Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement
Abstract
:1. Introduction
2. Experimental Methods and Materials
2.1. Materials
2.2. Experimental Details
3. Results and Discussion
3.1. Isothermal Conduction Calorimetry
3.2. Compressive Strength Tests
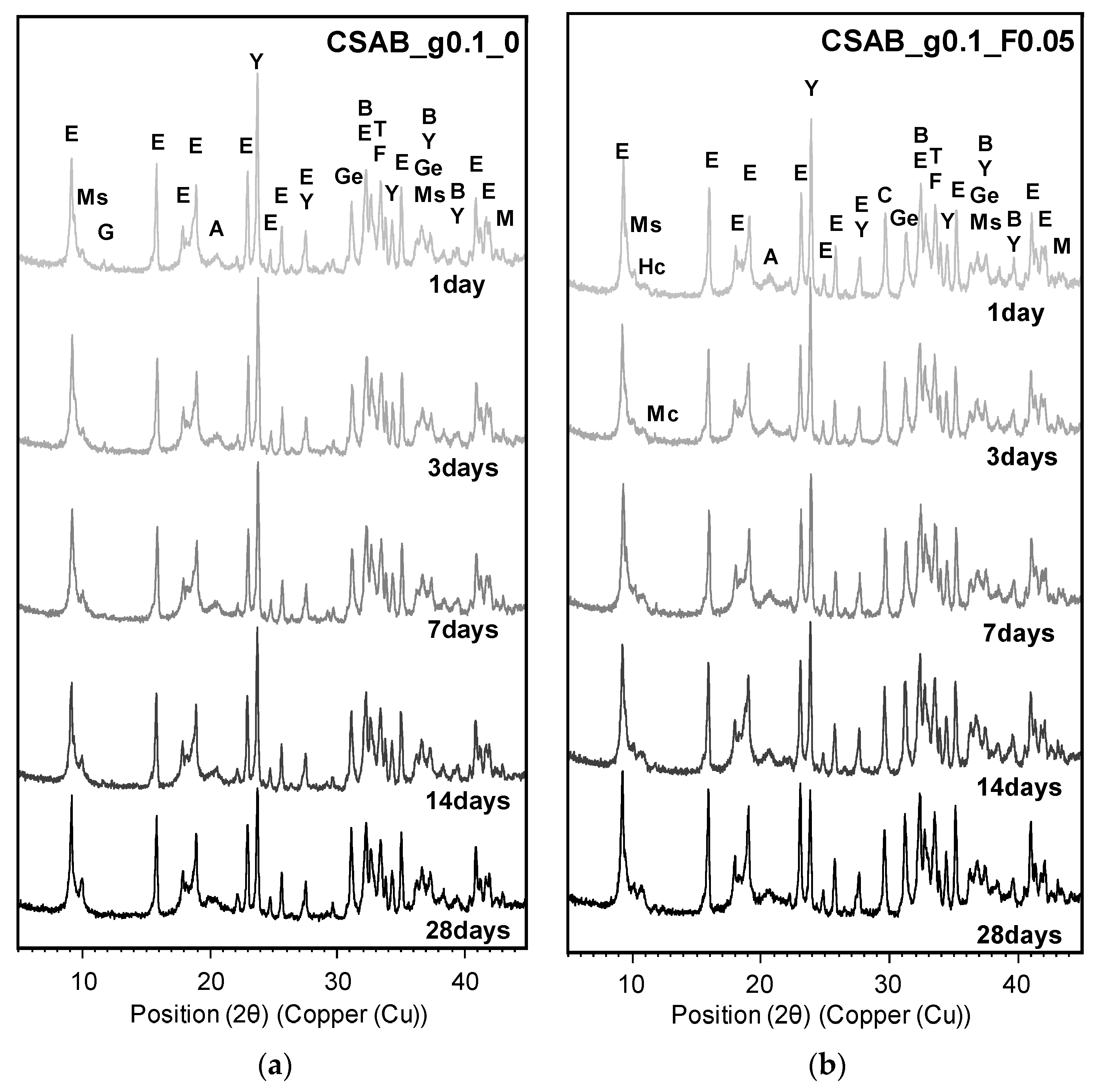
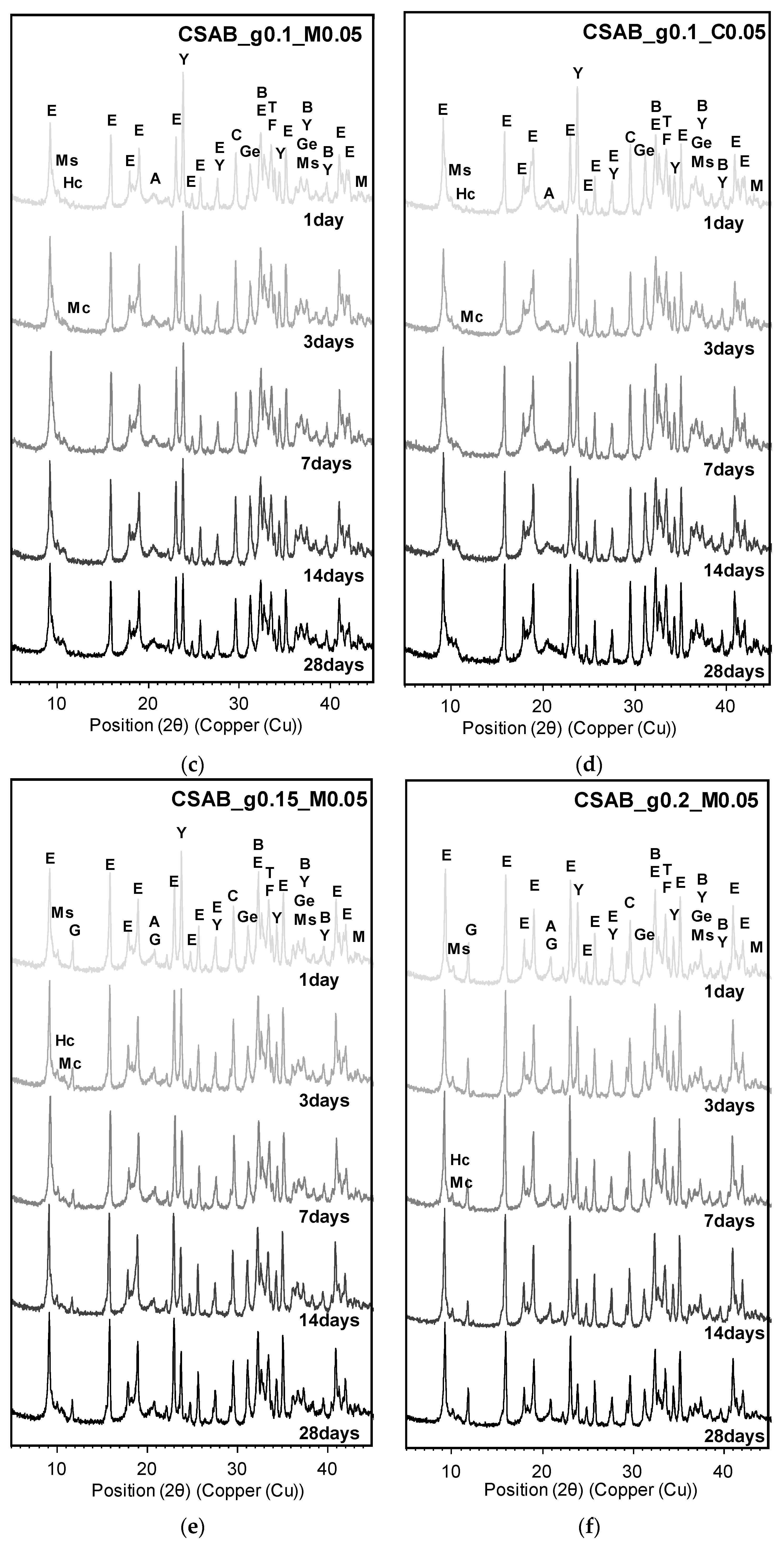
3.3. Powder X-ray Diffraction Analysis
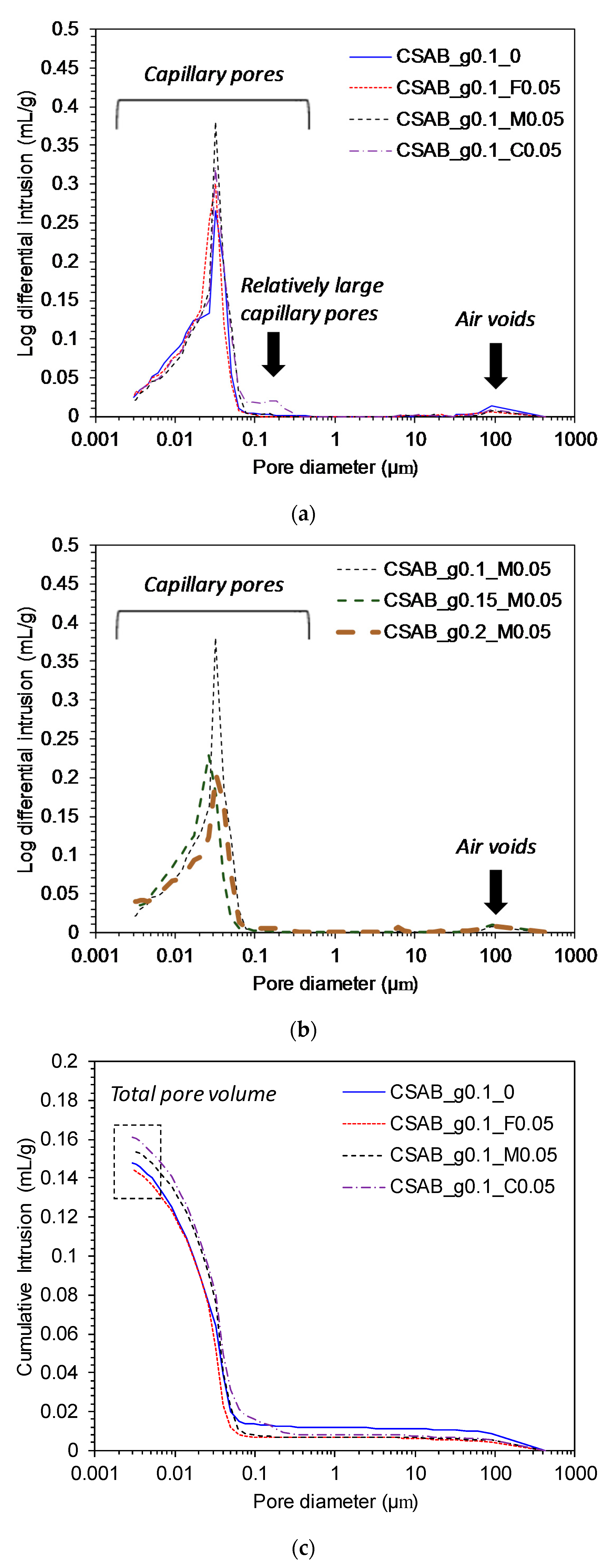
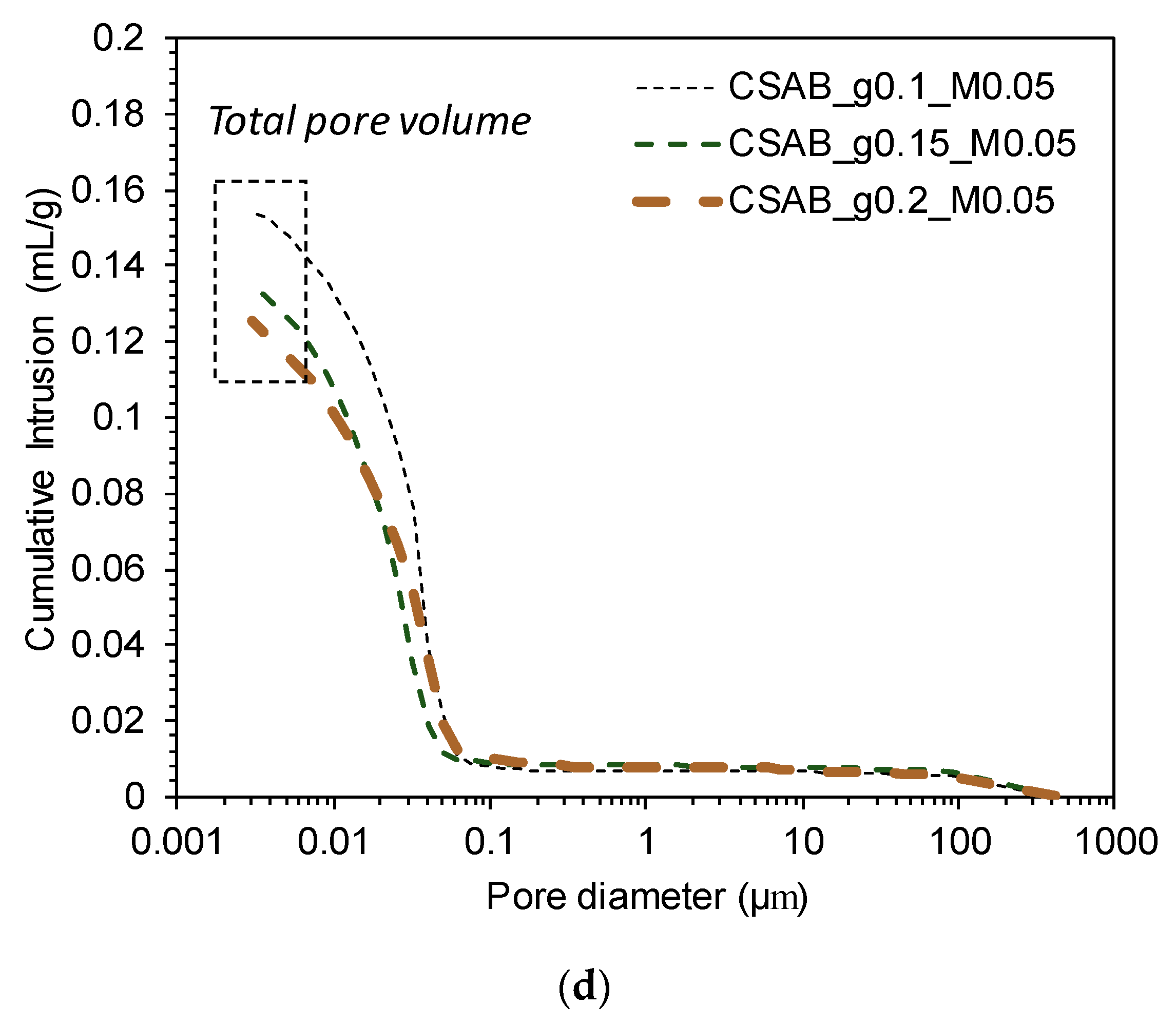
3.4. Mercury Intrusion Porosimetry
3.5. Modeling of Cement Hydration
4. Conclusions
- The presence of calcium carbonate powder accelerated the hydration of calcium sulfoaluminate-belite cement regardless of its fineness. The degree of acceleration became larger with increasing fineness of the calcium carbonate powder due to increased surface area of finer CC powder providing more nucleation sites for hydration product to form. On the other hand, increased gypsum content delayed the hydration at early ages.
- The use of calcium carbonate powder having a similar fineness as the clinker decreased the total heat of hydration released due to the reduction in anhydrous clinker phases (dilution effect). However, finer calcium carbonate powder compensated for the dilution effect by accelerating the hydration through increased nucleation sites, as evidenced by the pastes’ increased total heat of hydration and acceleration of the second peak in heat evolution.
- The calcium carbonate powders took part in the hydration of calcium sulfoaluminate-belite cement regardless of their fineness and gypsum content, forming hemicarboaluminate and monocarboaluminate, which could potentially decrease the paste’s porosity and increase its strength with additional ettringite formation.
- Nevertheless, the strength improvement was only observed in the cases where finer calcium carbonates than the clinker were used. This implies that the positive influence of forming hemicarboaluminate and/or monocarboaluminate with additional ettringite might be diluted by the reduction in clinker components. The use of finer calcium carbonate provides more surface area for nucleation at early ages and faster dissolution due to more surface area at later ages, helping it to overcome the negative effect of diluting the clinker phases.
- Increased gypsum content contributed to strength improvement while total early-age heat of hydration decreased due to the dilution of the clinker phases.
- The compressive strength of the pastes showed a clear inverse relationship with the total pore volumes, indicating that total pore volume was a key factor for determining the strength of calcium sulfoaluminate-belite cements.
- Thermodynamic modeling of calcium sulfoaluminate-belite cement hydration depending on varying amounts of calcium carbonate powder presented that the total solid volume of the cement increased with increasing calcium carbonate powder up to 6 wt %, implying that calcium carbonate amounts used in this study were possibly helpful to improve the strength and reduce the pore volume of the cements. Nevertheless, as confirmed by conducted experiments, only finer powders contributed to improving the compressive strength and reducing the pore volume.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharp, J.H.; Lawrence, C.D.; Yang, R. Calcium sulfoaluminate cements—Low-energy cements, special cements or what? Adv. Cem. Res. 1999, 11, 3–13. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate—Belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C.G. Understanding expansion in calcium sulfoaluminate—Belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Beltagui, H.; Jen, G.; Whittaker, M.; Imbabi, M.S. The influence of variable gypsum and water content on the strength and hydration of a belite-calcium sulphoaluminate cement. Adv. Appl. Ceram. 2017, 116, 199–206. [Google Scholar] [CrossRef]
- Zhang, L. Microstructure and Performance of Calcium Sulfoaluminate Cements. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2000. [Google Scholar]
- Berger, S.; Coumes, C.C.D.; Le Bescop, P.; Damidot, D. Influence of a thermal cycle at early age on the hydration of calcium sulphoaluminate cements with variable gypsum contents. Cem. Concr. Res. 2011, 41, 149–160. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Compos. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial use of limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Aranda, M.A.G.; Santacruz, I. Hydration studies of calcium sulfoaluminate cements blended with fly ash. Cem. Concr. Res. 2013, 54, 12–20. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Chaunsali, P.; Mondal, P. Influence of calcium sulfoaluminate (CSA) cement content on expansion and hydration behavior of various ordinary Portland cement-CSA blends. J. Am. Ceram. Soc. 2015, 98, 2617–2624. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Mehta, P.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2006. [Google Scholar]
- Aqel, M.; Panesar, D.K. Hydration kinetics and compressive strength of steam-cured cement pastes and mortars containing limestone filler. Constr. Build. Mater. 2016, 113, 359–368. [Google Scholar] [CrossRef]
- Courard, L.; Michel, F.; Perkowicz, S.; Garbacz, A. Effects of limestone fillers on surface free energy and electrical conductivity of the interstitial solution of cement mixes. Cem. Concr. Compos. 2014, 45, 111–116. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Li, L.G.; Kwan, A.K.H. Adding limestone fines as cementitious paste replacement to improve tensile strength, stiffness and durability of concrete. Cem. Concr. Compos. 2015, 60, 17–24. [Google Scholar] [CrossRef]
- Lollini, F.; Redaelli, E.; Bertolini, L. Effects of portland cement replacement with limestone on the properties of hardened concrete. Cem. Concr. Compos. 2014, 46, 32–40. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Mounanga, P.; Khokhar, M.I.A.; El Hachem, R.; Loukili, A. Improvement of the early-age reactivity of fly ash and blast furnace slag cementitious systems using limestone filler. Mater. Struct. 2011, 44, 437–453. [Google Scholar] [CrossRef]
- Oey, T.; Kumar, A.; Bullard, J.W.; Neithalath, N.; Sant, G. The filler effect: The influence of filler content and surface area on cementitious reaction rates. J. Am. Ceram. Soc. 2013, 96, 1978–1990. [Google Scholar] [CrossRef]
- Kadri, E.H.; Aggoun, S.; De Schutter, G.; Ezziane, K. Combined effect of chemical nature and fineness of mineral powders on Portland cement hydration. Mater. Struct. 2010, 43, 665–673. [Google Scholar] [CrossRef]
- Zajac, M.; Rossberg, A.; Le Saout, G.; Lothenbach, B. Influence of limestone and anhydrite on the hydration of Portland cements. Cem. Concr. Compos. 2014, 46, 99–108. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Ramezanianpour, A.M.; Hooton, R.D. A study on hydration, compressive strength, and porosity of Portland-limestone cement mixes containing SCMs. Cem. Concr. Compos. 2014, 51, 1–13. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Properties of alkali activated slag-fly ash blends with limestone addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Pelletier, L.; Winnefeld, F.; Lothenbach, B. The ternary system Portland cement-calcium sulphoaluminate clinker-anhydrite: Hydration mechanism and mortar properties. Cem. Concr. Compos. 2010, 32, 497–507. [Google Scholar] [CrossRef]
- Hargis, C.W.; Moon, J.; Lothenbach, B.; Winnefeld, F.; Wenk, H.-R.; Monteiro, P.J.M. Calcium sulfoaluminate sodalite (Ca4Al6O12SO4) crystal structure evaluation and bulk modulus determination. J. Am. Ceram. Soc. 2014, 97, 892–898. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- PANalytical. X’Pert HighScore Plus, version 3.0.5; PANalytical: Almelo, The Netherlands, 2012.
- Allmann, R.; Hinek, R. The introduction of structure types into the Inorganic Crystal Structure Database ICSD. Acta Crystallogr. A 2007, 63, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef]
- Wagner, T.; Kulik, D.A.; Hingerl, F.F.; Dmytrieva, S.V. GEM-Selektor geochemical modeling package: TSolMod library and data interface for multicomponent phase models. Can. Mineral. 2012, 50, 1173–1195. [Google Scholar] [CrossRef]
- Hummel, W.; Berner, U.; Curti, E.; Pearson, F.; Thoenen, T. Nagra/PSI chemical thermodynamic data base 01/01. Radiochim. Acta 2002, 90, 805–813. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. Thermodynamic properties of Portland cement hydrates in the system CaO-Al2O3-SiO2-CaSO4-CaCO3-H2O. Cem. Concr. Res. 2007, 37, 1379–1410. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Lothenbach, B.; Pelletier-Chaignat, L.; Winnefeld, F. Stability in the system CaO-Al2O3-H2O. Cem. Concr. Res. 2012, 42, 1621–1634. [Google Scholar] [CrossRef]
- Dilnesa, B.Z.; Lothenbach, B.; Le Saout, G.; Renaudin, G.; Mesbah, A.; Filinchuk, Y.; Wichser, A.; Wieland, E. Iron in carbonate containing AFm phases. Cem. Concr. Res. 2011, 41, 311–323. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase compositions. J. Mater. Sci. 2011, 46, 2568–2577. [Google Scholar] [CrossRef]
- Ballim, Y.; Graham, P.C. The effects of supplementary cementing materials in modifying the heat of hydration of concrete. Mater. Struct. 2009, 42, 803–811. [Google Scholar] [CrossRef]
- Cyr, M.; Lawrence, P.; Ringot, E. Efficiency of mineral admixtures in mortars: Quantification of the physical and chemical effects of fine admixtures in relation with compressive strength. Cem. Concr. Res. 2006, 36, 264–277. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Carbonation of calcium sulfoaluminate mortars. Cem. Concr. Compos. 2017, 80, 123–134. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.H.; Oh, J.E. Influence of slag characteristics on strength development and reaction products in a CaO-activated slag system. Cem. Concr. Compos. 2016, 72, 155–167. [Google Scholar] [CrossRef]
- Park, H.; Jeong, Y.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem. Concr. Compos. 2016, 68, 57–65. [Google Scholar] [CrossRef]
- Olson, R.A.; Neubauer, C.M.; Jennings, H.M. Damage to the pore structure of hardened portland cement paste by mercury intrusion. J. Am. Ceram. Soc. 1997, 80, 2454–2458. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Bizzozero, J.; Scrivener, K.L. Limestone reaction in calcium aluminate cement-calcium sulfate systems. Cem. Concr. Res. 2015, 76, 159–169. [Google Scholar] [CrossRef]
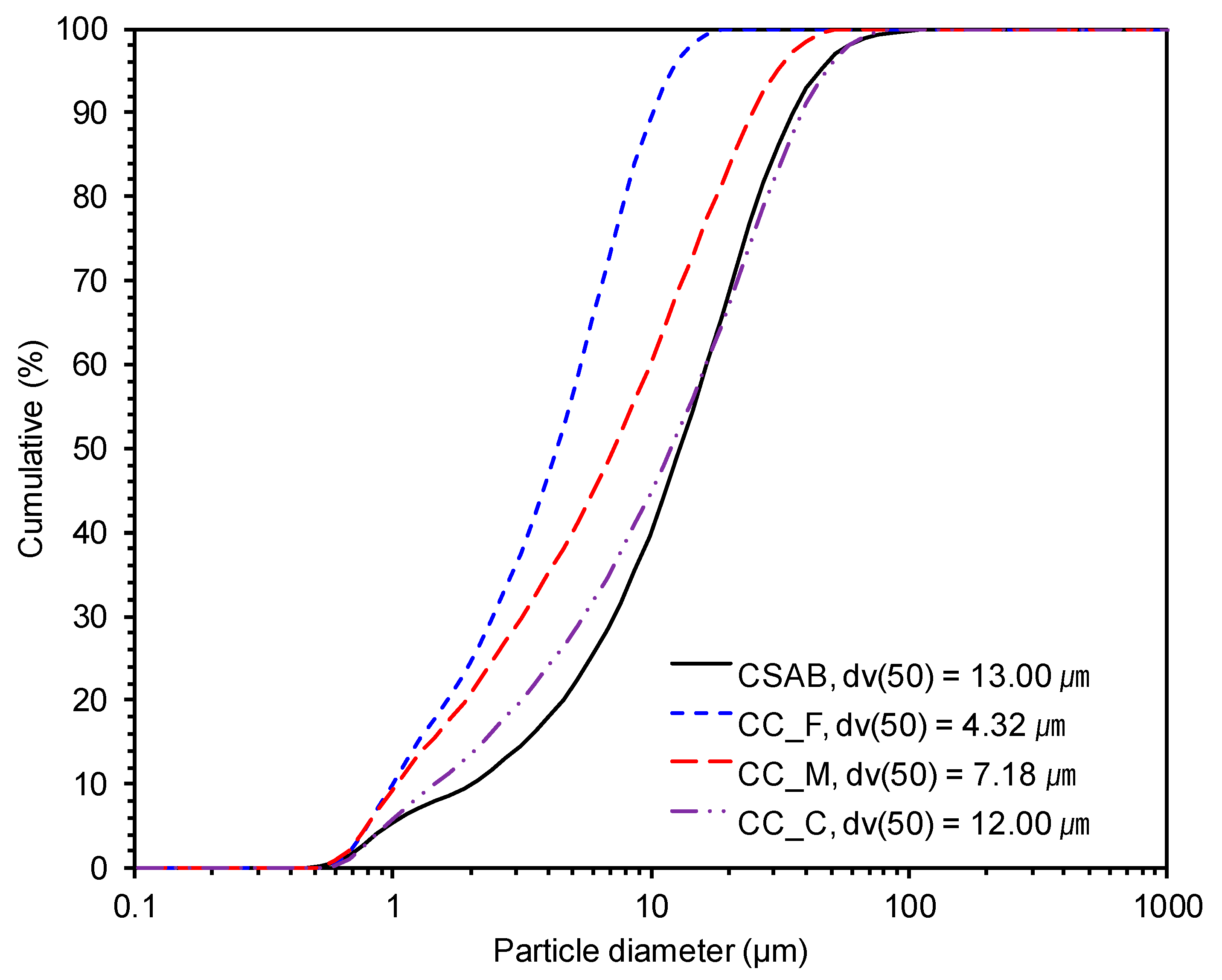
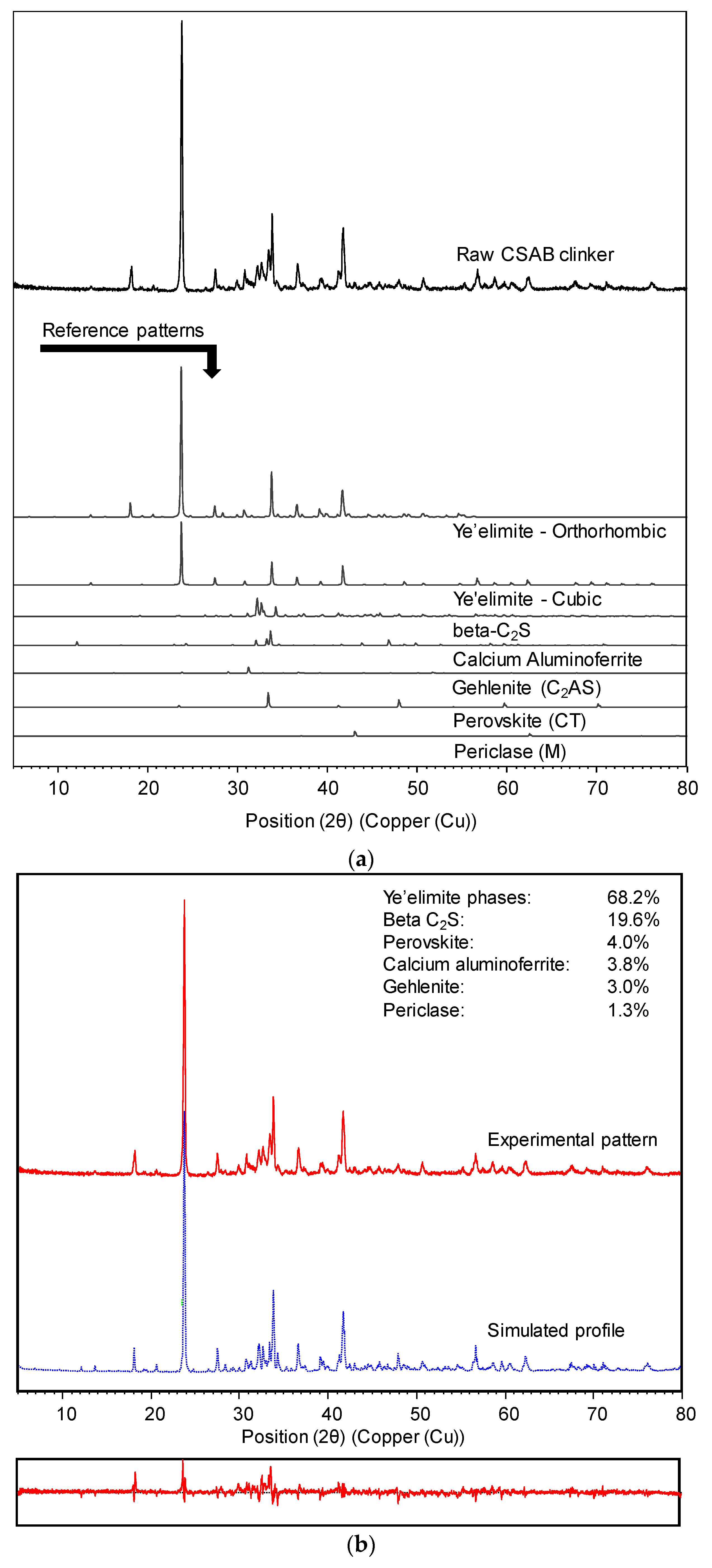
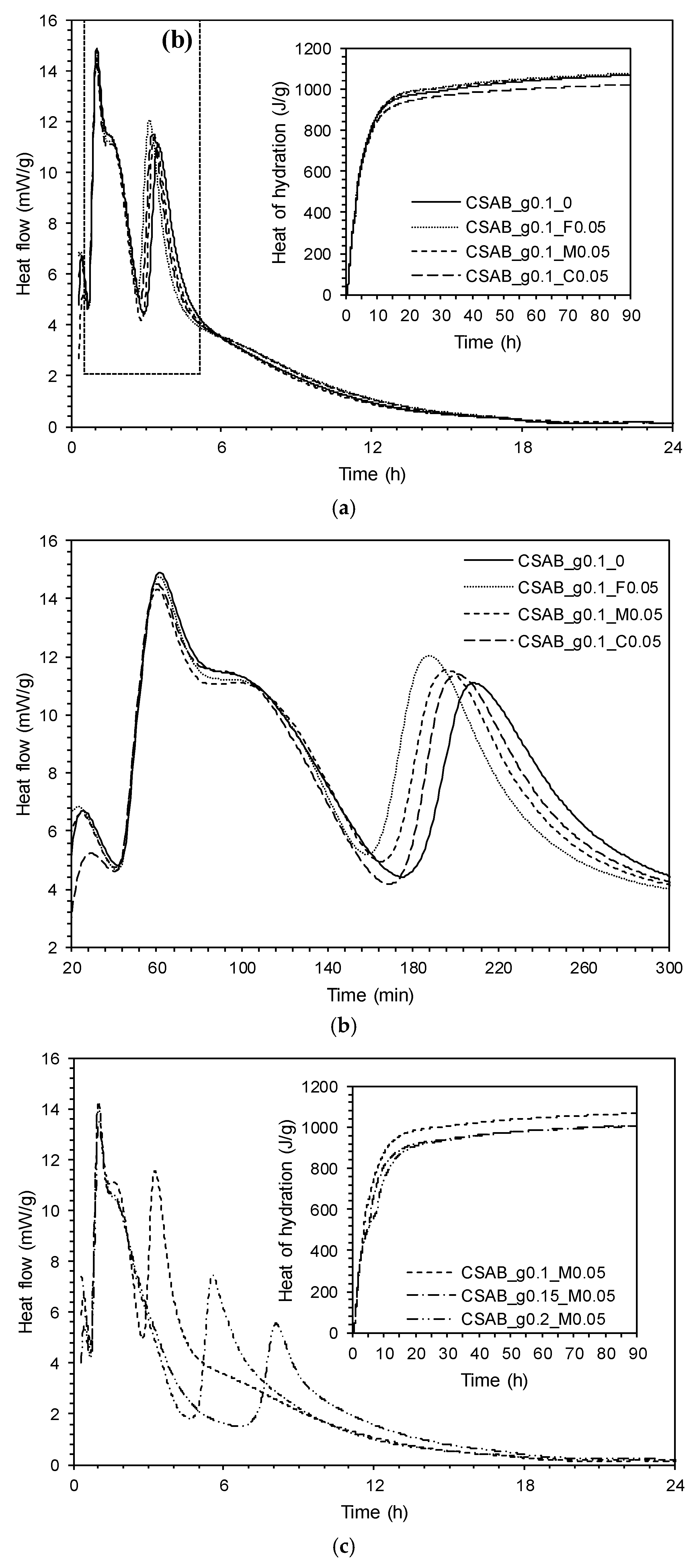
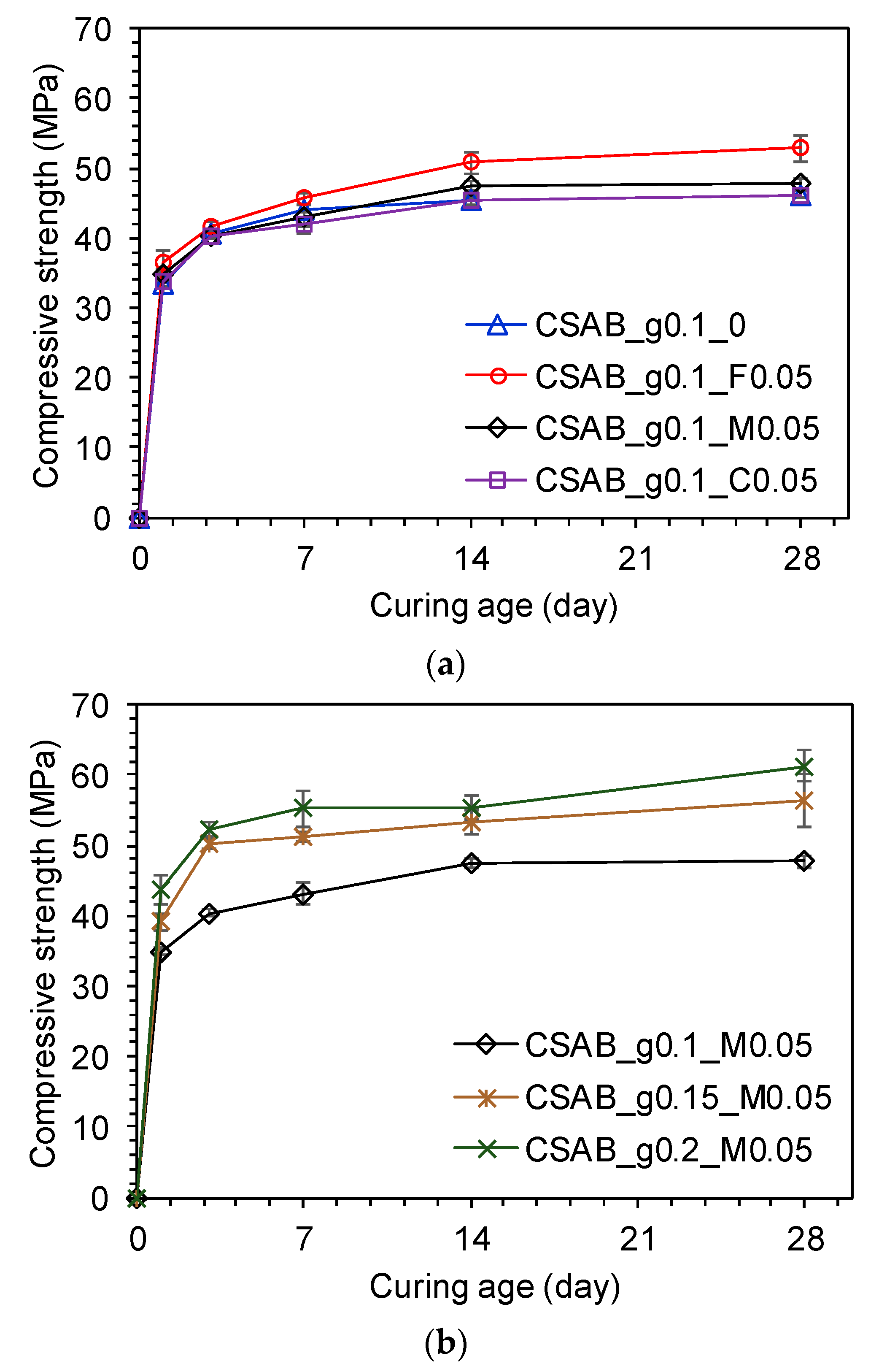


| Formula | Content (wt %) | |||
|---|---|---|---|---|
| CSAB | CC_F | CC_M | CC_C | |
| CaO | 44.6 | 57.6 | 58.7 | 55.8 |
| Al2O3 | 32.8 | N.D. | N.D. | 0.4 |
| SiO2 | 8.7 | N.D. | 0.4 | 0.3 |
| SO3 | 7.7 | N.D. | N.D. | N.D. |
| Fe2O3 | 2.1 | 0.1 | 0.1 | 0.1 |
| MgO | 2.0 | 1.8 | 2.6 | 2.6 |
| TiO2 | 1.4 | N.D. | N.D. | N.D. |
| K2O | 0.4 | N.D. | N.D. | N.D. |
| ZrO2 | 0.1 | N.D. | N.D. | N.D. |
| LOI | 0.3 | 40.3 | 37.9 | 40.8 |
| Phases | Mineral Name | Rietveld Quantification (wt %) | |||
|---|---|---|---|---|---|
| CSAB | CC_F | CC_M | CC_C | ||
| C4A3 | Ye’elimite | 68.2 | N.D. | N.D. | N.D. |
| β-C2S | Larnite | 19.6 | N.D. | N.D. | N.D. |
| C2AS | Gehlenite | 3.0 | N.D. | N.D. | N.D. |
| CT | Pervoskite | 4.0 | N.D. | N.D. | N.D. |
| M | Periclase | 1.3 | N.D. | N.D. | N.D. |
| C4AF | Brownmillerite | 3.8 | N.D. | N.D. | N.D. |
| C | Calcite | N.D. | 96.9 | 95.2 | 88.1 |
| CM2 | Dolomite | N.D. | 3.1 | 4.8 | 11.9 |
| SUM | 99.9 | 100 | 100 | 100 | |
| Label | CSAB | Gypsum | Calcium Carbonate | Water | Gypsum/Ye’elimite Molar Ratio (m) | ||
|---|---|---|---|---|---|---|---|
| CC_F | CC_M | CC_C | |||||
| CSAB_g0.1_0 | 89.5 | 10.5 | 0.0 | 0.0 | 0.0 | 50.0 | 0.65 |
| CSAB_g0.1_F0.05 | 85.0 | 10.0 | 5.0 | 0.0 | 0.0 | 50.0 | 0.65 |
| CSAB_g0.1_M0.05 | 85.0 | 10.0 | 0.0 | 5.0 | 0.0 | 50.0 | 0.65 |
| CSAB_g0.1_C0.05 | 85.0 | 10.0 | 0.0 | 0.0 | 5.0 | 50.0 | 0.65 |
| CSAB_g0.15_M0.05 | 80.0 | 15.0 | 0.0 | 5.0 | 0.0 | 50.0 | 1.04 |
| CSAB_g0.2_M0.05 | 75.0 | 20.0 | 0.0 | 5.0 | 0.0 | 50.0 | 1.47 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Hargis, C.W.; Chun, S.; Moon, J. Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement. Materials 2017, 10, 900. https://doi.org/10.3390/ma10080900
Jeong Y, Hargis CW, Chun S, Moon J. Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement. Materials. 2017; 10(8):900. https://doi.org/10.3390/ma10080900
Chicago/Turabian StyleJeong, Yeonung, Craig W. Hargis, Sungchul Chun, and Juhyuk Moon. 2017. "Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement" Materials 10, no. 8: 900. https://doi.org/10.3390/ma10080900





