeolites. Cryst. Growth Des. 2013, 13, 2697–2702. [Google Scholar] [CrossRef]
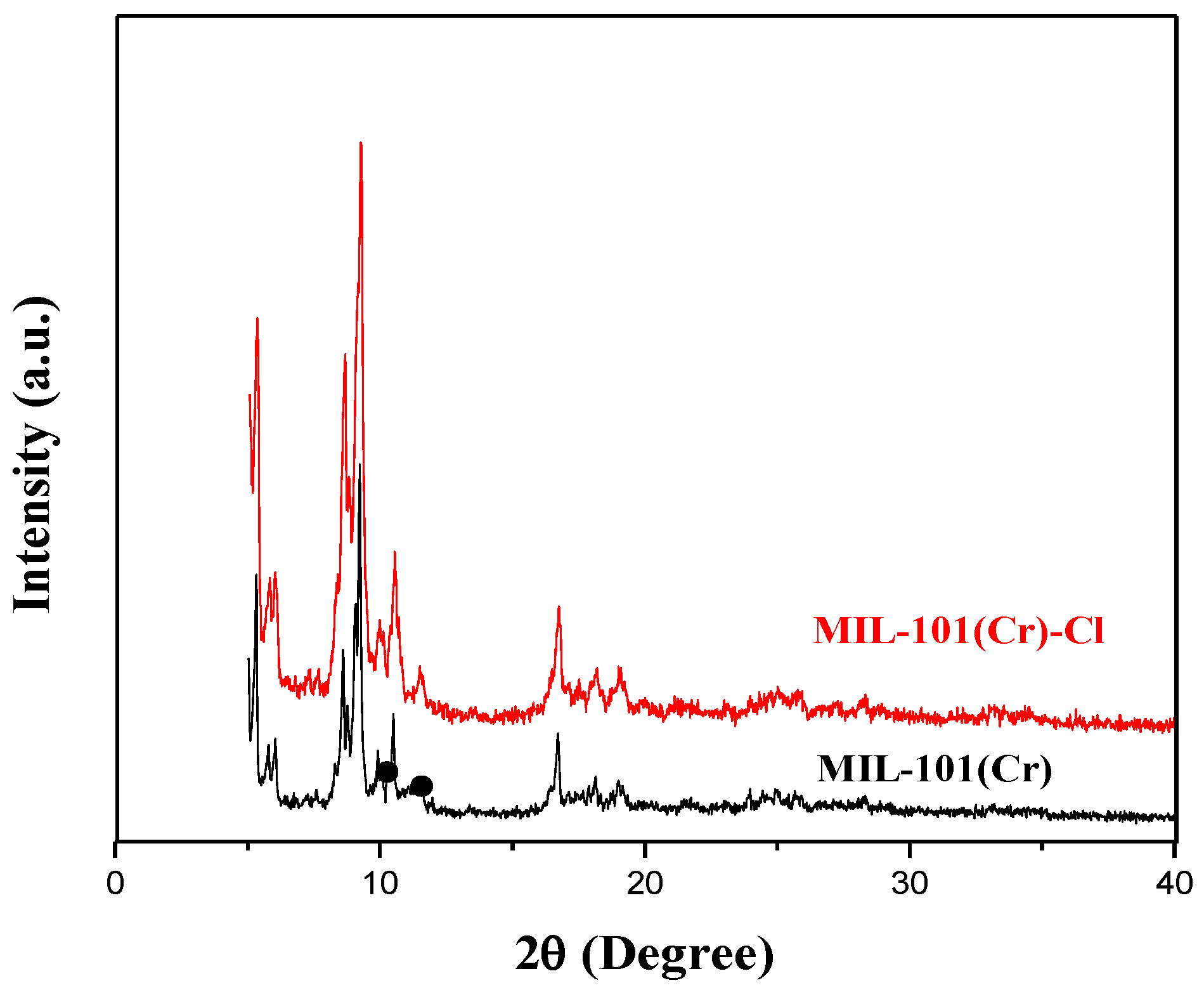
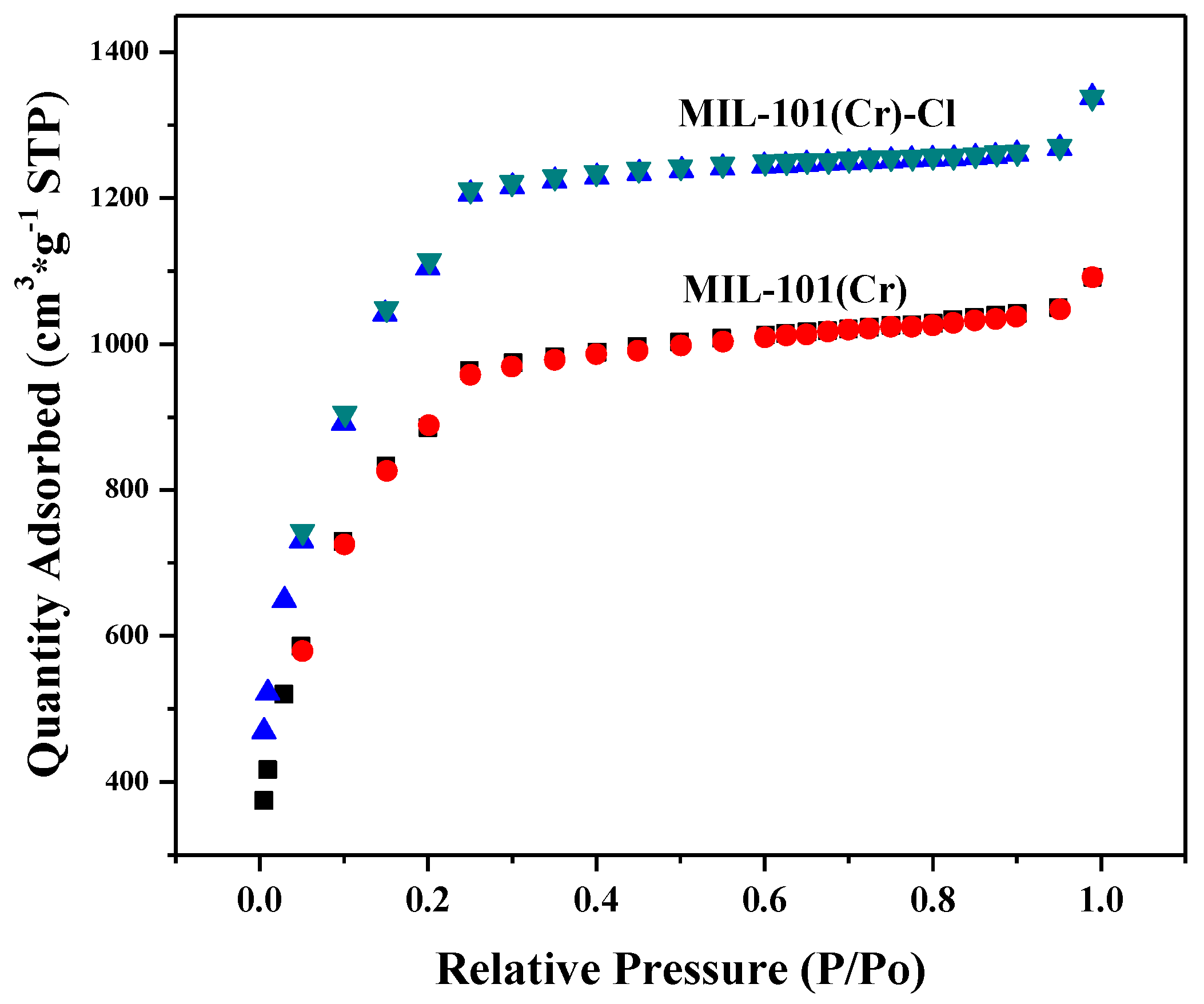
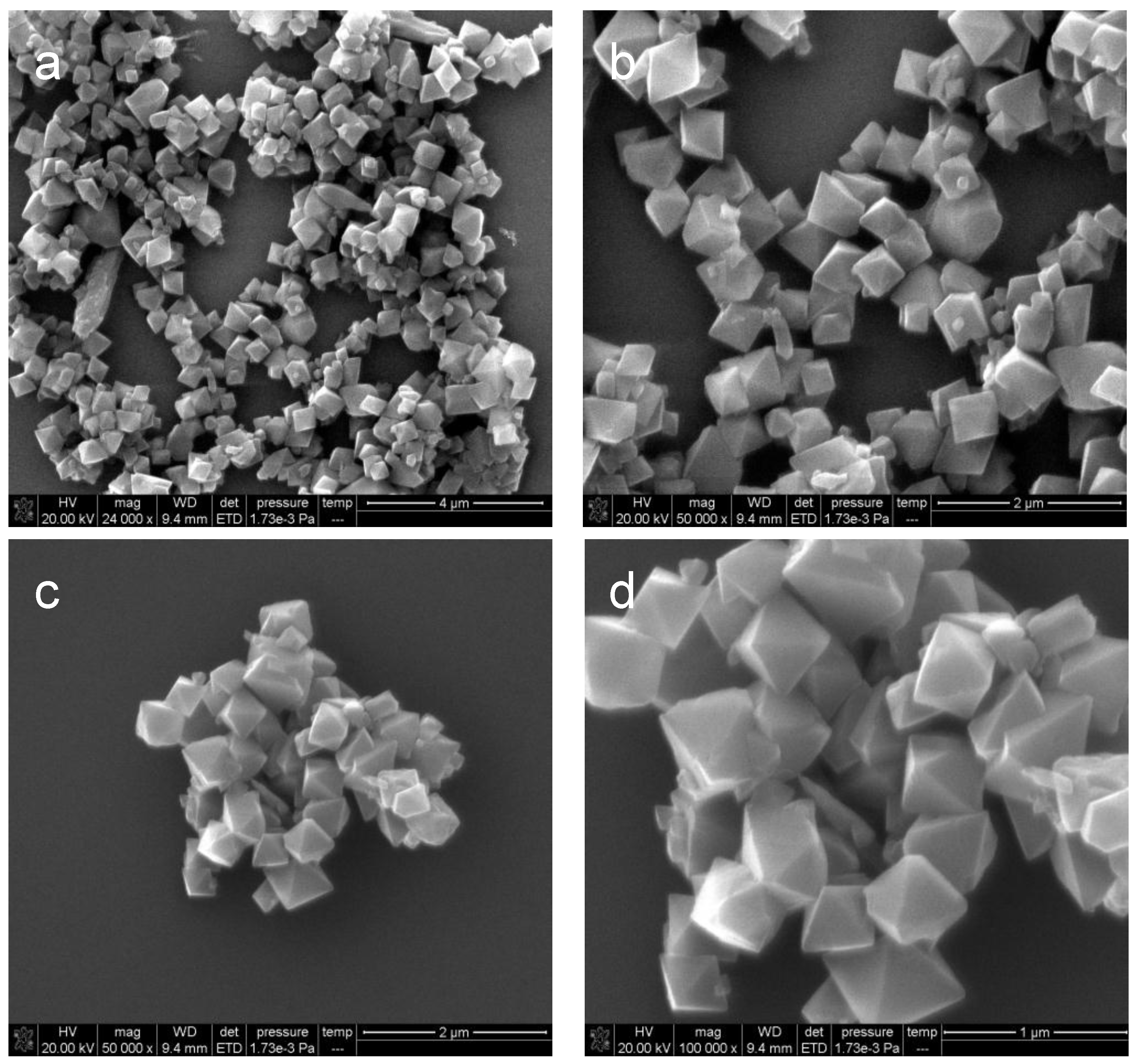
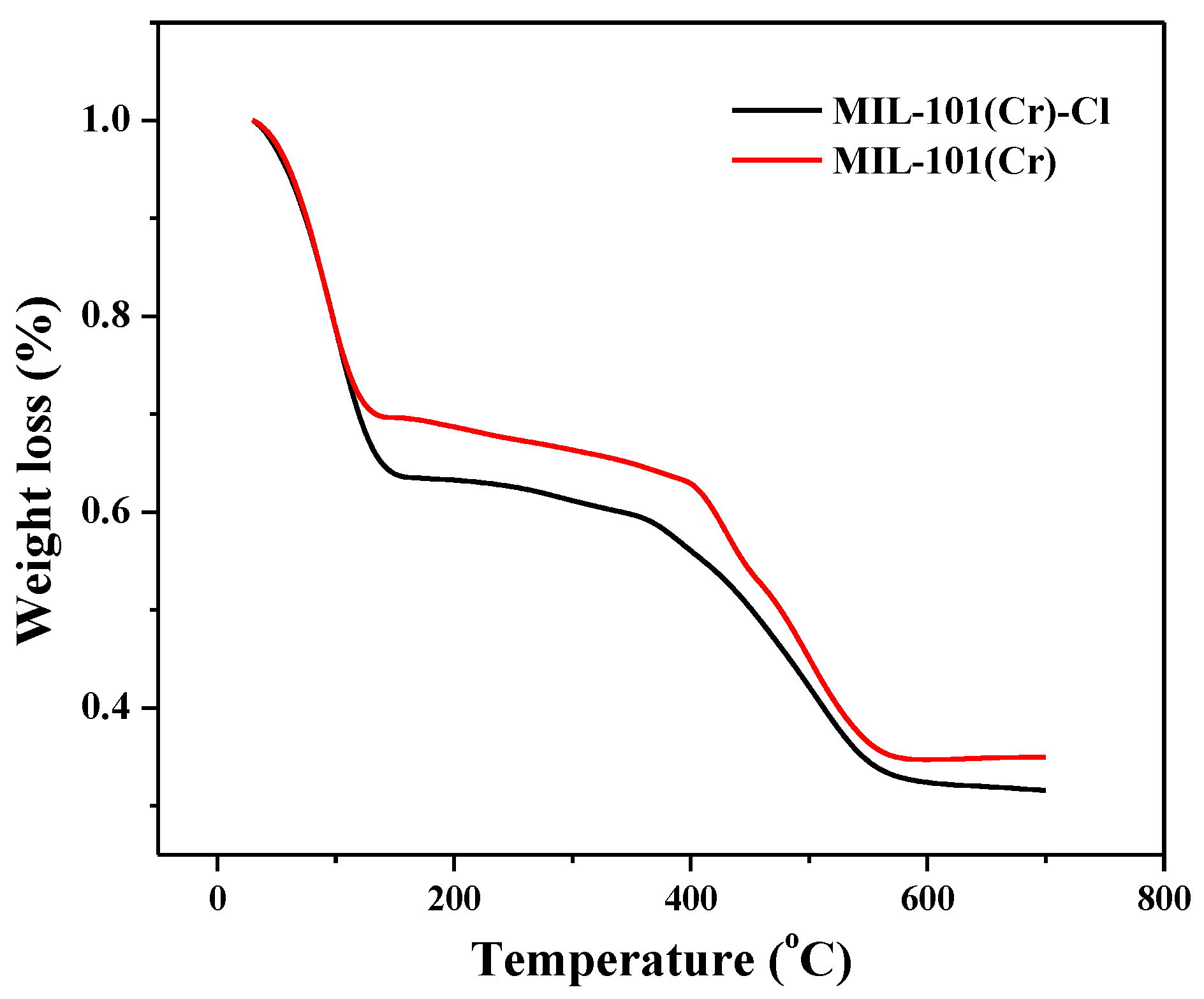

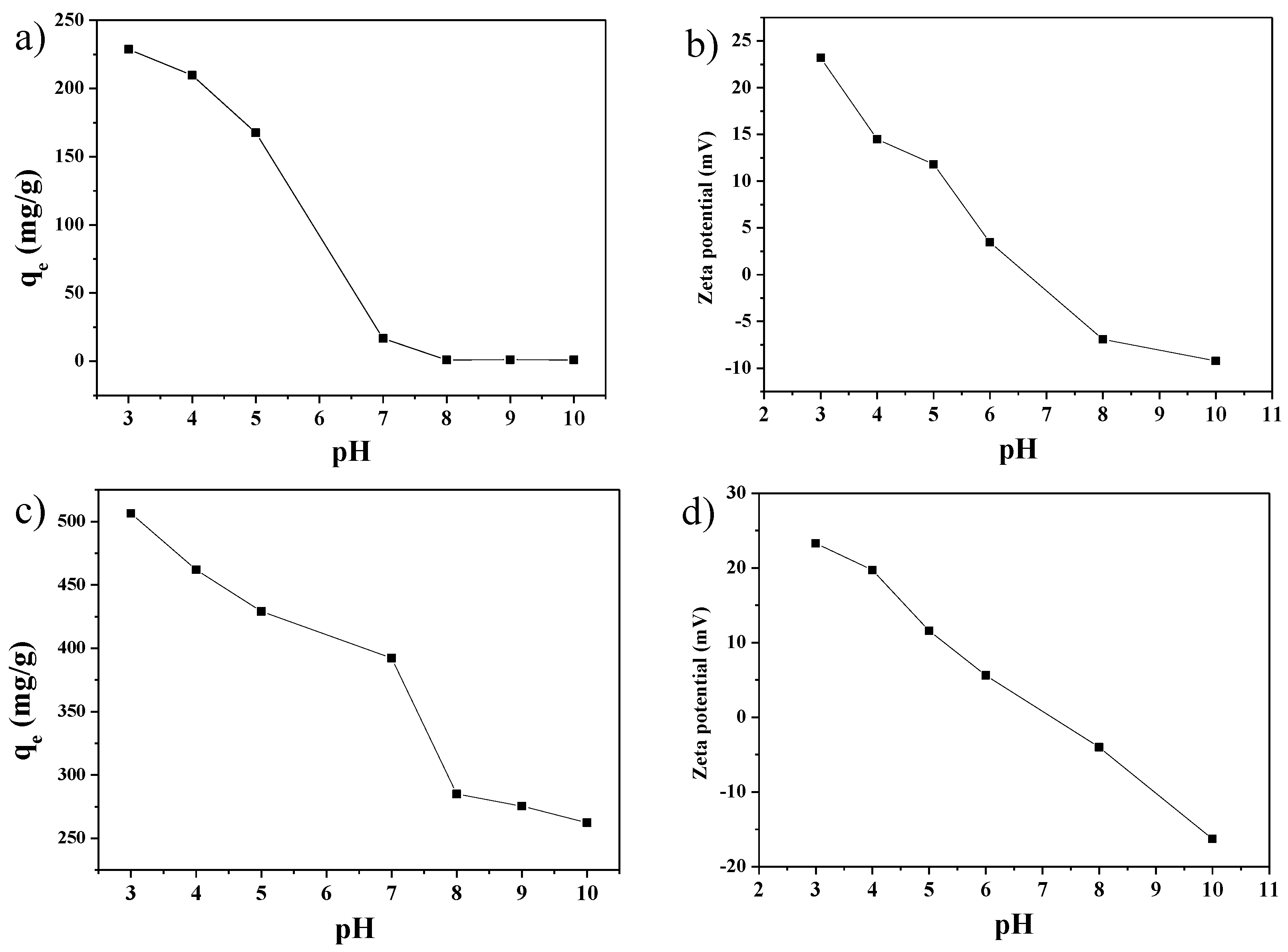

| Sample | SBET a (m2/g) | Vp b (cm3/g) | Dp c (nm) |
|---|---|---|---|
| MIL-101(Cr) | 3133.7 | 1.688 | 3.06 |
| MIL-101(Cr)-Cl | 3931.8 | 2.070 | 3.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Zhang, C.; Qin, Y.; Yang, H.; Zhang, P.; Ye, F. Preparation of Cationic MOFs with Mobile Anions by Anion Stripping to Remove 2,4-D from Water. Materials 2017, 10, 879. https://doi.org/10.3390/ma10080879
Chen T, Zhang C, Qin Y, Yang H, Zhang P, Ye F. Preparation of Cationic MOFs with Mobile Anions by Anion Stripping to Remove 2,4-D from Water. Materials. 2017; 10(8):879. https://doi.org/10.3390/ma10080879
Chicago/Turabian StyleChen, Tao, Cong Zhang, Yuemei Qin, Haiguan Yang, Peng Zhang, and Fanggui Ye. 2017. "Preparation of Cationic MOFs with Mobile Anions by Anion Stripping to Remove 2,4-D from Water" Materials 10, no. 8: 879. https://doi.org/10.3390/ma10080879





