Hot Press as a Sustainable Direct Recycling Technique of Aluminium: Mechanical Properties and Surface Integrity
Abstract
:1. Introduction
2. Experimental Material and Procedure
2.1. Experimental Material and Processing
2.2. Material Characterization Measurement
2.2.1. Mechanical Characterization
2.2.2. Microstructural Characterization
2.2.3. Hardness
2.2.4. Density
3. Results and Discussion
3.1. Mechanical Properties
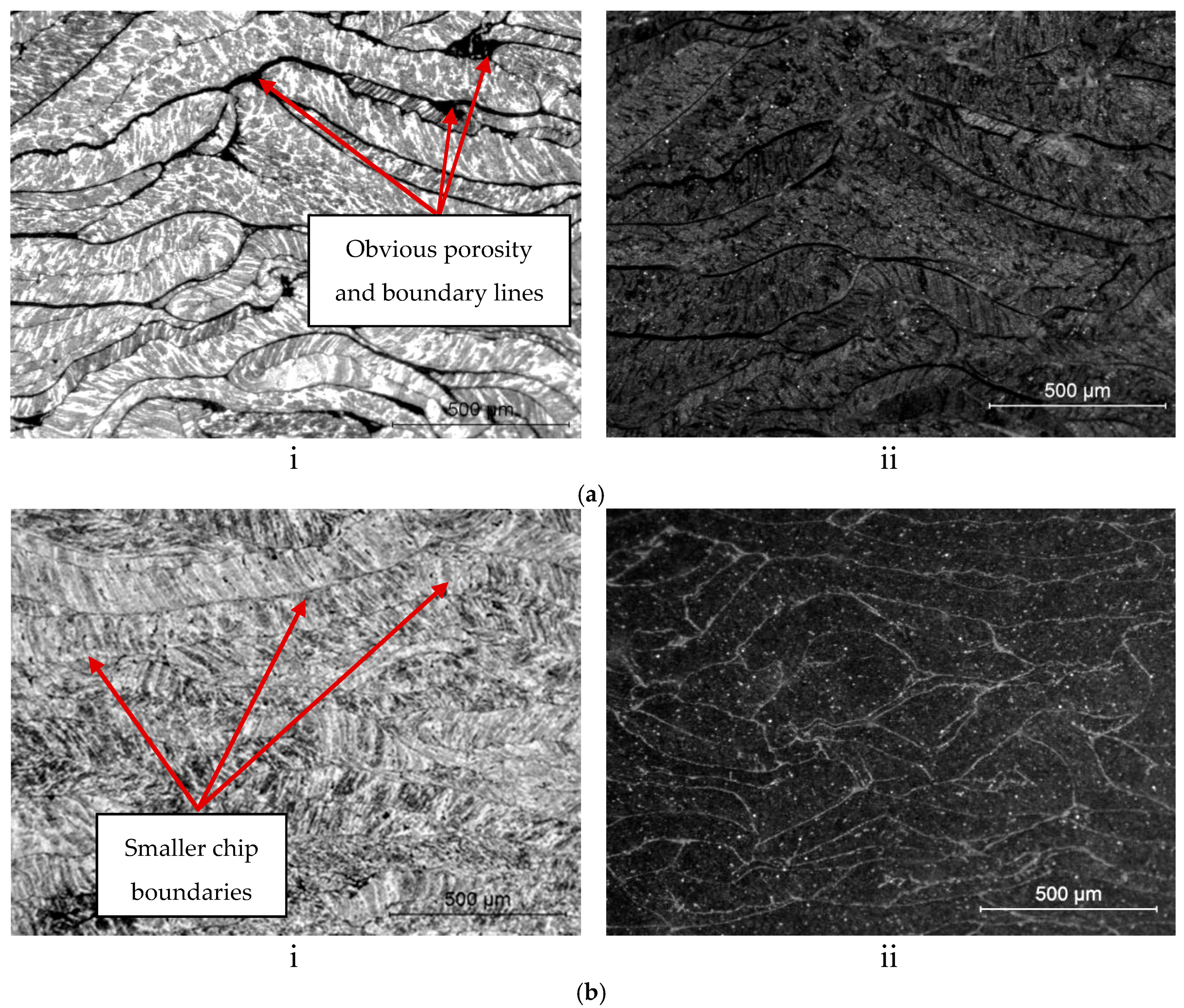
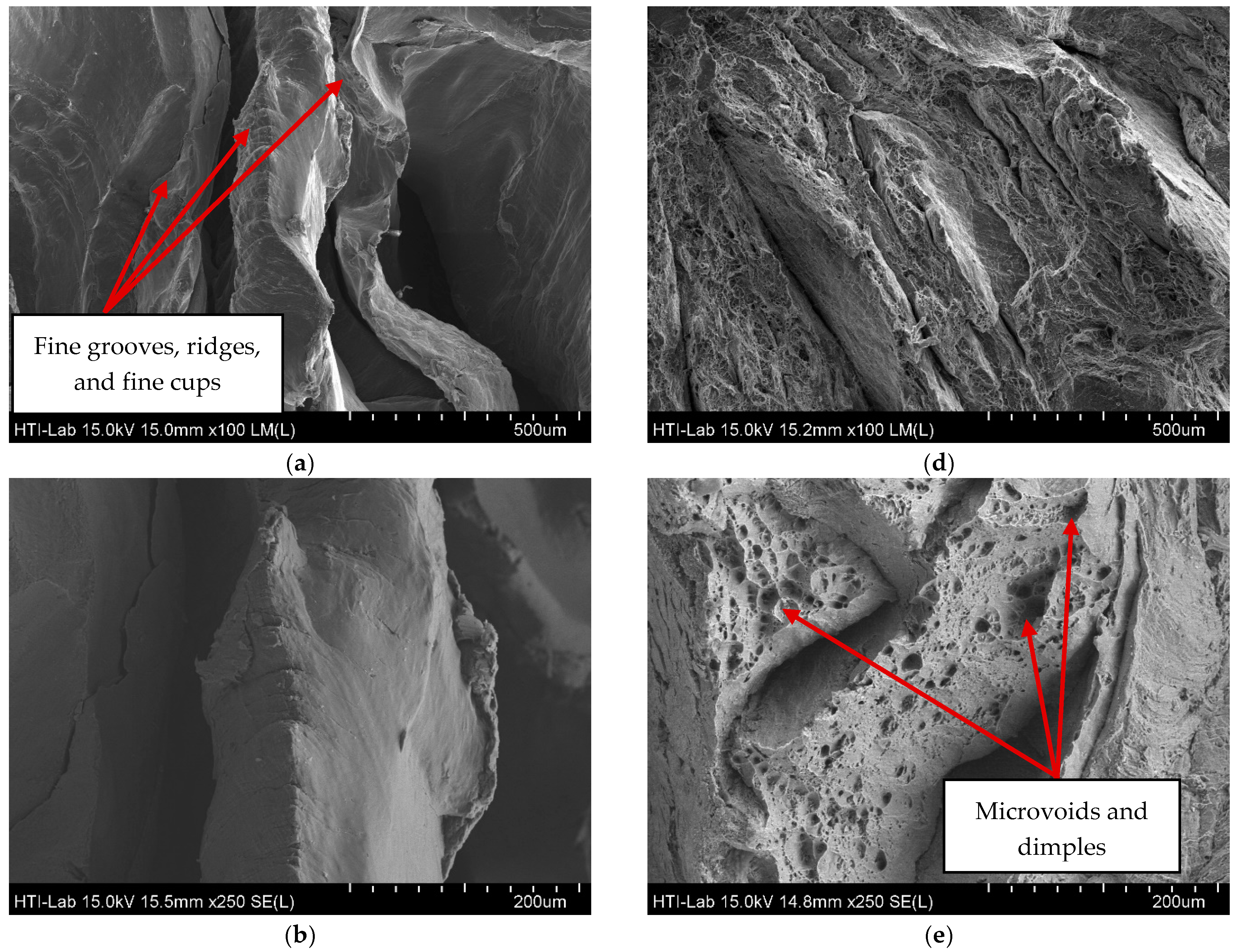
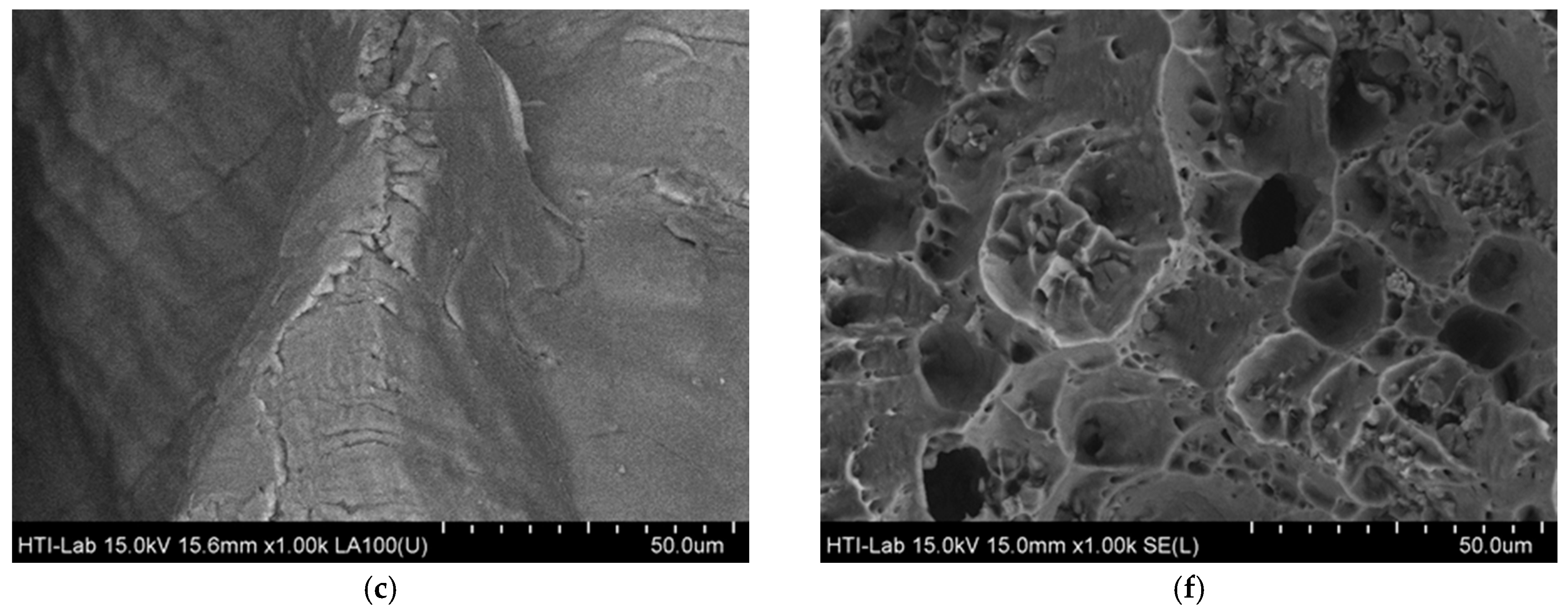
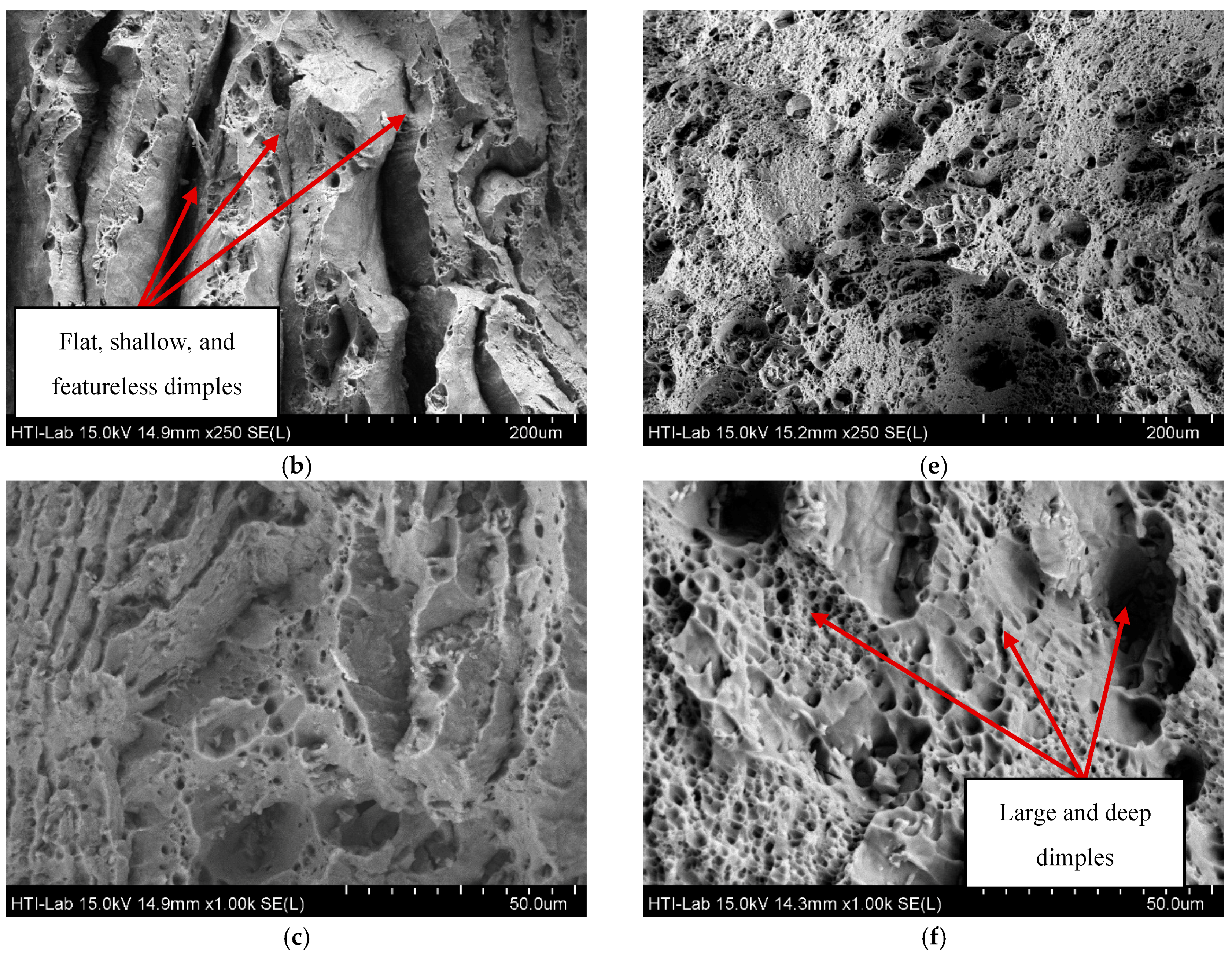
3.2. Surface Integrity
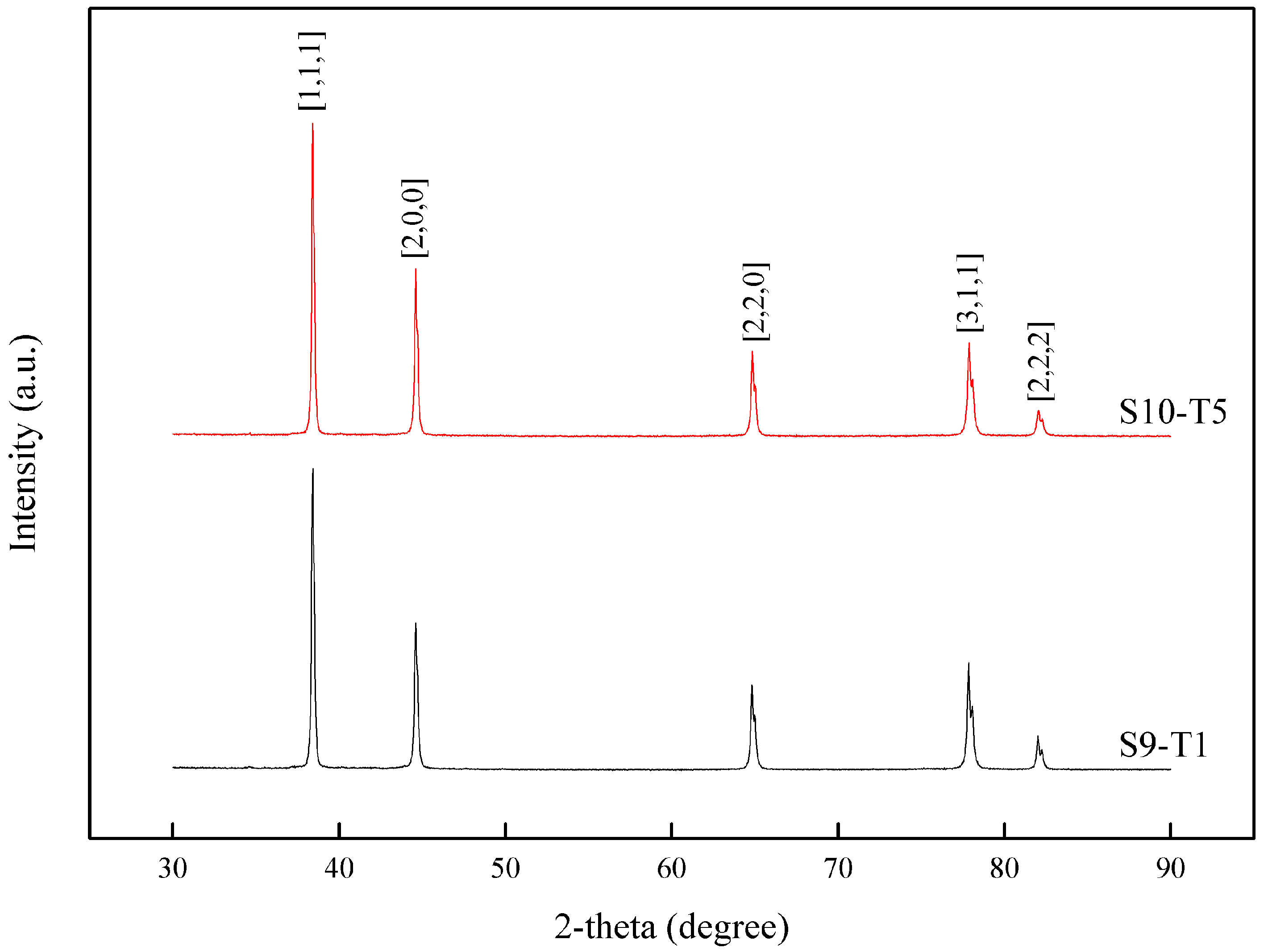
3.3. Post-Process (Artificial Aging-T5 Temper)
4. Conclusions
- Results on the effect of operating temperature give most significant impact to the forging process:
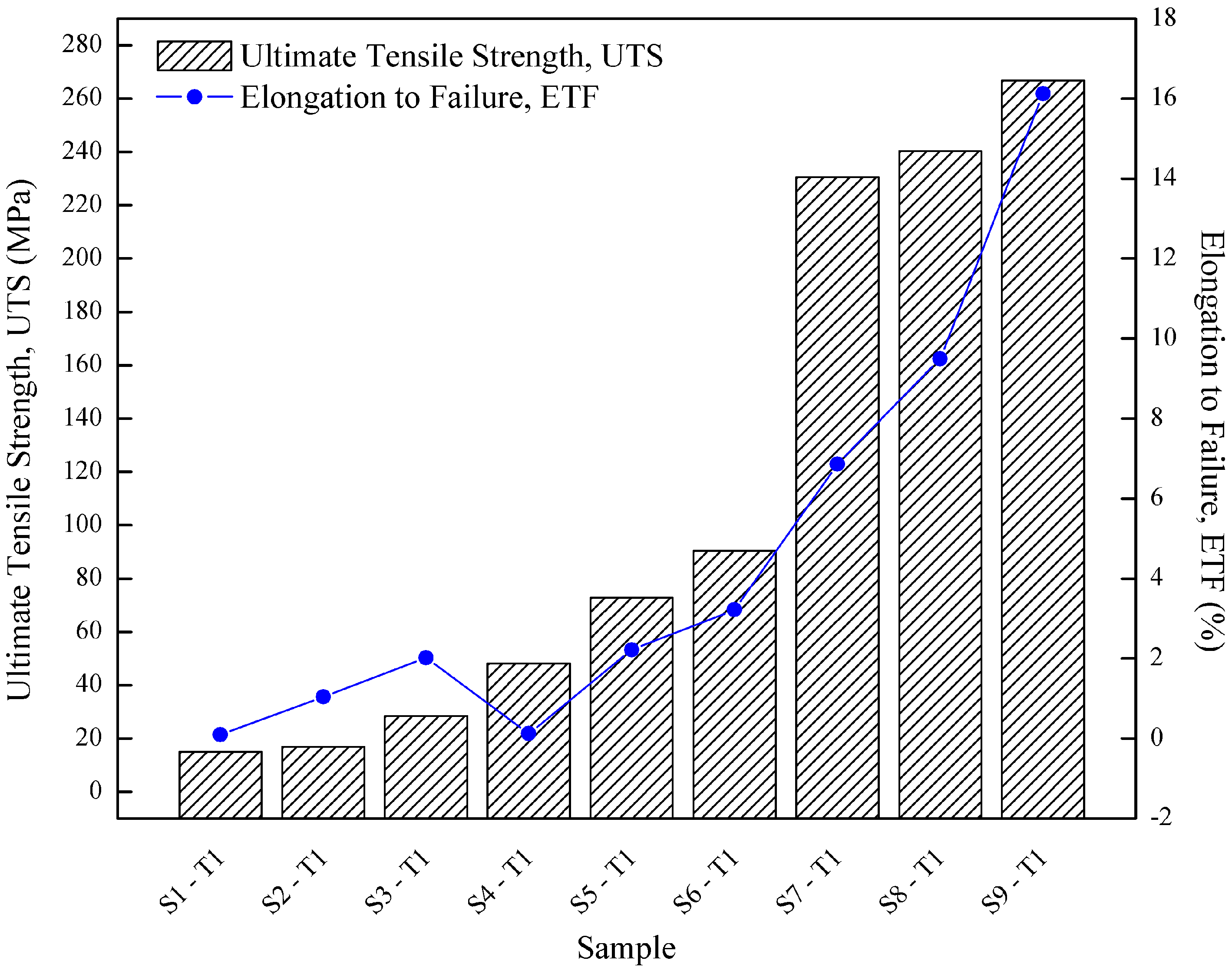
- UTS increased 89.51–93.35% from 14.97–266.78 MPa by increasing the operating temperature from 430 °C to 530 °C.
- ETF increased 86.32–98.67% from 0.091–16.12% by increasing the operating temperature from 430 °C to 530 °C.
- Microhardness reached 7.88–10.28 HV by increasing the operating temperature from 430 °C to 530 °C.
- Maximum mechanical properties and surface integrity of recycled chip (T1-temper) are considered comparable with theoretical AA6061 T4-temper:
- Maximum UTS are notably raised up to 9.27% (266.78 MPa) at parameter condition (530 °C/120 min).
- Maximum hardness surged up to 20.48% (81.74 HV) at parameter condition (530 °C/120 min).
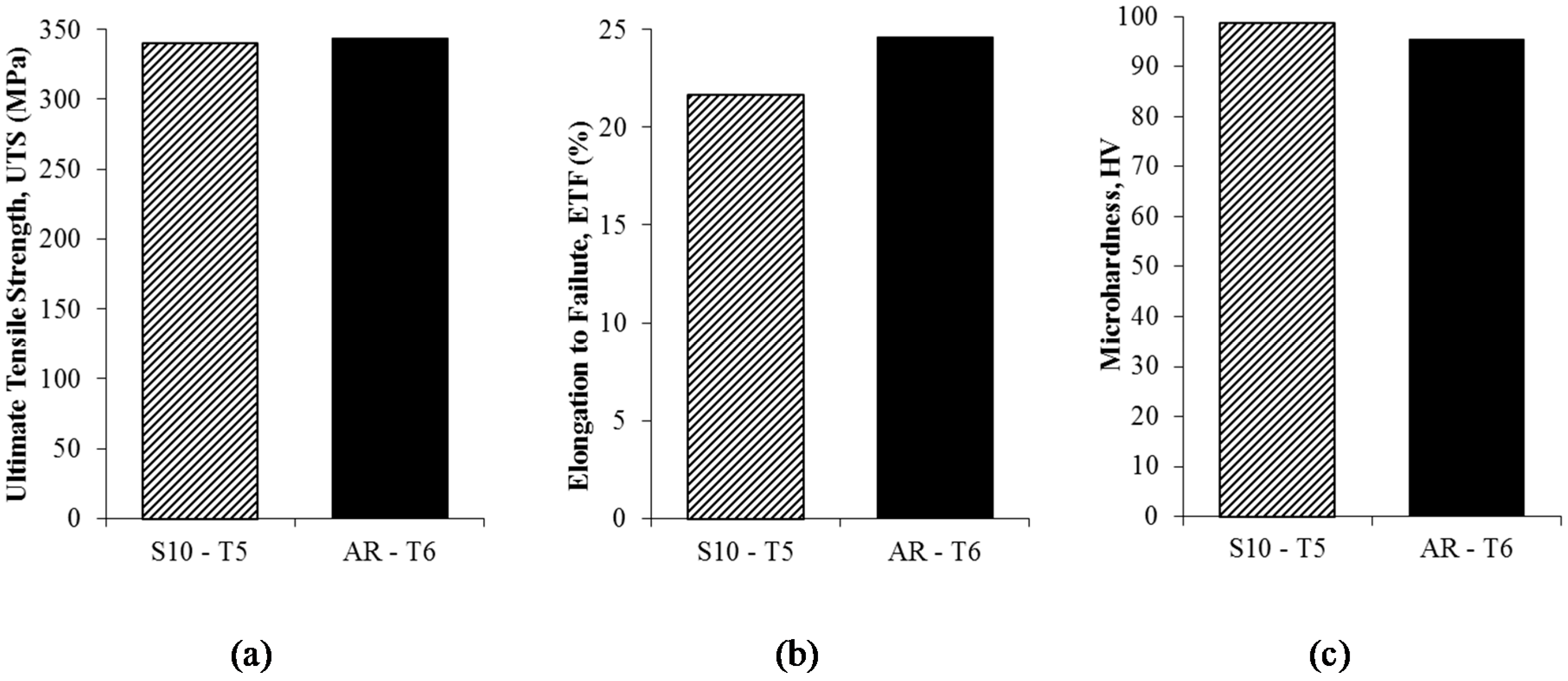
- Heat treated recycled billet (T5-temper) are considered comparable with as-received AA6061 T6-temper:
- Value of microhardness peak after 175 °C for 120 min aging increased 3.18% (98.64 HV).
- Value of UTS (340.47 MPa) and ETF (21.70%) reached close to the as-received value.
Acknowledgments
Author Contributions
Conflicts of Interests
References
- National Research Council. Advancing the Science of Climate Change; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Jayal, D.; Badurdeen, F.; Dillon, O.W.; Jawahir, I.S. Sustainable manufacturing: Modeling and optimization challenges at the product, process and system levels. CIRP J. Manuf. Sci. Technol. 2010, 2, 144–152. [Google Scholar] [CrossRef]
- Rashid, M.W.A.; Yacob, F.F.; Lajis, M.A.; Abid, M.A.A.M.; Mohamad, E.; Ito, T. A review: The potential of powder metallurgy in recycling aluminum chips (Al 6061 & Al 7075). In Proceedings of the 24th Design Engineering Systems Division JSME Conference Japan Society of Mechanical Engineers, Tokushima, Japan, 17–19 September 2014. [Google Scholar]
- Fuziana, Y.F.; Warikh, A.R.M.; Lajis, M.A.; Azam, M.A.; Muhammad, N.A. Recycling aluminium (Al 6061) chip through powder metallurgy route. Mater. Res. Innov. 2014, 18, 354–358. [Google Scholar] [CrossRef]
- Mashhadi, H.A.; Moloodi, A.; Golestanipour, M.; Karimi, E.Z.V. Recycling of aluminium alloy turning scrap via cold pressing and melting with salt flux. J. Mater. Process. Technol. 2009, 209, 3138–3142. [Google Scholar] [CrossRef]
- Schlesinger, M.E. Aluminum Recycling; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Hatayama, H.; Daigo, I.; Matsuno, Y.; Adachi, Y. Evolution of aluminum recycling initiated by the introduction of next-generation vehicles and scrap sorting technology. Resour. Conserv. Recycl. 2012, 66, 8–14. [Google Scholar] [CrossRef]
- Gronostajski, J.Z.; Kaczmar, J.W.; Marciniak, H.; Matuszak, A. Direct recycling of aluminium chips into extruded products. J. Mater. Process. Technol. 1997, 64, 149–156. [Google Scholar] [CrossRef]
- Fogagnolo, J.; Ruiz-Navas, E.; Simón, M.; Martinez, M. Recycling of aluminium alloy and aluminium matrix composite chips by pressing and hot extrusion. J. Mater. Process. Technol. 2003, 143, 792–795. [Google Scholar] [CrossRef]
- Haase, M.; Tekkaya, A.E. Cold extrusion of hot extruded aluminum chips. J. Mater. Process. Technol. 2015, 217, 356–367. [Google Scholar] [CrossRef]
- Gronostajski, J.; Marciniak, H.; Matuszak, A. New methods of aluminium and aluminium-alloy chips recycling. J. Mater. Process. Technol. 2000, 106, 34–39. [Google Scholar] [CrossRef]
- Tekkaya, A.E.; Schikorra, M.; Becker, D.; Biermann, D.; Hammer, N.; Pantke, K. Hot profile extrusion of AA-6060 aluminum chips. J. Mater. Process. Technol. 2009, 209, 3343–3350. [Google Scholar] [CrossRef]
- Güley, V.; Güzel, A.; Jäger, A.; Khalifa, N.B.; Tekkaya, A.E.; Misiolek, W.Z. Effect of die design on the welding quality during solid state recycling of AA6060 chips by hot extrusion. Mater. Sci. Eng. A 2013, 574, 163–175. [Google Scholar] [CrossRef]
- Ab Rahim, S.N.; Lajis, M.A.; Ariffin, S. A review on recycling aluminum chips by hot extrusion process. Procedia CIRP 2015, 26, 761–766. [Google Scholar] [CrossRef]
- Ab Rahim, S.N.; Lajis, M.A.; Ariffin, S. Effect of extrusion speed and temperature on hot extrusion process of 6061 aluminum alloy chip. ARPN J. Eng. Appl. Sci. 2016, 11, 2272–2277. [Google Scholar]
- Paraskevas, D.; Vanmeensel, K.; Vleugels, J.; Dewulf, W.; Deng, Y.; Duflou, J.R. Spark plasma sintering as a solid-state recycling technique: The case of aluminum alloy scrap consolidation. Materials 2014, 7, 5664–5687. [Google Scholar] [CrossRef]
- Paraskevas, D.; Dadbakhsh, S.; Vleugels, J.; Vanmeensel, K.; Dewulf, W.; Duflou, J.R. Solid state recycling of pure Mg and AZ31 Mg machining chips via spark plasma sintering. Mater. Des. 2016, 109, 520–529. [Google Scholar] [CrossRef]
- Ingarao, G.; Di Lorenzo, R.; Micari, F. Sustainability issues in sheet metal forming processes: An overview. J. Clean. Prod. 2011, 19, 337–347. [Google Scholar] [CrossRef]
- Duflou, J.R.; Tekkaya, A.E.; Haase, M.; Welo, T.; Vanmeensel, K.; Kellens, K.; Dewulf, W.; Paraskevas, D. Environmental assessment of solid state recycling routes for aluminium alloys: Can solid state processes significantly reduce the environmental impact of aluminium recycling? CIRP Ann. Manuf. Technol. 2015, 64, 37–40. [Google Scholar] [CrossRef]
- Yusuf, N.K.; Lajis, M.A.; Daud, M.I.; Noh, M.Z. Effect of Operating Temperature on Direct Recycling Aluminium Chips (AA6061) in Hot Press Forging Process. Appl. Mech. Mater. 2013, 315, 728–732. [Google Scholar] [CrossRef]
- Lajis, M.A.; Khamis, S.S.; Yusuf, N.K. Optimization of Hot Press Forging Parameters in Direct Recycling of Aluminium Chip (AA 6061). Key Eng. Mater. 2014, 622, 223–230. [Google Scholar]
- Ahmad, A.; Lajis, M.A.; Yusuf, N.K.; Wagiman, A. Hot Press Forging as The Direct Recycling Technique of Aluminium—A Review. ARPN J. Eng. Appl. Sci. 2016, 11, 2258–2265. [Google Scholar]
- Yusuf, N.K. Effects of Hot Press Forging Parameter And Life Cycle Assessment In Direct Recycling of AA6061 Aluminium. Ph.D. Thesis, Universiti Tun Hussein Onn Malaysia, Johor, Malaysia, May 2017. [Google Scholar]
- Naka, T.; Nakayama, Y.; Uemori, T.; Hino, R.; Yoshida, F. Effects of temperature on yield locus for 5083 aluminum alloy sheet. J. Mater. Process. Technol. 2003, 140, 494–499. [Google Scholar] [CrossRef]
- Tiryakiouglu, M.; Campbell, J.; Staley, J.T. On macrohardness testing of Al-7 wt % Si-Mg alloys: II. An evaluation of models for hardness-yield strength relationships. Mater. Sci. Eng. A 2003, 361, 240–248. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Yuan, D.; Peng, D. Hot deformation behavior of the new Al-Mg-Si-Cu aluminum alloy during compression at elevated temperatures. Mater. Charact. 2007, 58, 168–173. [Google Scholar] [CrossRef]
- Handbook Committee. ASM Handbook; ASM International: Materials Park, OH, USA, 2006; Volume 4. [Google Scholar]
- Monteiro, V.M.; Diniz, S.B.; Meirelles, B.G.; Silva, L.C.; Paula, A.S. Microstructural and Mechanical study of aluminium alloys submitted to distinct soaking times during solution heat treatment. Tecnol. Metal. Mater. Miner. 2014, 11, 332–339. [Google Scholar] [CrossRef]
- Pezda, J. The effect of the T6 heat treatment on hardness and microstructure of the En Ac-AlSi12CuNiMg alloy. Metalurgija 2014, 53, 63–66. [Google Scholar]
- Friis, J.; Marthinsen, K.; Furu, T.; Nes, E.; Myhr, O.R.; Grong, Ø.; Holmedal, B.; Ryen, Ø. Work hardening behaviour of heat-treatable Al-Mg-Si-alloys. Mater. Sci. Forum 2006. [Google Scholar] [CrossRef]
- Van de Langkruis, J.; Kuijpers, N.C.W.; Kool, W.H.; Vermolen, F.J.; van der Zwaag, S. Modelling Mg2Si Dissolution in an AA6063 Alloy During Pre-heating to the Extrusion Temperature. In Proceedings of the International Aluminum Extrusion Technology Seminar, Chicago, IL, USA, 16–19 May 2000. [Google Scholar]
- Rometsch, P.A.; Schaffer, G.B. An age hardening model for Al-7Si-Mg casting alloys. Mater. Sci. Eng. A 2002, 325, 424–434. [Google Scholar] [CrossRef]
- Meng, C. Effect of Preheating Condition on Strength of AA6060 Aluminium Alloy for Extrusion. Master’s Thesis, Auckland University of Technology, Auckland, New Zealand, 2010. [Google Scholar]
- Oppenheim, T.; Tewfic, S.; Scheck, T.; Klee, V.; Lomeli, S.; Dahir, W.; Youngren, P.; Aizpuru, N.; Clark, R.; Lee, E.W.; et al. On the correlation of mechanical and physical properties of 6061-T6 and 7249-T76 aluminum alloys. Eng. Fail. Anal. 2007, 14, 218–225. [Google Scholar] [CrossRef]
- Russell, A.M.; Lee, K.L. Structure-Property Relations in Nonferrous Metals; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chino, Y.; Mabuchi, M.; Otsuka, S.; Shimojima, K.; Hosokawa, H.; Yamada, Y.; Wen, C.; Iwasaki, H. Corrosion and mechanical properties of recycled 5083 aluminum alloy by solid state recycling. Mater. Trans. 2003, 44, 1284–1289. [Google Scholar] [CrossRef]
- Tan, E.; Ogel, B. Influence of heat treatment on the mechanical properties of AA6066 alloy. Turkish J. Eng. Environ. Sci. 2007, 31, 53–60. [Google Scholar]
- Rashid, M. Mathematical Modeling and Optimization of Precipitation Hardening of Extrudable Aluminum Alloys. Ph.D. Thesis, King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia, June 1997. [Google Scholar]
- Krishnan, K.N. The effect of post weld heat treatment on the properties of 6061 friction stir welded joints. J. Mater. Sci. 2002, 7, 473–480. [Google Scholar] [CrossRef]
- Moustafa, S.; Daoush, W.; Ibrahim, A.; Neubaur, E. Hot Forging and Hot Pressing of AlSi Powder Compared to Conventional Powder Metallurgy Route. Mater. Sci. Appl. 2011, 2, 1127. [Google Scholar] [CrossRef]
- Ceschini, L.; Minak, G.; Morri, A.; Tarterini, F. Forging of the AA6061/23 vol.%Al2O3p composite: Effects on microstructure and tensile properties. Mater. Sci. Eng. A 2009, 513, 176–184. [Google Scholar] [CrossRef]
- Demir, H.; Gündüz, S. The effects of aging on machinability of 6061 aluminium alloy. Mater. Des. 2009, 30, 1480–1483. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Abdullah, H.A.; Al-Belushi, K.R. Influence of aging parameters on the mechanical properties of 6063 aluminium alloy. J. Mater. Process. Technol. 2000, 102, 234–240. [Google Scholar] [CrossRef]
- Risanti, D.D.; Yin, M.; del Castillo, P.E.J.R.D.; van der Zwaag, S. A systematic study of the effect of interrupted ageing conditions on the strength and toughness development of AA6061. Mater. Sci. Eng. A 2009, 523, 99–111. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Nejadseyfi, O. A comparison of the effects of severe plastic deformation and heat treatment on the tensile properties and impact toughness of aluminum alloy 6061. Mater. Sci. Eng. A 2014, 594, 140–148. [Google Scholar] [CrossRef]
- Kuijpers, N.C.W.; Kool, W.H.; Koenis, P.T.G.; Nilsen, K.E.; Todd, I.; van der Zwaag, S. Assessment of different techniques for quantification of α-Al(FeMn)Si and β-AlFeSi intermetallics in AA 6xxx alloys. Mater. Charact. 2002, 49, 409–420. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, W.; Zhao, Y.; Li, Y. Solid-state transformation of Fe-rich intermetallic phases in Al-5.0Cu-0.6Mn squeeze cast alloy with variable Fe contents during solution heat treatment. Mater. Charact. 2015, 104, 124–131. [Google Scholar] [CrossRef]
- Ezatpour, H.R.; Haddad Sabzevar, M.; Sajjadi, S.A.; Huang, Y. Investigation of work softening mechanisms and texture in a hot deformed 6061 aluminum alloy at high temperature. Mater. Sci. Eng. A 2014, 606, 240–247. [Google Scholar] [CrossRef]
- Kuijpers, N.C.W.; Vermolen, F.J.; Vuik, C.; Koenis, P.T.G.; Nilsen, K.E.; van der Zwaag, S. The dependence of the α-AlFeSi to β-Al(FeMn)Si transformation kinetics in Al-Mg-Si alloys on the alloying elements. Mater. Sci. Eng. A 2005, 394, 9–19. [Google Scholar] [CrossRef]
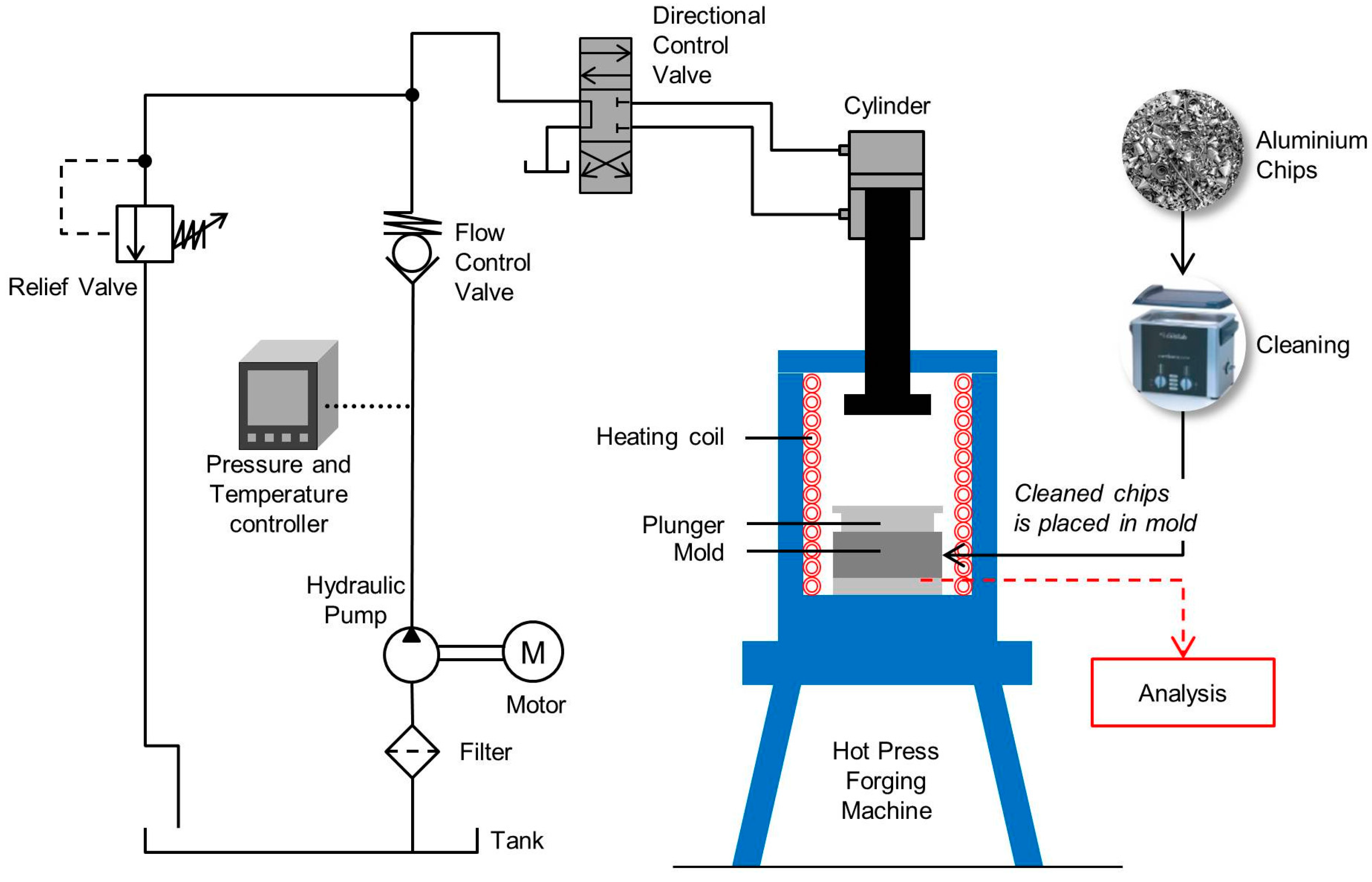
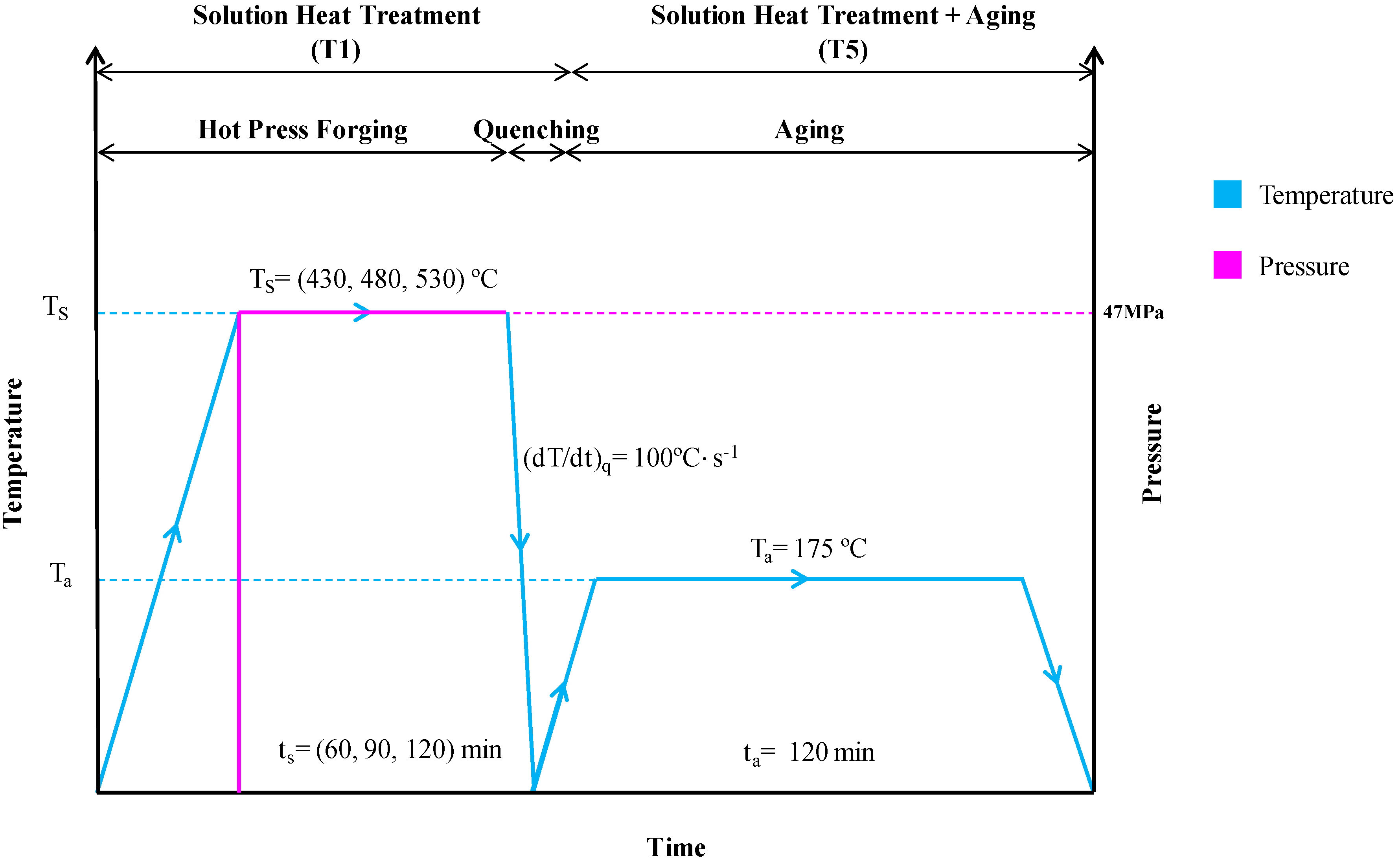
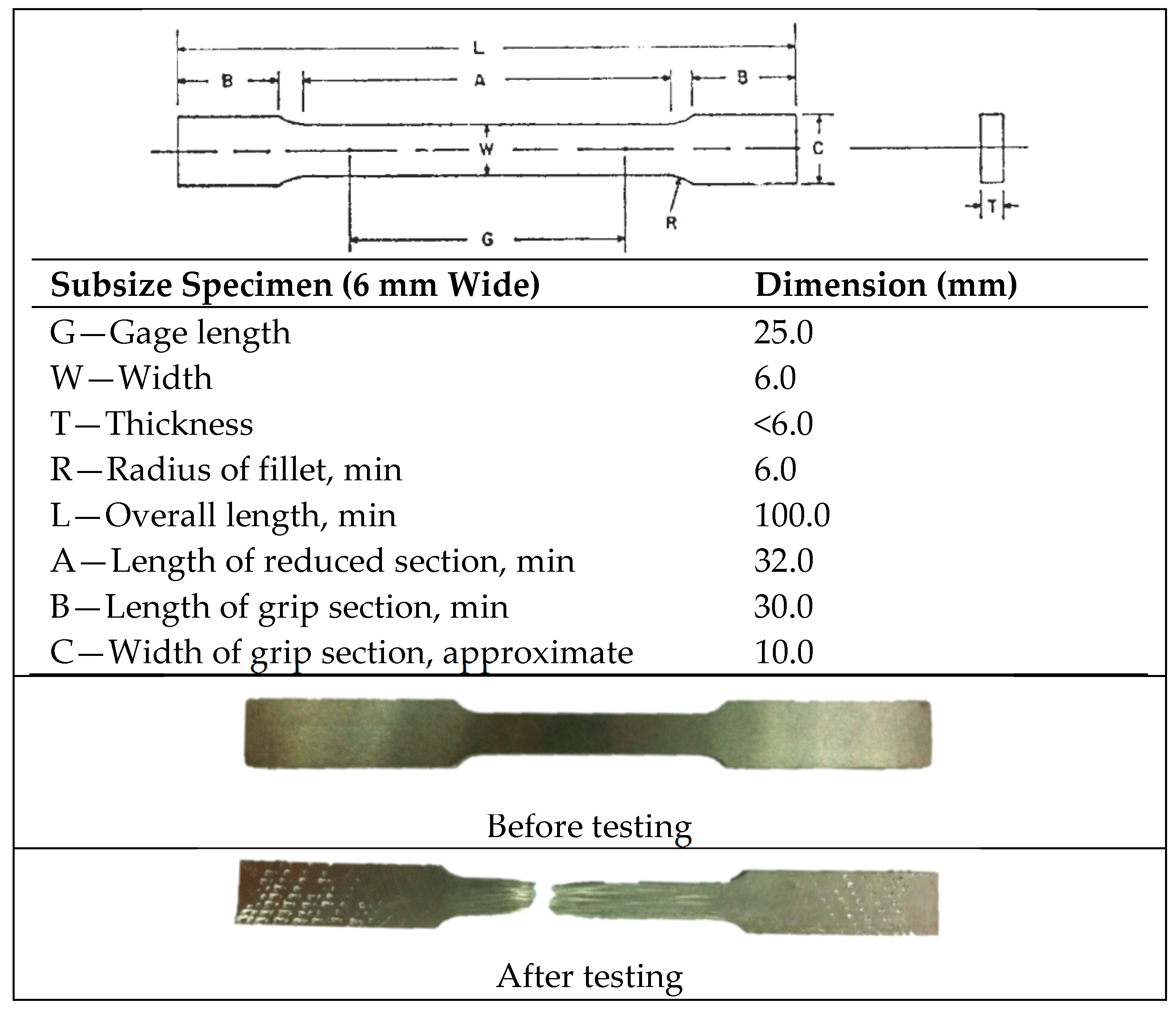



| Elements | Weight Percent (wt %) |
|---|---|
| Aluminium | 95.03 |
| Magnesium | 1.17 |
| Silicon | 0.34 |
| Iron | 0.46 |
| Copper | 0.15 |
| Manganese | 0.14 |
| Others | Balance |
| Sample Designation | Solution Heat Treatment (Hot Press) | Solution Heat Treatment + Aging | ||
|---|---|---|---|---|
| Operating Temperature, Ts (°C) | Holding Time, ts (min) | Aging Temp, Ta (°C) | Aging Time, ta (min) | |
| S1-T1 | 430 | 60 | - | - |
| S2-T1 | 430 | 90 | - | - |
| S3-T1 | 430 | 120 | - | - |
| S4-T1 | 480 | 60 | - | - |
| S5-T1 | 480 | 90 | - | - |
| S6-T1 | 480 | 120 | - | - |
| S7-T1 | 530 | 60 | - | - |
| S8-T1 | 530 | 90 | - | - |
| S9-T1 | 530 | 120 | - | - |
| S10-T5 | 530 | 120 | 175 | 120 |
| Theory-T4 | ASM Theory AA6061-T4 | |||
| AR-T6 | As-Received AA6061-T6 | |||
| Sample Designation | Ultimate Tensile Strength, UTS | Elongation to Failure, ETF | Microhardness | Density |
|---|---|---|---|---|
| (MPa) | (%) | HV | g/cc | |
| S1-T1 | 14.97 | 0.09 | 69.76 | 1.92 |
| S2-T1 | 16.91 | 1.04 | 70.37 | 2.08 |
| S3-T1 | 28.44 | 2.02 | 71.45 | 2.16 |
| S4-T1 | 48.11 | 0.12 | 72.62 | 2.29 |
| S5-T1 | 72.84 | 2.22 | 74.69 | 2.38 |
| S6-T1 | 90.42 | 3.22 | 75.63 | 2.51 |
| S7-T1 | 230.55 | 6.86 | 77.64 | 2.56 |
| S8-T1 | 240.27 | 9.50 | 79.81 | 2.63 |
| S9-T1 | 266.78 | 16.12 | 81.74 | 2.65 |
| S10-T5 | 340.47 | 21.70 | 98.64 | 2.69 |
| Theory-T4 | 240.00 | 22.00 | 65.00 | 2.70 |
| AR-T6 | 343.43 | 24.62 | 95.51 | 2.67 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, N.K.; Lajis, M.A.; Ahmad, A. Hot Press as a Sustainable Direct Recycling Technique of Aluminium: Mechanical Properties and Surface Integrity. Materials 2017, 10, 902. https://doi.org/10.3390/ma10080902
Yusuf NK, Lajis MA, Ahmad A. Hot Press as a Sustainable Direct Recycling Technique of Aluminium: Mechanical Properties and Surface Integrity. Materials. 2017; 10(8):902. https://doi.org/10.3390/ma10080902
Chicago/Turabian StyleYusuf, Nur Kamilah, Mohd Amri Lajis, and Azlan Ahmad. 2017. "Hot Press as a Sustainable Direct Recycling Technique of Aluminium: Mechanical Properties and Surface Integrity" Materials 10, no. 8: 902. https://doi.org/10.3390/ma10080902





