3.1. Microstructure of Sn-Bi Solder Alloys
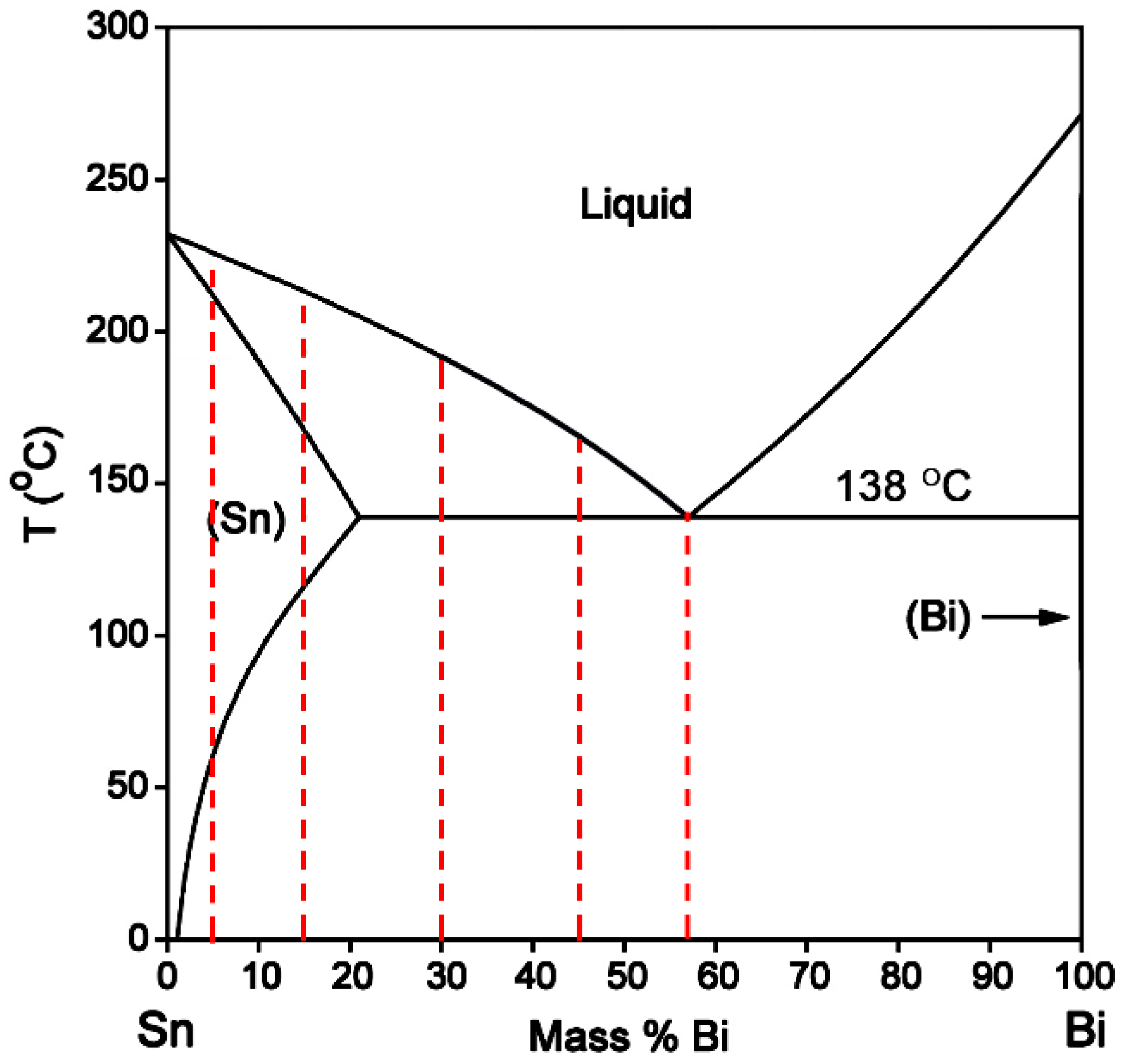
From the Sn-Bi binary phase diagram shown in
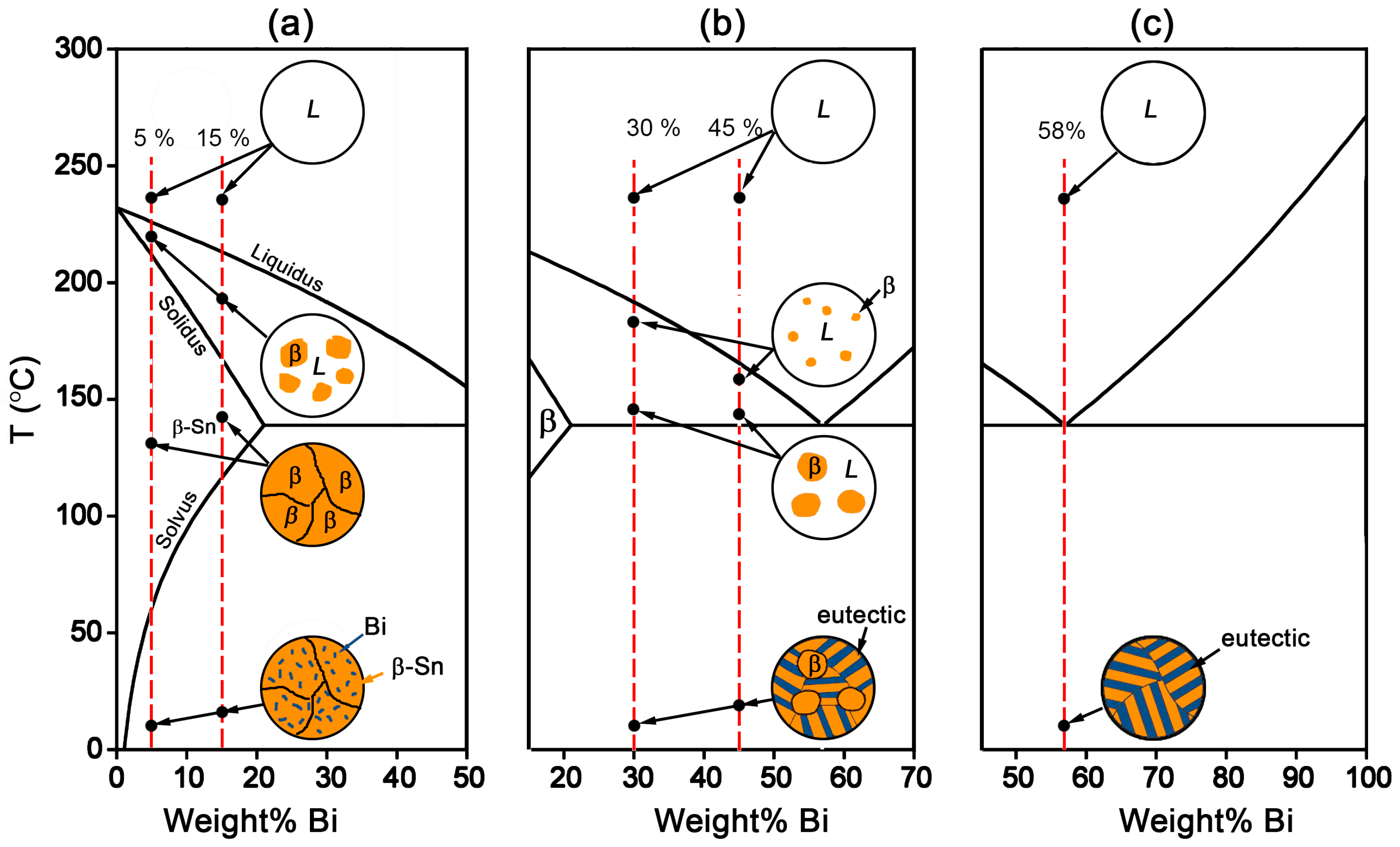
Figure 1, there exists an eutectic reaction at 138 °C with the composition of Sn-58Bi. β-Sn phase is the solid solution rich in Bi with the solubility limit of 21 wt % occurring at eutectic temperature, while there is almost no solution for Sn in Bi. Accordingly, the microstructural evolution for Sn-Bi solder system is illustrated in
Figure 3. Sn-5Bi or Sn-15Bi belongs to the solid solution region. With the temperature reaching the liquidus line, β-Sn solid solution phases will gradually crystallize from the liquid phase until reaching the solidus line. After the solvus line, Bi will precipitate from the solid solution phases, and the final microstructure for the solder is composed of β-Sn solid solution matrix with finely distributed Bi precipitates, as illustrated in
Figure 3a. For Sn-30Bi and Sn-45Bi hypoeutectic solder alloys, the Bi composition in Sn-Bi solder is over the solubility limit in the β-Sn, but less than the eutectic composition. After reaching the liquidus line during solidification, the primary β-Sn phases will be formed in the liquid solder. When the solder temperature continually decreases to 138 °C, eutectic reaction occurs in the left liquid phase in order to produce a striped eutectic microstructure with alternating Sn and Bi phases. Therefore, the final microstructure for Sn-30Bi and Sn-45Bi is then composed of the primary β-Sn phases surrounded by stripes of the eutectic phases, as illustrated in
Figure 3b. Sn-58Bi is the solder with an eutectic composition, and therefore an eutectic reaction directly occurs to produce the eutectic structure when the temperature of liquid solder reaches 138 °C. The final microstructure of Sn-58Bi solder is fully composed of the striped eutectic structure with alternating β-Sn and Bi phases, as seen in
Figure 3c.
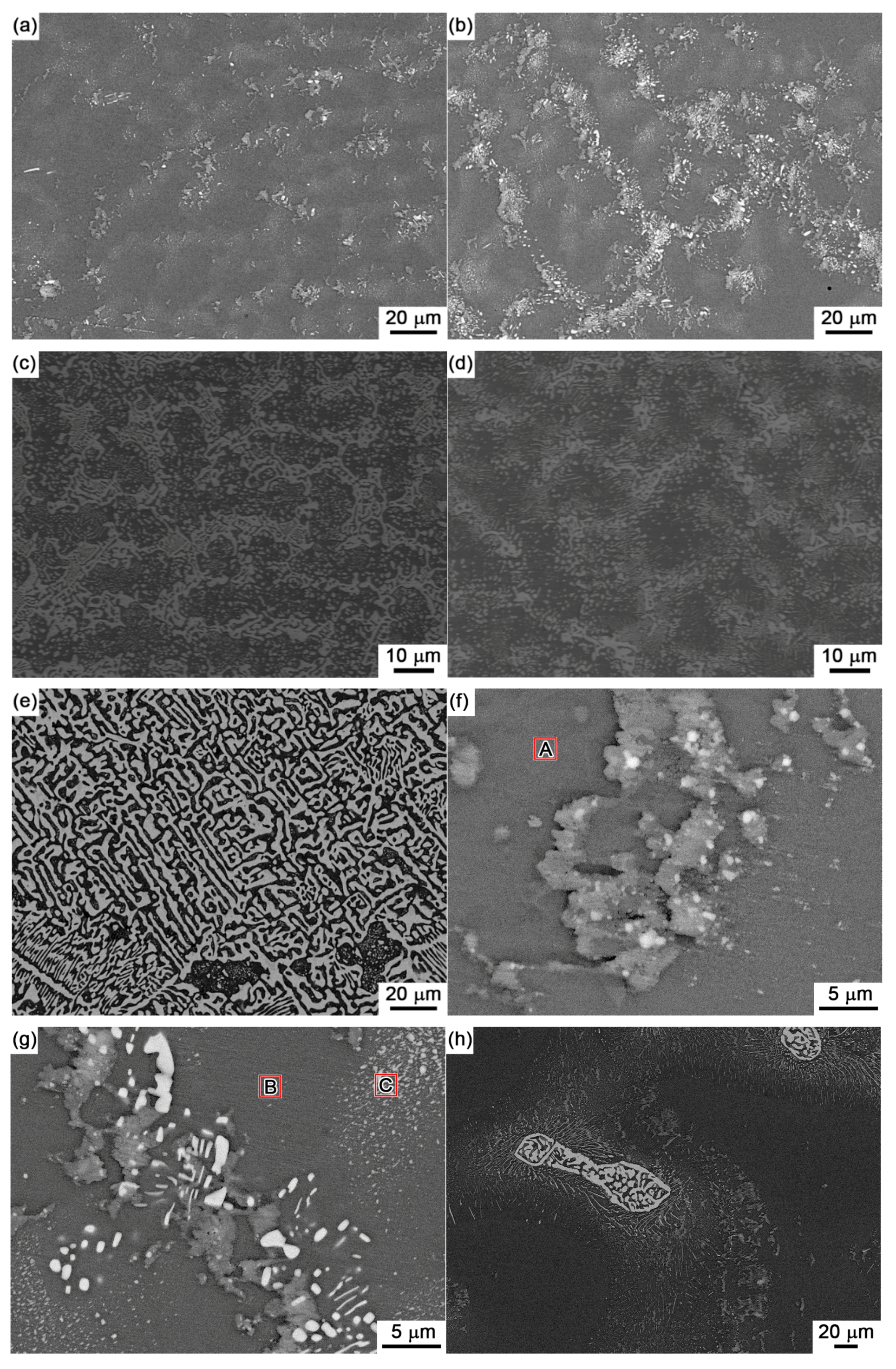
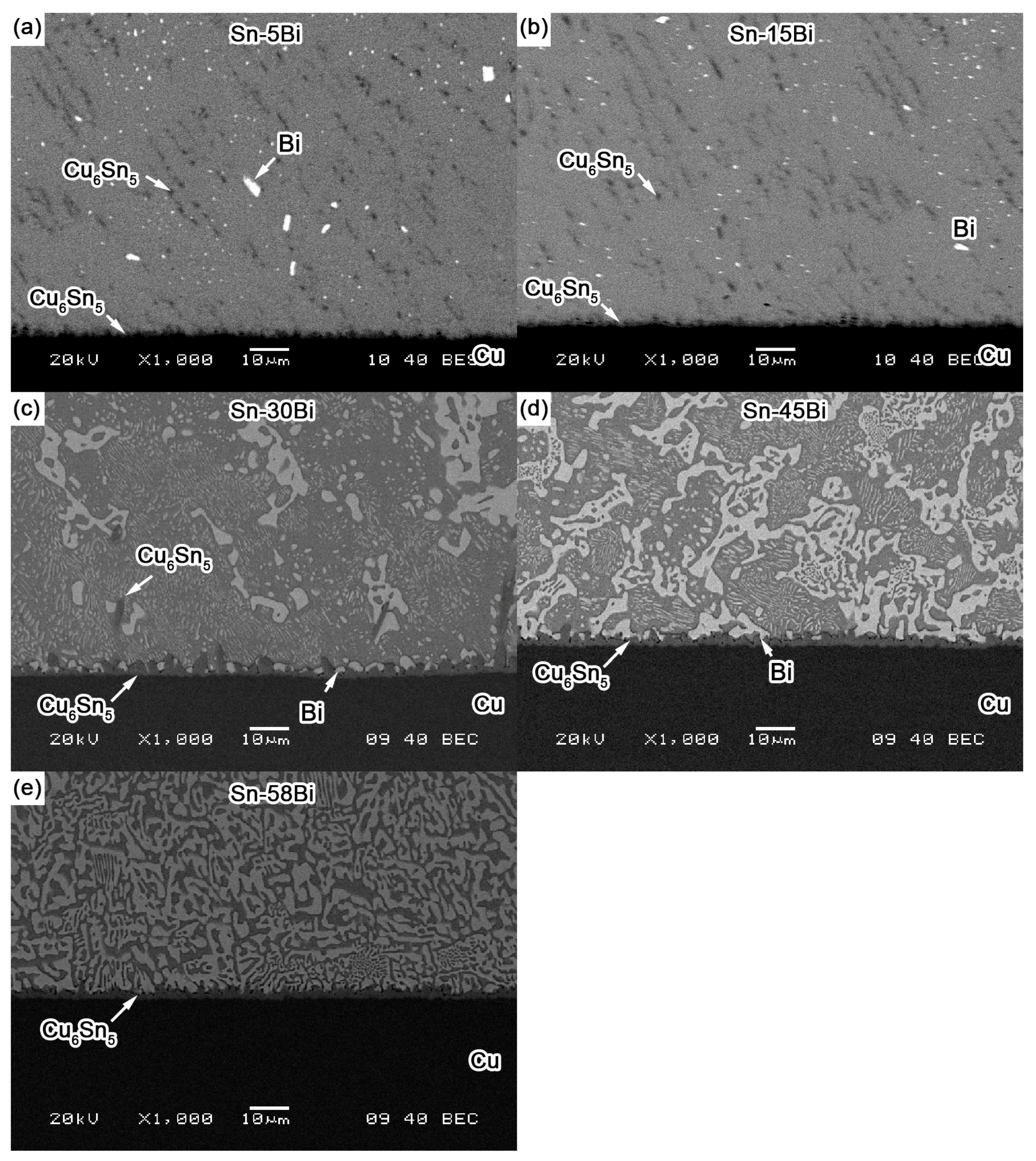
Figure 4 shows the back scattered-electron (BSE) images on the microstructure for Sn-5Bi, Sn-15Bi, Sn-30Bi, Sn-45Bi and Sn-58Bi solder alloys. There are two kinds of phases in them: grey β-Sn phases and white Bi-rich phase. The distribution of Bi phases was obviously changed by Bi content. Meanwhile, the compositions of phases for the regions marked in
Figure 4 were also detected by EDS analysis.
As shown in
Figure 4a for Sn-5Bi solder, the microstructure was mainly composed of β-Sn grains surrounded by rarely distributed small Bi particles. Bi distribution was magnified with the image shown in
Figure 4f. It can be clearly seen that Bi phases were mainly precipitated from the Sn phases. From EDS results on β-Sn phase (region A), there still exists about 3.17 wt % Bi with solid solution in β-Sn after solidification. With Bi content in Sn-Bi increased from 5 wt % to 15 wt %, large amounts of Bi particles were obviously observed both along and within the β-Sn grains. The more greatly magnified BSE image on Sn-15Bi solder is shown in
Figure 4g. In region B, the solid solubility of Bi atoms in β-Sn phase was about 5.49 wt % from EDS analysis. Therefore, other Bi atoms were precipitated both along β-Sn phase grains and within β-Sn phase grains with very small sizes such as the region C in
Figure 4g, due to the limited solubility of Bi in Sn at room temperature. Therefore, just as shown in
Figure 3, the microstructure of Sn-5Bi and Sn-15Bi solid solution solders is composed of the β-Sn matrix embedded with fine Bi particles along or within the β-Sn phase grains. The microstructure in
Figure 3 was predicted from the equilibrium crystallization. However, it should be mentioned that the cooling rate within the graphite mold should be much higher than the equilibrium solidification in the present study. There should be some differences between experimental and predicted structures. Therefore, we especially observed the microstructure of Sn-15Bi solder under furnace cooling, as seen in
Figure 4h, which was completely different from microstructure in
Figure 4b under rapid cooling conditions. Due to the adequate precipitation of Bi atoms from β-Sn, large amounts of bulk Bi phases were observed along β-Sn grains. Because the substrate and the component also provide the cooling channels for Sn-Bi in the solder joints, most Bi atoms will also be kept in β-Sn phases due to the non-equilibrium crystallization.
Figure 4c,d show the BSE microstructure of Sn-30Bi and Sn-45Bi hypoeutectic solders. The dark and bright regions are primary β-Sn phases and Sn-Bi eutectic structure, respectively. As mentioned above, the primary β-Sn phases were firstly solidified from the liquid solder, and then the eutectic reaction occurred on the remaining liquid to form the eutectic structure among them. The increase in Bi content in Sn-Bi hypoeutectic solder promotes the increase of the amount of eutectic structure and a decrease in the primary β-Sn phases. Therefore, the microstructure of Sn-30Bi and Sn-45Bi hypoeutectic solders is composed of the primary β-Sn phases surrounded by the networked Sn-Bi eutectic phases.
Finally,
Figure 4e shows the microstructure of Sn-58Bi solder. It is a typical striped eutectic structure with alternated β-Sn phases and Bi phases due to the instantaneous phase separation during solidification. Similarly, the primary β-Sn phases still formed in the right corner of solder microstructure due to the non-equilibrium solidification.
3.2. Microstructure of Sn-Bi Solder Joints at the Interface
Figure 5a–e show the BSE images on the interfacial structure in Sn-Bi solder joints after wetting. It is clearly seen that an IMC layer was produced between Sn-Bi solder and Cu substrate. Its composition was confirmed as Cu
6Sn
5 by EDS analysis and was independent of the Bi content in the solder. At the interfaces of Sn-5Bi and Sn-15Bi solder shown in
Figure 5a,b, the morphology of IMC layer shows a typical scallop type, and the thickness of IMC layer is about 1 μm. Besides the interfacial Cu
6Sn
5, it is interesting to find that a huge quantity of Cu
6Sn
5 IMC flakes was also produced in the solder matrix, but the amount of these IMC flakes in Sn-15Bi solder was less than that in Sn-5Bi. The precipitated Bi particles from soldering were uniformly distributed within the solder matrix. In Sn-30Bi solder joint shown in
Figure 5c, the IMC thickness grew a little more than that in Sn-5Bi or Sn-15Bi solder joints. The scallop-type morphology was also observed for the IMC layer. Different from the interfaces in Sn-5Bi or Sn-15Bi, Bi-rich phase segregation was clearly observed in the grooves between the scallop-type grains. In the solder matrix, Bi atoms existed with both small particles and large bulk phases, while Cu
6Sn
5 IMC flakes were seldom observed in the bulk solder compared with
Figure 5a or
Figure 5b. In Sn-45Bi and Sn-58Bi solder joints, as shown in
Figure 5d,e, more Bi-rich phases accumulated at the interface between Sn-Bi solder and the Cu
6Sn
5 IMC layer, while no Cu
6Sn
5 flakes were observed in the solder matrix. The morphology of the IMC layer also became slightly flattened but thicker. The IMC thickness is about 2 μm.
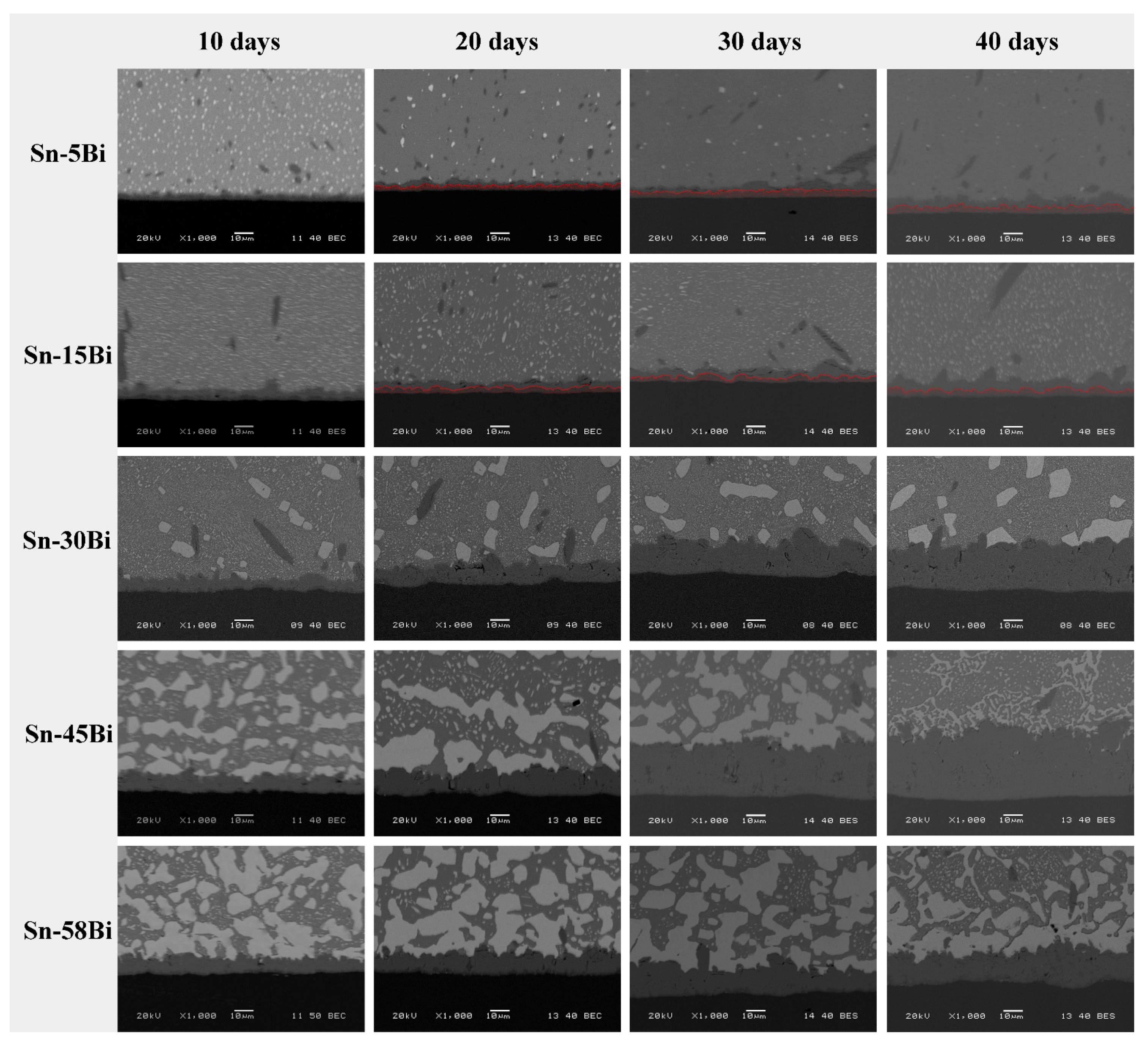
The effect of aging on the interfacial behaviors of solder joints was then studied.
Figure 6 illustrates the representative backscattered electron (BSE) images on Sn-Bi/Cu samples aged at 135 °C with aging duration of 10, 20, 30 and 40 days, respectively. The IMC thickness obviously increased with the aging time. In Sn-5Bi and Sn-15Bi solder joints, with increasing of the IMC thickness at the interface, the Cu
3Sn IMC layer was produced between Cu
6Sn
5 and Cu, which was sketched by the red area in
Figure 6, and its thickness also increased with the aging time. In the solder matrix, because some Bi atoms were dissolved into the β-Sn grains during the soldering condition, they were segregated to form small Bi particles during isothermal aging, which were uniformly distributed in the solder matrix as observed in
Figure 6. The large amounts of Cu
6Sn
5 IMC flakes formed in the soldering condition, as seen in
Figure 5a,b, were coarsened and combined with the aging time. In Sn-30Bi and Sn-45Bi hypoeutectic solder joints, the most important observation at the interface is the obvious growth of Cu
6Sn
5 layer and the prohibition on the formation of Cu
3Sn layer between Cu
6Sn
5 and Cu. Bi-rich phases were obviously coarsened in the solder matrix and were also accumulated at the interface between solder and IMC layer due to the Sn consumption during the reaction to form Cu
6Sn
5. In the Sn-58Bi solder joint, the rapid growth on the Cu
6Sn
5 layer and the inhibition on the Cu
3Sn layer were similarly observed at the interface, but it is interesting to find that the IMC thickness in the Sn-58Bi solder joint was thinner than that in Sn-30Bi and Sn-45Bi solder joints under the same aging time. At the interface between Sn-58Bi solder and IMC layer, the continuous Bi-rich layer always existed during the aging duration.
It is well known that the IMC growth at the interface in solder joints follows the empirical diffusion formula:
where
X is the total IMC thickness,
X0 is the initial IMC thickness,
t is the time and
D is the diffusivity of the IMC layer. Therefore, the growth of IMC thickness (
X −
X0) was plotted with the square root of aging days (
t1/2) with the results shown in
Figure 7. It is clearly seen that Sn-5Bi and Sn-15Bi had the lowest rate of 1.0 μm/day
1/2 and 1.2 μm/day
1/2, respectively. With Bi content in Sn-Bi solder increasing to 30 wt % and 45 wt %, the rapid growth on the IMC layer occurred at the interface with the growing rate of 4.6 μm/day
1/2 and 5.4 μm/day
1/2, respectively. However, with Bi content in the solder reaching the eutectic composition of Sn-58Bi, the growth rate of the IMC layer at the interface decreased to 3.0 μm/day
1/2. Therefore, in all of these Sn-Bi solders, Sn-5Bi and Sn-45Bi solder had the lowest and highest growth rate on IMC thickness, respectively.
In the case of the formation of Cu
6Sn
5 IMC layer at the interface, it is commonly obtained from the reaction between Sn and Cu atoms with the following reaction:
where Cu and Sn atoms are from the solder matrix and Cu substrate, respectively. In the case of the formation of the Cu
3Sn IMC layer at the interface during isothermal aging, it is obtained from the following reactions:
where the latter reaction plays the dominant role by consuming the Cu
6Sn
5 IMC layer. In the case of the formation of Cu
6Sn
5 particles or flakes in the solder matrix, it is also obtained from the reaction between Cu and Sn, which means that Cu atoms must diffuse through the Cu-Sn IMC layer into the solder matrix with a longer distance.
From
Figure 7, it can be noted that Bi addition into Sn had the obvious effect on the growth rate of IMC at the interface. In the Sn-Bi/Cu system, Zou et al. [
18] verified that Bi atoms were also an important diffusing component to be partly dissolved into Cu
6Sn
5 IMC. From
Figure 5 and
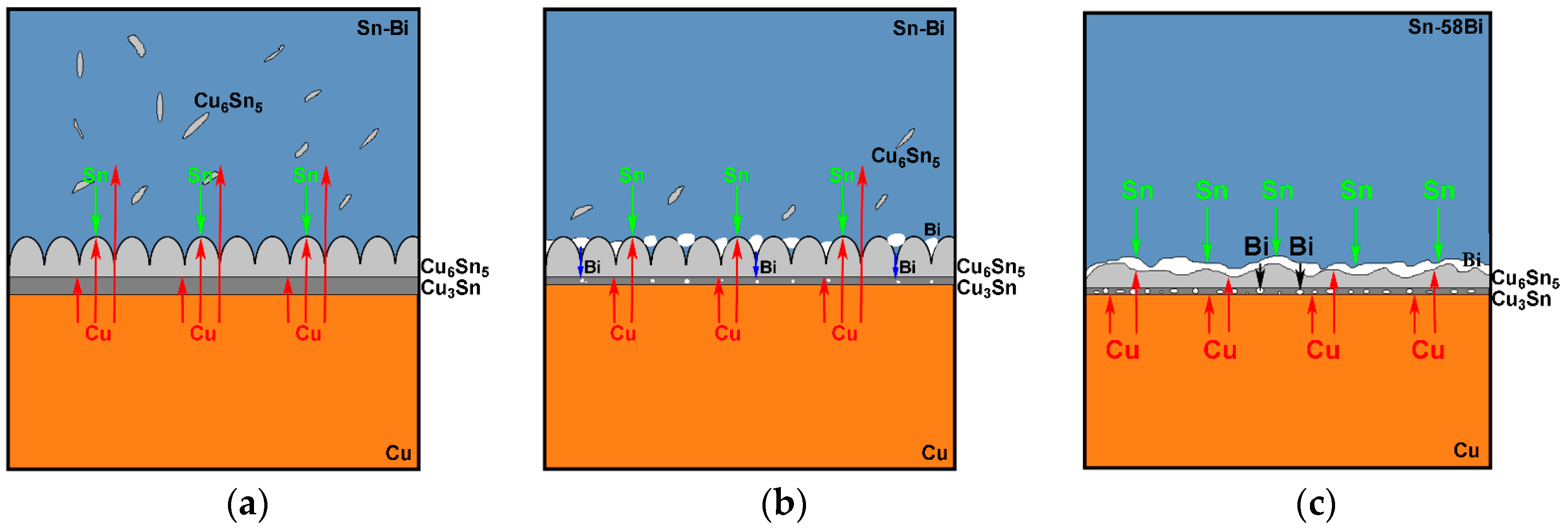
Figure 6, the addition of Bi into Sn solder changed not only the distribution of Cu-Sn IMC in the solder matrix but also the morphology of Cu-Sn IMC at the interface. Therefore, we try to explain the interfacial behaviors from the change on the diffusing fluxes of atoms caused from different Bi addition. The schematic diagrams on the effect of different Bi content in the Sn-Bi solder on the interfacial morphology and IMC growth behavior are illustrated in
Figure 8. It should be noted that Cu was the dominant species in Cu-Sn interdiffusion [
19]. In Sn-5Bi or Sn-15Bi solder joints illustrated in
Figure 8a, a Cu
6Sn
5 IMC layer with the scallop-type morphology was produced during soldering. Cu atoms were also diffused from the substrate into solder through the groove between two scallops, and then reacted with Sn atoms to form huge Cu
6Sn
5 precipitates within the solder matrix. During isothermal aging, Cu atoms diffused from the substrate not only reacted with Sn atoms diffused from the solder to promote the continuous growth of Cu
6Sn
5 IMC layer, but also reacted with the existing Cu
6Sn
5 IMC layer to form a Cu
3Sn layer between Cu
6Sn
5 and Cu. For the Cu
6Sn
5 IMC layer at the interface, besides the transition to Cu
3Sn, it was also broken to produce Cu
6Sn
5 flakes in the solder matrix close to the interface. The growth on IMC thickness was attributed to them. In Sn-30Bi or Sn-45Bi solder joint, as shown in
Figure 8b, Bi particles were precipitated in the grooves of the scallop-type Cu
6Sn
5 grains during soldering, which was not observed in
Figure 8a because Bi atoms were mainly dissolved in β-Sn phases to produce the solid solution phases for Sn-5Bi or Sn-15Bi. Therefore, the diffusion of Cu atoms into the solder matrix through the grooves of scallops was prohibited. On the other hand, with the growing on interfacial Cu
6Sn
5 IMC, more Bi atoms were also accumulated at the interface between solder and the IMC layer due to the depletion of Sn atoms, but were not enough to produce a continuous Bi-rich layer. These enriched Bi phases would inevitably promote the diffusion of Bi atoms from interface into the Cu
6Sn
5 phases. During the transition from Cu
6Sn
5 to Cu
3Sn according to Equation (4), the Bi atoms were then precipitated to produce Bi segregation at the interface between Cu
3Sn and Cu accompanied with the formation of Cu
3Sn.
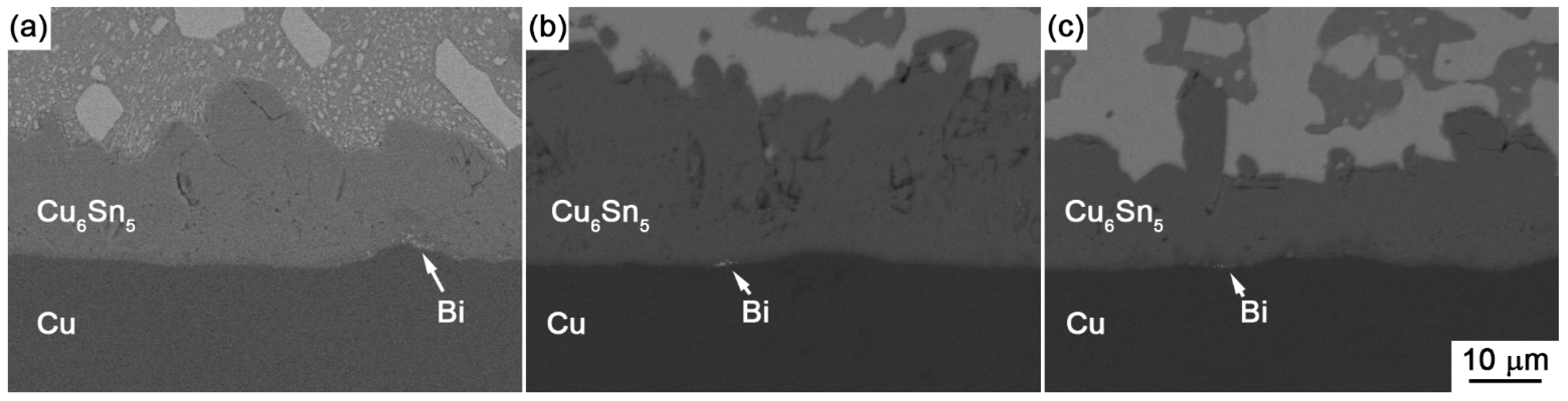
Figure 9a,b show the enlarged images on the interfaces of Sn-30Bi/Cu and Sn-45Bi/Cu aged joints with 30 days aging, respectively. The Bi segregation is clearly observed between Cu
6Sn
5 and Cu, which may be attributed to the reason that the solubility of Bi atoms in Cu
3Sn was lower than that in Cu
6Sn
5 [
20].
From
Figure 6, it is interesting to find that the formation of Cu
3Sn was very little. The transition from Cu
6Sn
5 to Cu
3Sn was almost depressed at the interface because the dissolution of Bi atoms into IMC possibly increased the driving force on the formation of Cu
3Sn. On the other hand, Bi precipitation in the grooves of Cu
6Sn
5 also inhibited the diffusion of Cu atoms into the solder matrix. Therefore, the diffusing Cu atoms were mainly accumulated at the interface, which promoted the rapid growth of the thickness of the Cu
6Sn
5 layer during isothermal aging due to the lack of formation of Cu
3Sn at the interface and Cu
6Sn
5 phases in the solder matrix. Similarly, as illustrated in
Figure 8c for Sn-58Bi solder, the difference from Sn-30Bi or Sn-45Bi hypoeutectic solder joint is the formation of a continuous Bi-rich layer close to the interface during isothermal aging. The transition from Cu
6Sn
5 to Cu
3Sn was also depressed by Bi segregation at the interface observed in
Figure 9c. The continuous Bi-rich layer partly blocked the interdiffusion of the Cu-Sn couple, and accordingly caused a slower growth rate of IMC at the interface compared with Sn-30Bi or Sn-45Bi hypoeutectic solder joint. In all of these solder joints, another effect on the IMC growth rate is the ratio of aging temperature to the solidus of solder, which is calculated as 0.84, 0.93, 0.99, 0.99 and 0.99 for Sn-5Bi, Sn-15Bi, Sn-30Bi, Sn-45Bi and Sn-58Bi, respectively. It means that there exists a higher diffusion rate of Cu and Sn atoms in Sn-Bi solders with higher Bi content.
3.3. Shear Properties on Sn-Bi Solder Joints
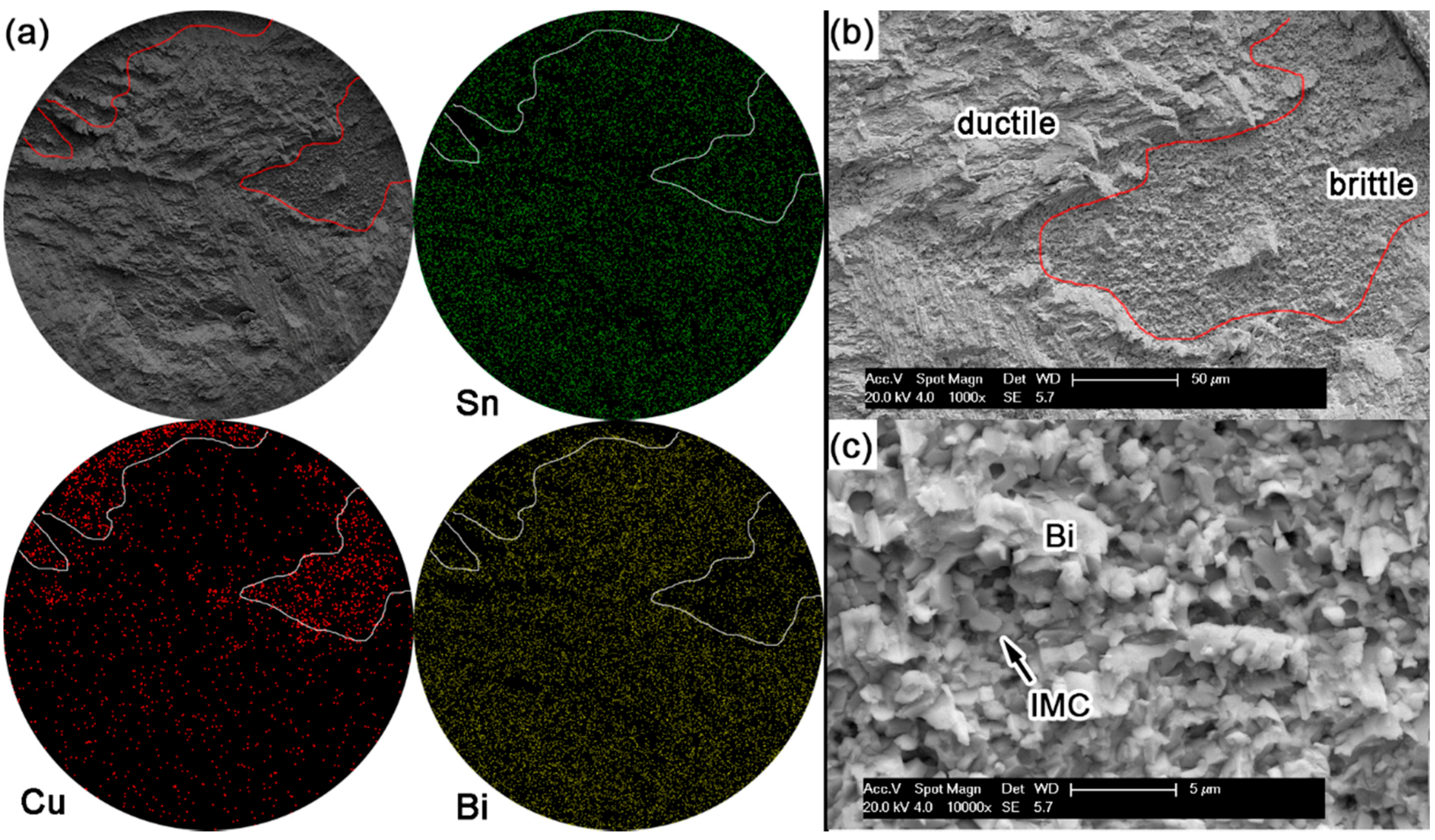
Figure 10 shows the displacement-load curves after ball shear tests on Sn-Bi as-soldered joints and the corresponding average shear strength with the effect of Bi content. The shear strength of Sn-Bi solder joints decreased with the increase in Bi content, and then slightly increased for Sn-58Bi eutectic solder. To obtain the effect of Bi content in Sn-Bi solder on the crack propagation and failure mode, the fracture surface morphologies and the corresponding element mapping were detected by EDS, and the results are shown in
Figure 11,
Figure 12,
Figure 13,
Figure 14 and
Figure 15. The shear direction was always from up to down for all solder joints in these images.
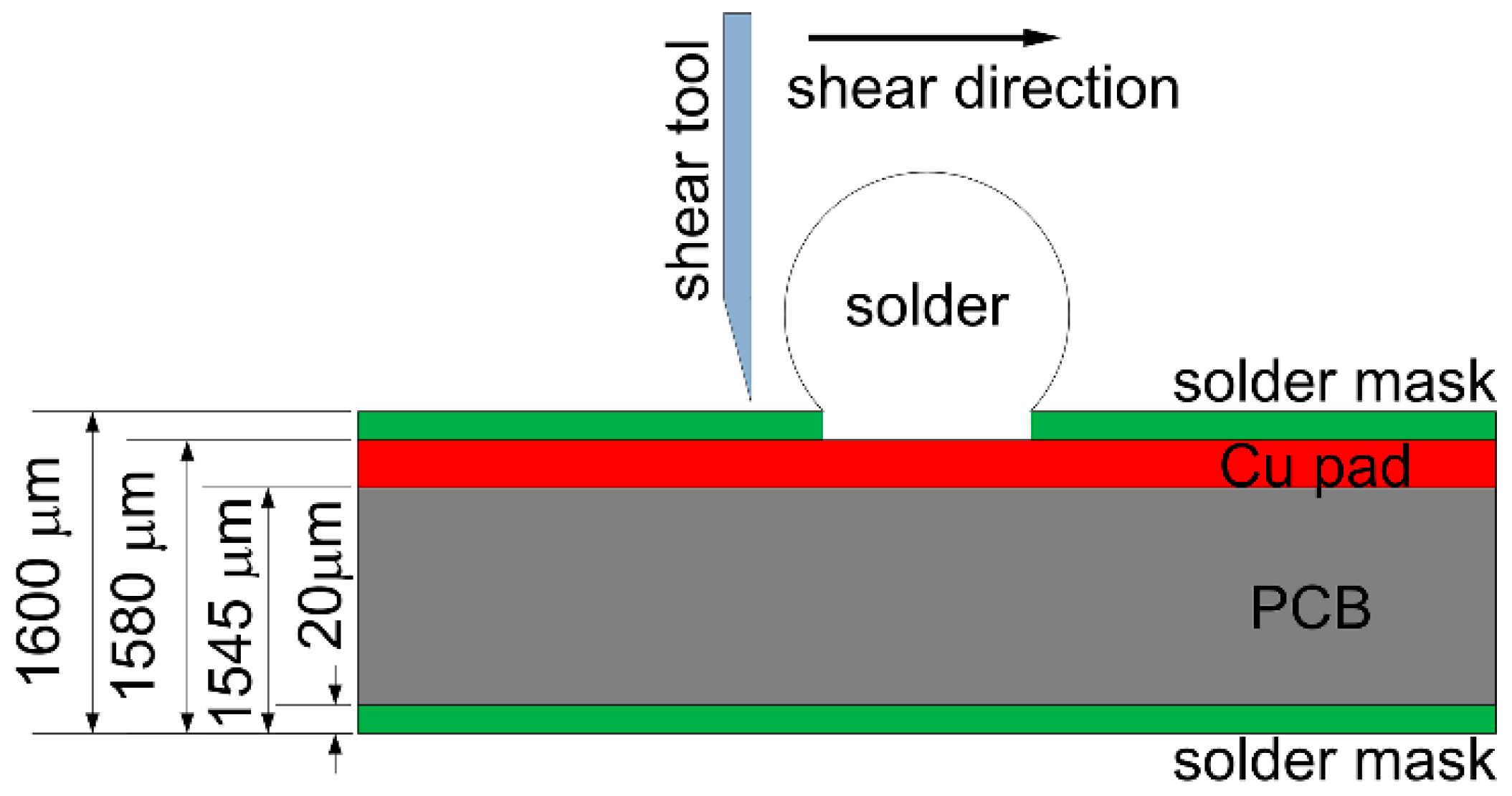
During the ball shear test, because there exists a shear height about 20 μm between the shear tool and the PCB surface, the shear force is always imposed on the bulk solder ball, and the joint strength accordingly reflects the bulk solder strength or the interfacial adhesion strength between solder and Cu substrate. If the shear strength of the bulk solder ball is higher than the interfacial adhesion strength, the fracture propagates along the interface between solder and Cu substrate, which represents a brittle failure mode. Otherwise, ductile failure mode will be observed with the fracture occurring inside the bulk solder ball. Meanwhile, there also exists the quasi-ductile failure mode with more than 50% fracture occurring inside the solder or the quasi-brittle failure mode with more than 50% fracture occurring along the interface, which represents a combination of partial ductile failure mode and partial brittle failure mode. The brittle failure area with fracture propagated along the interface is distinguished with the prominent Cu element mapping from the ductile failure area, and is also marked in
Figure 11,
Figure 12,
Figure 13,
Figure 14 and
Figure 15. It seems that Bi addition into Sn-based solder, even with 5 wt % Bi content, inevitably produces brittle failure mode, which is completely different from the results on Sn-Ag-Cu Pb-free solder. The ball shear test on Sn-Ag-Cu as-soldered joint always produces the ductile failure inside the bulk solder [
21,
22]. Their difference is attributed to the reason that Sn-Bi solder exhibits a higher strength but a weaker ductility than Sn-Ag-Cu solder [
23] and the Bi element is very prone to embrittlement [
6]. As observed from the fracture morphologies, the fracture surfaces almost contain all the above-mentioned failure modes: quasi-ductile mode for Sn-5Bi (
Figure 11), quasi-brittle mode for Sn-15Bi (
Figure 12), brittle mode for Sn-30Bi and Sn-45Bi (
Figure 13 and
Figure 14) and ductile to quasi-ductile mode for Sn-58Bi (
Figure 15).
In Sn-5Bi solder joints, the performance of bulk solder matrix was obviously improved by both Bi solid solution in the β-Sn phases and large amounts of Cu
6Sn
5 flakes produced from the soldering reaction, as observed in
Figure 5a. Therefore, during the ball shear test, partial brittle fracture occurred at the interface with the circular area marked in
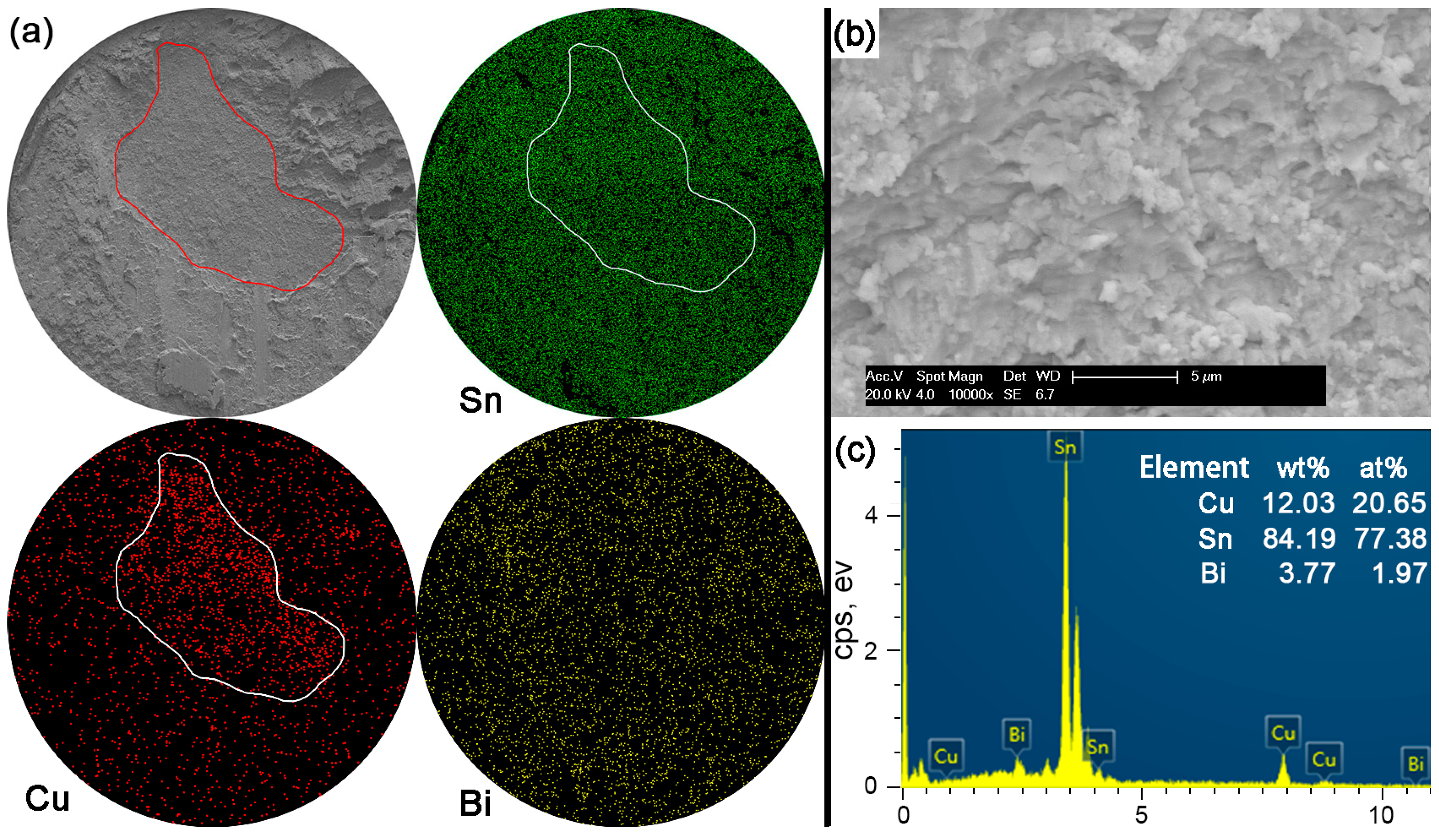
Figure 11a for Sn-5Bi solder bump, which represents the quasi-ductile failure mode because the retained solder area is more than 50%. The circular area was then observed with a 10,000× magnification and the fracture morphology is shown in
Figure 11b. EDS analysis was used to confirm the composition at this fracture surface with the result shown in
Figure 11c. The interfacial fractured area still contains much Sn-Bi solder with large amounts of Sn and a small amount of Bi besides the Cu atoms from Cu
6Sn
5.
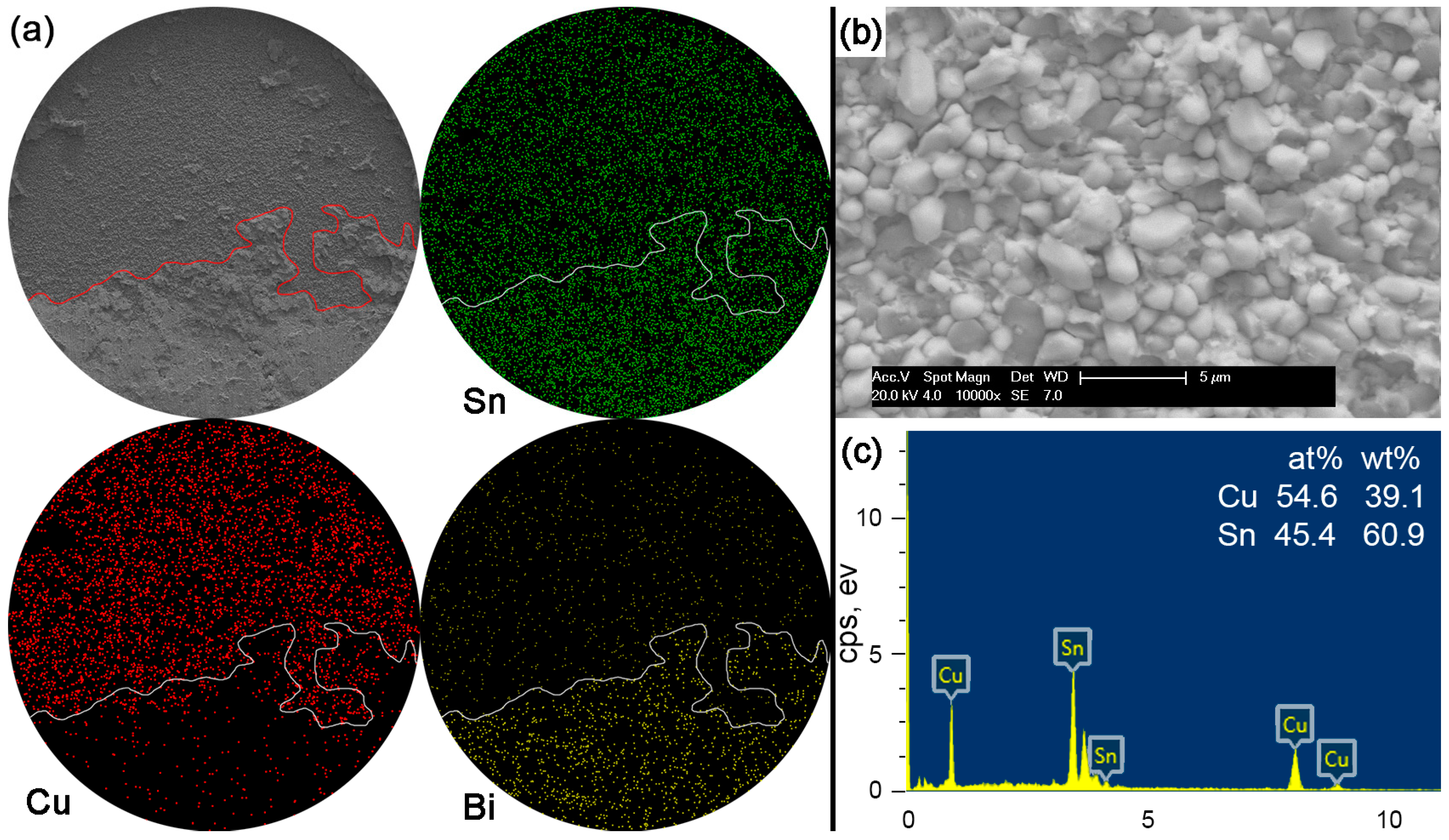
Figure 12 shows the fracture morphologies, elemental mapping and the EDS result on the interfacial fracture area for Sn-15Bi solder joints. Compared with the Sn-5Bi solder bump, a more brittle result occurred along the interface to reach a quasi-brittle failure mode with the interfacial fracture area more than 50%. Similarly, the brittle fracture was also observed with a higher magnification with the morphology shown in
Figure 12b. It is a typical scallop-type IMC exposed fracture morphology with very little retained solder. The composition of scallops was confirmed as the Cu
6Sn
5 phase by the EDS spectrum in
Figure 12c. Therefore, although both of the solder alloys were solid solution compositions, more Bi content in Sn-15Bi solder further increased the solder strength and resulted in more fractures along the interface instead of inside the bulk solder and less retained solder on the IMC exposed area compared with Sn-5Bi solder.
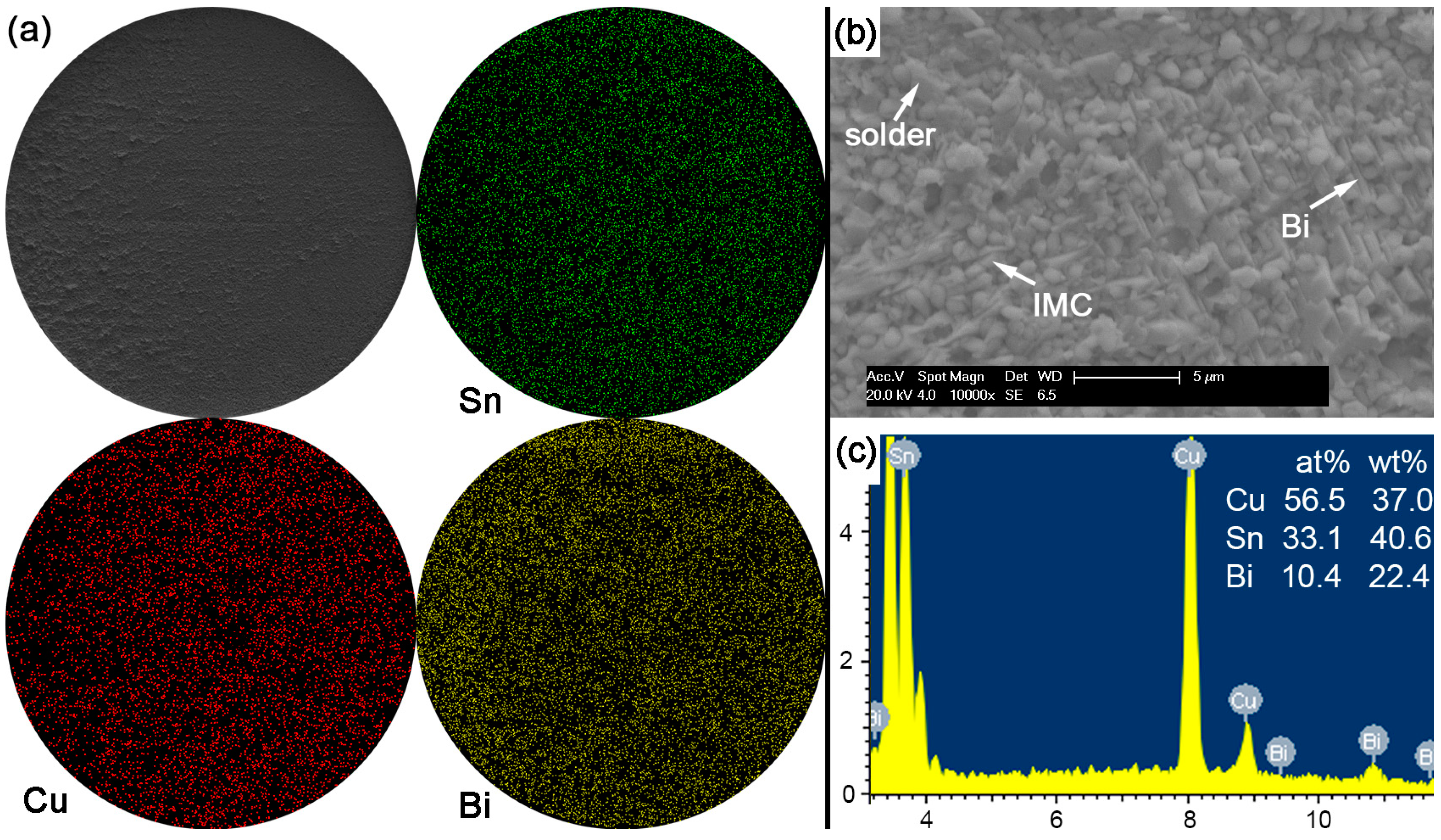
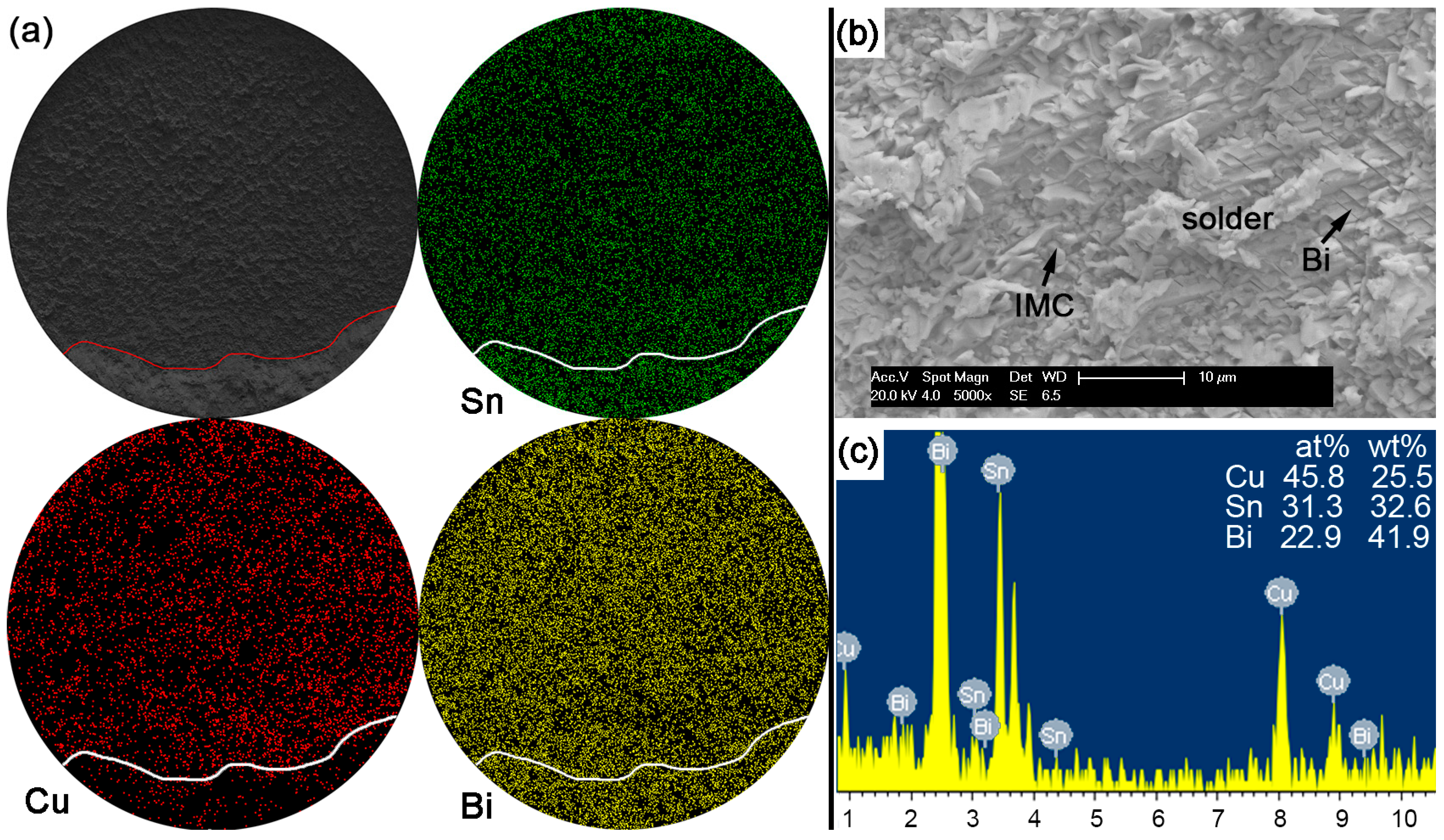
With Bi content in Sn-based solder increased to 30% or 45% in order to constitute the Sn-Bi hypoeutectic solder alloy, the fracture of solder joints in
Figure 13 or
Figure 14 completely occurred along the interface between solder and Cu substrate with a full brittle failure mode, although there still existed a small retained solder area in
Figure 14a for the Sn-45Bi solder joint. From the elemental mapping on fracture morphologies of Sn-30Bi and Sn-45Bi, it is interesting to find that the whole fracture was covered with the uniform distribution of Bi atoms besides the Cu atoms and Sn atoms from Cu
6Sn
5.
Figure 13b,c and
Figure 14b,c show the magnified observation on the brittle fracture area and the corresponding compositional analysis in Sn-30Bi and Sn-45Bi, respectively. In contrast to Sn-5Bi or Sn-15Bi solder bump, the solder residues and cleavage fracture from coarsened Bi phases were easily observed with the amounts increased with Bi content, which came from the Bi precipitation in the grooves or near the interface in
Figure 5c,d.
Figure 15 shows the elemental mapping on Sn-58Bi eutectic solder bump and the enlarged fracture morphologies. Most fractures occurred inside the solder. From the fracture morphology on the Sn-58Bi solder joint in
Figure 15a, most fractures occurred inside the bulk solder ball, which illustrates a ductile or ductile-to brittle fracture mode. With a highly magnified observation on the interfacial brittle area shown in
Figure 15c, small Bi particles were uniformly distributed on the IMC layer.
The shear strength of Sn-Bi solder bumps should be decided by the performances of solder and the fracture modes during ball shear tests. Ye et al. [
6] studied the effect of Bi content in Sn-Bi solder on the mechanical performance of bulk solder alloys, and found that the hardness of Sn-Bi solder reached the highest with Bi content at 7–15 wt %, and then decreased with continuous increasing on Bi content in Sn-Bi solder. Therefore, Sn-5Bi and Sn-15Bi solder bumps show a higher shear strength in
Figure 10, and the fracture mode also transits from quasi-ductile to quasi-brittle with the cracks mainly occurring inside the solder bump to the interface between the solder and IMC layer. However, it is interesting to discover that the fractures for Sn-30Bi and Sn-45Bi solder bumps fully occurred at the interface because they had a lower strength than Sn-5Bi and Sn-15Bi solder bumps. As shown in
Figure 8b, large amounts of Bi elements were segregated at the interface between the bulk solder and Cu
6Sn
5 IMC layer. In
Figure 13b and
Figure 14b, we also observed the existence of Bi cleavage at the fracture surfaces. It seems that the degradation on Sn-30Bi and Sn-45Bi solder joints were mainly attributed to Bi segregation at the interface. The brittle fracture mode occurring along the interface produces a lower shear strength on Sn-30Bi and Sn-45Bi solder bumps. Sn-58Bi solder had the lowest strength out of all of the Sn-Bi solder alloys in this study; accordingly, the fracture mainly occurred inside the solder bump because the shear strength of solder bump was lower than the interfacial strength, but we still observed the existence of Bi segregation at the interface from the small brittle area in
Figure 15c. Sn-58Bi solder joint also shows a slightly higher shear strength due to its ductile fracture mode.