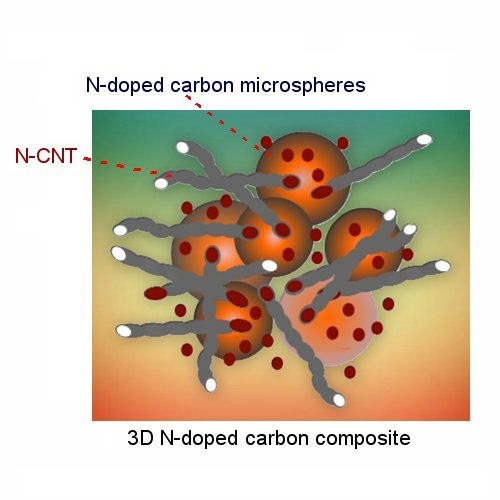
A Unique 3D Nitrogen-Doped Carbon Composite as High-Performance Oxygen Reduction Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Maghemite Nanoparticles
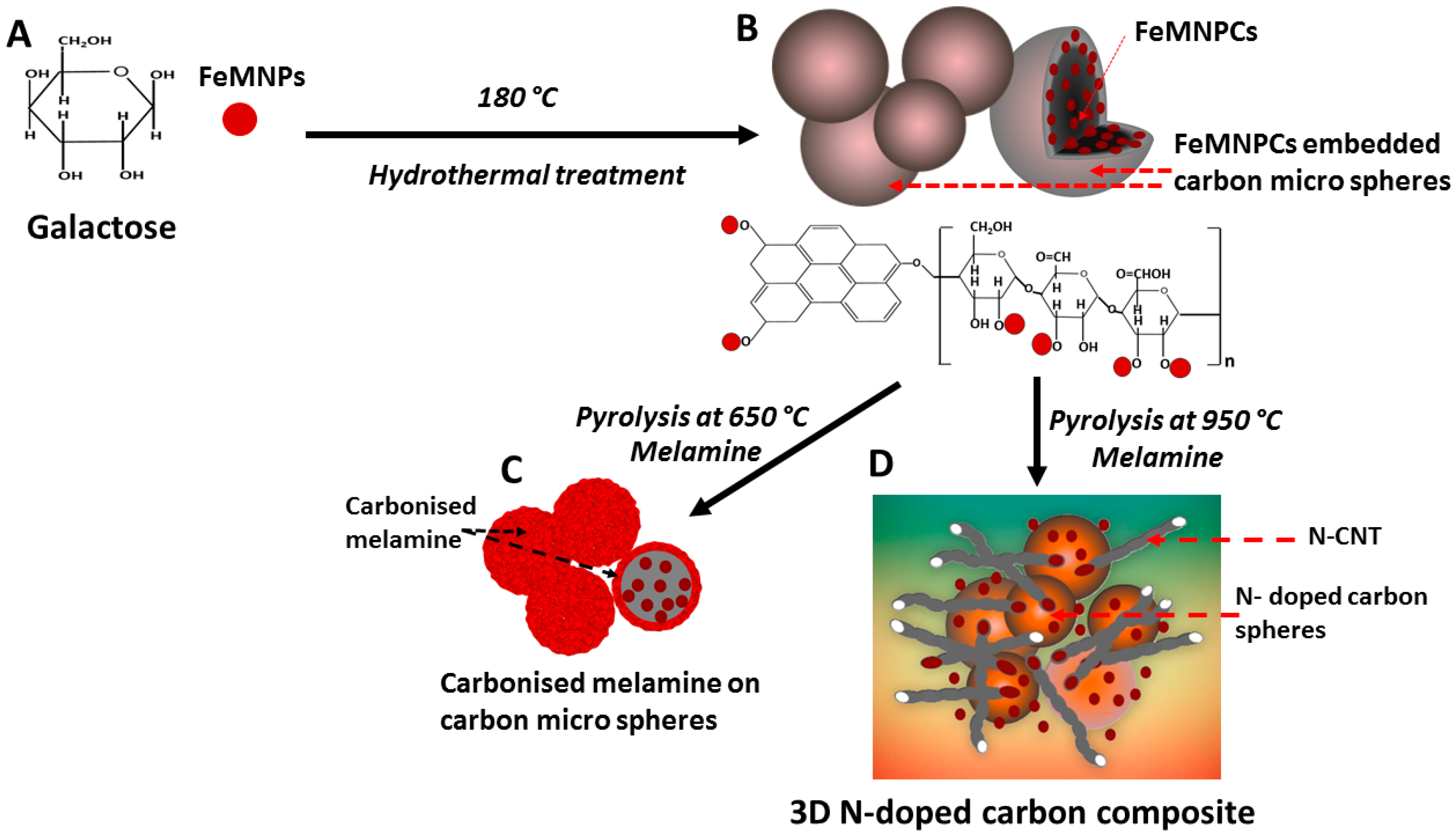
2.2.2. Synthesis of Iron Oxide Embedded Carbonaceous Spheres from Galactose
2.2.3. Synthesis of N-do ped Carbon Spheres with Iron Oxide Nanoparticles
2.2.4. Synthesis of Carbon Spheres Without Iron Oxide Nanoparticles
2.3. Preparation of Catalytic Inks
2.4. Characterisation
Electrochemical Characterisation
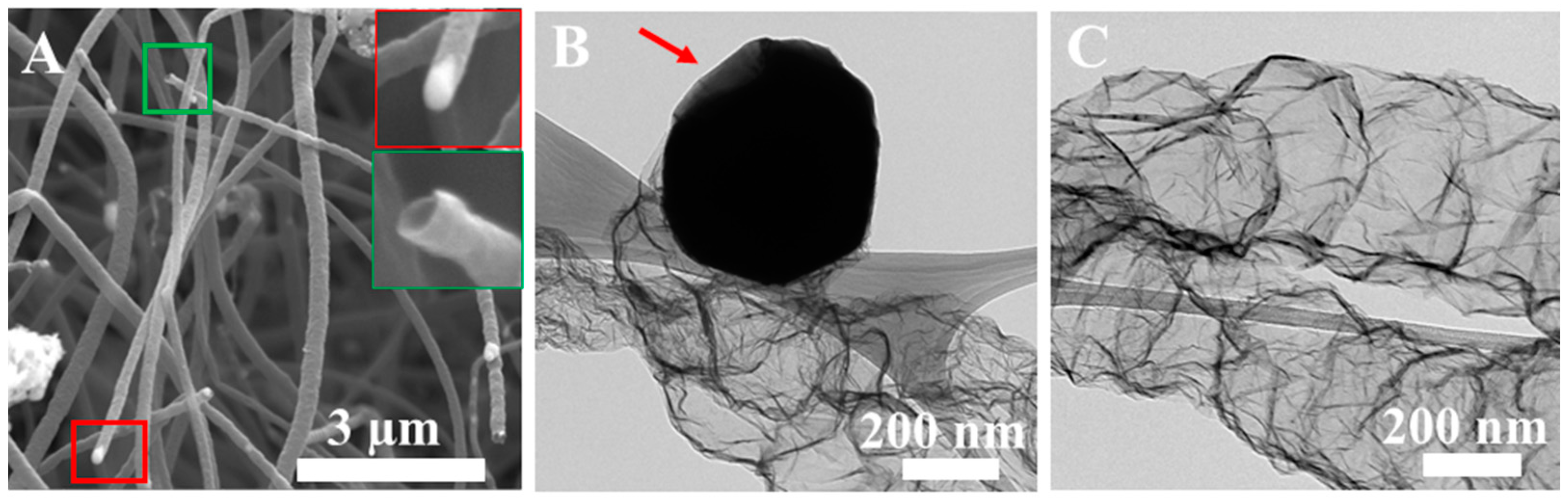
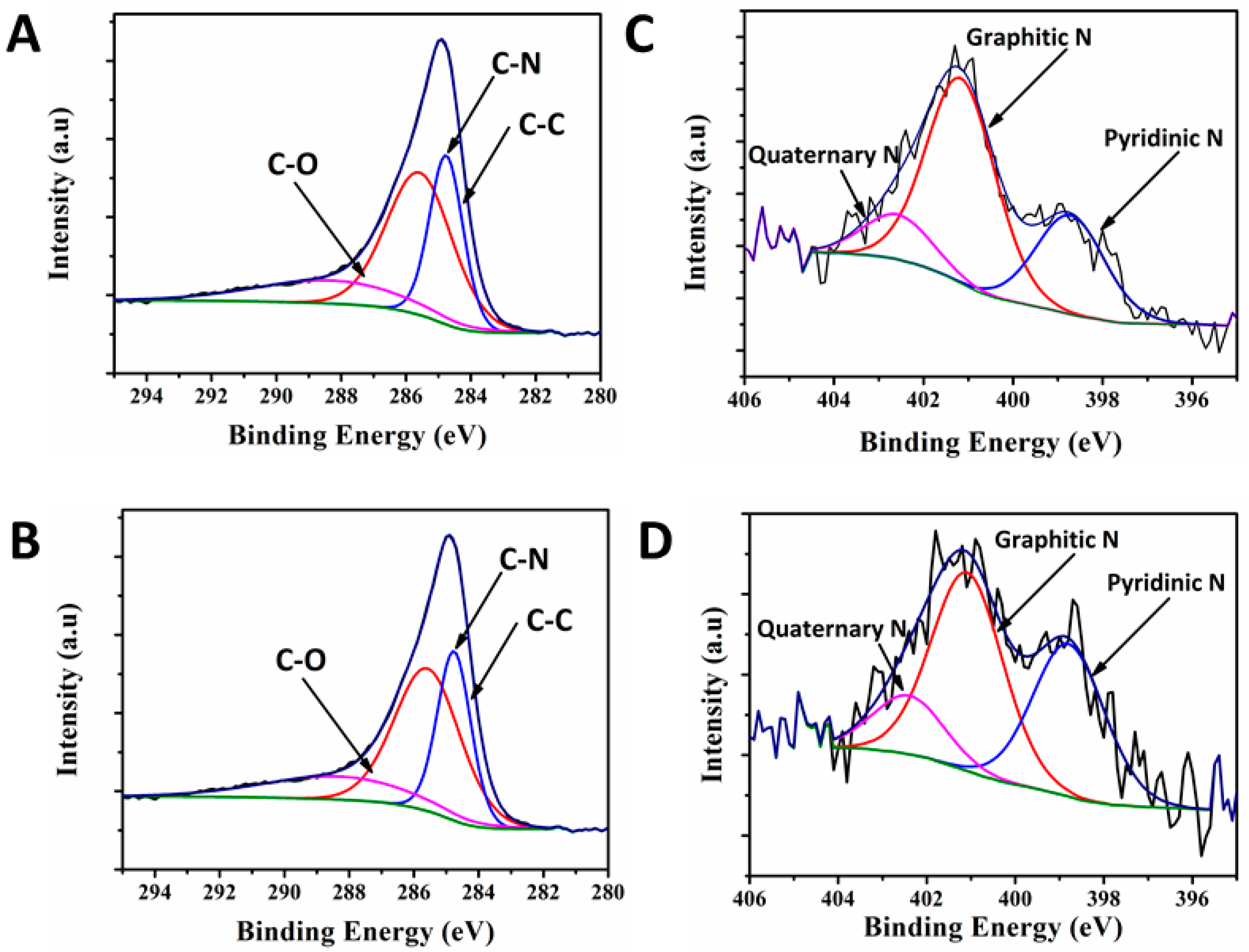
3. Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balat, M. Global Bio-Fuel Processing and Production Trends. Energy Explor. Exploit. 2007, 25, 1–25. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, E.; Ruan, M.; Song, P.; Xu, W. Recent progress on Fe/N/C electrocatalysts for the oxygen reduction reaction in fuel cells. Catalysts 2015, 5, 1167–1192. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.T.; Hor, T.A.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanics to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Byon, H.R.; Suntivich, J.; Shao-Horn, Y. Graphene-based non-noble-metal catalysts for oxygen reduction reaction in acid. Chem. Mater. 2011, 23, 3421–3428. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with highelectrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Eviron. Sci. 2014, 7, 576–591. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Ganesan, P.; Popov, B.N. Development of non-precious metal oxygen-reduction catalysts for PEM fuel cells based on N-doped ordered porous carbon. Appl. Catal. B 2009, 93, 156–165. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Yao, M.; Liu, S. Metal-free nitrogen-doped hollow carbon spheres synthesized by thermal treatment of poly (O-phenylenediamine) for oxygen reduction reaction in direct methanol fuel cell applications. J. Mater. Chem. 2012, 22, 10911–10917. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Z.; Nie, H.; Yao, Z.; Zhang, L. Catalyst-free growth of large scale nitrogen-doped carbon spheres as efficient electrocatalysts for oxygen reduction in alkaline medium. J. Power Sources 2011, 196, 9970–9974. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Lieder, M.; Jablonska, M. N-doped mesoporous carbon nanosheets obtained by pyrolysis of a chitosan–melamine mixture for the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 44969–44977. [Google Scholar] [CrossRef]
- Feng, L.; Yang, L.; Huang, Z.; Luo, J.; Li, M.; Wang, D.; Chen, Y. Enhancing electrocatalytic oxygen reduction on nitrogen-doped graphene by active sites implantation. Sci. Rep. 2013, 3, 3306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Shi, C.X.; Xu, X.Y.; Sun, P.C.; Chen, T.H. Nitrogen-doped hierarchically porous carbon spheres as efficient metal-free electrocatalysts for an oxygen reduction reaction. J. Power Sources 2015, 283, 389–396. [Google Scholar] [CrossRef]
- Yang, T.; Liu, J.; Zhou, R.; Chen, Z.; Xu, H.; Qiao, S.Z.; Monteiro, M.J. N-doped mesoporous carbon spheres as the oxygen reduction reaction catalysts. J. Mater. Chem. A 2014, 2, 18139–18146. [Google Scholar] [CrossRef]
- He, Y.; Han, X.; Du, Y.; Song, B.; Xu, P.; Zhang, B. Bifunctional Nitrogen-Doped Microporous Carbon Microspheres Derived from Poly(O-methylaniline) for Oxygen Reduction and Supercapacitors. ACS Appl. Mater. Interfaces. 2016, 6, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Nallathambi, V.; Lee, J.W.; Kumaraguru, S.P.; Wu, G.; Popov, B.N. Development of high performance carbon composites catalyst for oxygen reduction reaction in PEM Proton Exchange Membrane Fuel Cell. J. Power Sources. 2008, 183, 34–42. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.-J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Zheng, Y.; Li, L.H.; Cowie, B.C.; Gunzelmann, D.; Qiao, S.Z.; Huang, S.; Chen, Y. Observation of Active Sites for Oxygen Reduction Reaction on Nitrogen-Doped Multilayer Graphene. ACS Nano 2014, 8, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.T.K.; Harris, P.S.; Terry, S. Unique form of filamentous carbon. Nature 1975, 253, 37–39. [Google Scholar] [CrossRef]
- Matter, P.H.; Ozkan, U.S. Non-metal catalysts for dioxygen reduction in an acidic electrolyte. Catal. Lett. 2006, 109, 15–123. [Google Scholar] [CrossRef]
- Darezereshki, E. Synthesis of maghemite (γ-Fe2O3) nanoparticles by wet chemical method at room temperature. Mater. Lett. 2010, 64, 1471–1472. [Google Scholar] [CrossRef]
- Yu, G.; Sun, B.; Pei, Y.; Xie, S.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. FexOy@C spheres as an excellent catalyst for Fischer− Tropsch synthesis. J. Am. Chem. Soc. 2009, 3, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Wirth, C.T.; Bayer, B.C.; Gamalski, A.D.; Esconjauregui, S.; Weatherup, R.S.; Ducati, C.; Baehtz, C.; Robertson, J.; Hofmann, S. The phase of iron catalyst nanoparticles during carbon nanotube growth. Chem. Mater. 2012, 24, 4633–4640. [Google Scholar] [CrossRef]
- Terrones, M.; Hsu, W.K.; Kroto, H.W.; Walton, D.R. Nanotubes: A revolution in materials science and electronics. In Fullerenes and Related Structures; Springer: Berlin, Germany, 1999; Volume 199, pp. 189–234. [Google Scholar]
- Terrones, M.; Terrones, H.; Grobert, N.; Hsu, W.; Zhu, Y.; Hare, J.; Kroto, H.; Walton, D.; Kohler-Redlich, P.; Rühle, M. Efficient route to large arrays of CNx nanofibers by pyrolysis of ferrocene/melamine mixtures. Appl. Phys. Lett. 1999, 75, 3932–3934. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Huang, X.; Wang, Q.; Mei, A.; Shen, P.K. Highly stable electrocatalysts supported on nitrogen-self-doped three-dimensional graphene-like networks with hierarchical porous structures. J. Mater. Chem. A 2015, 3, 1492–1497. [Google Scholar] [CrossRef]
- Wu, Z.S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Peng, H.; Mo, Z.; Liao, S.; Liang, H.; Yang, L.; Luo, F.; Song, H.; Zhong, Y.; Zhang, B. High Performance Fe- and N- Doped Carbon Catalyst with Graphene Structure for Oxygen Reduction. Sci. Rep. 2013, 3, 1765. [Google Scholar] [CrossRef]
- Liu, Y.L.; Xu, X.Y.; Shi, C.X.; Ye, X.W.; Sun, P.C.; Chen, T.H. Iron- nitrogen co doped hieratchically mesoporous carbon spheres as highly efficient electrocatalysts for oxugen reduction reaction. RSC Adv. 2017, 7, 8879–8885. [Google Scholar] [CrossRef]
- Narayanamoorthy, B.; Datta, K.; Balaji, S. Kinetics and mechanism of electrochemical oxygen reduction using Platinum/clay/Nafion catalyst layer for polymer electrolyte membrane fuel cells. J. Colloid Interface Sci. 2012, 387, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Xu, A.W. Noble-metal-free Fe-N/C catalysts for Highly Efficient Oxygen Reduction Reaction under both Alkaline and Acidic Conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.P.; Li, X.; Nallathambi, V.; Kumaraguru, S.P.; Colon-Mercado, H.; Wu, G.; Lee, J.W.; Popov, B.N. Nitrogen-modified carbon-based catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. J. Power Sources 2009, 188, 38–44. [Google Scholar] [CrossRef]
- Damjanovic, A.; Genshaw, M.A.; Bockris, J.O. Distinction between Intermediates Produced in Main and Side Electrodic Reactions. J. Chem. Phys. 1966, 45, 4057–4059. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]

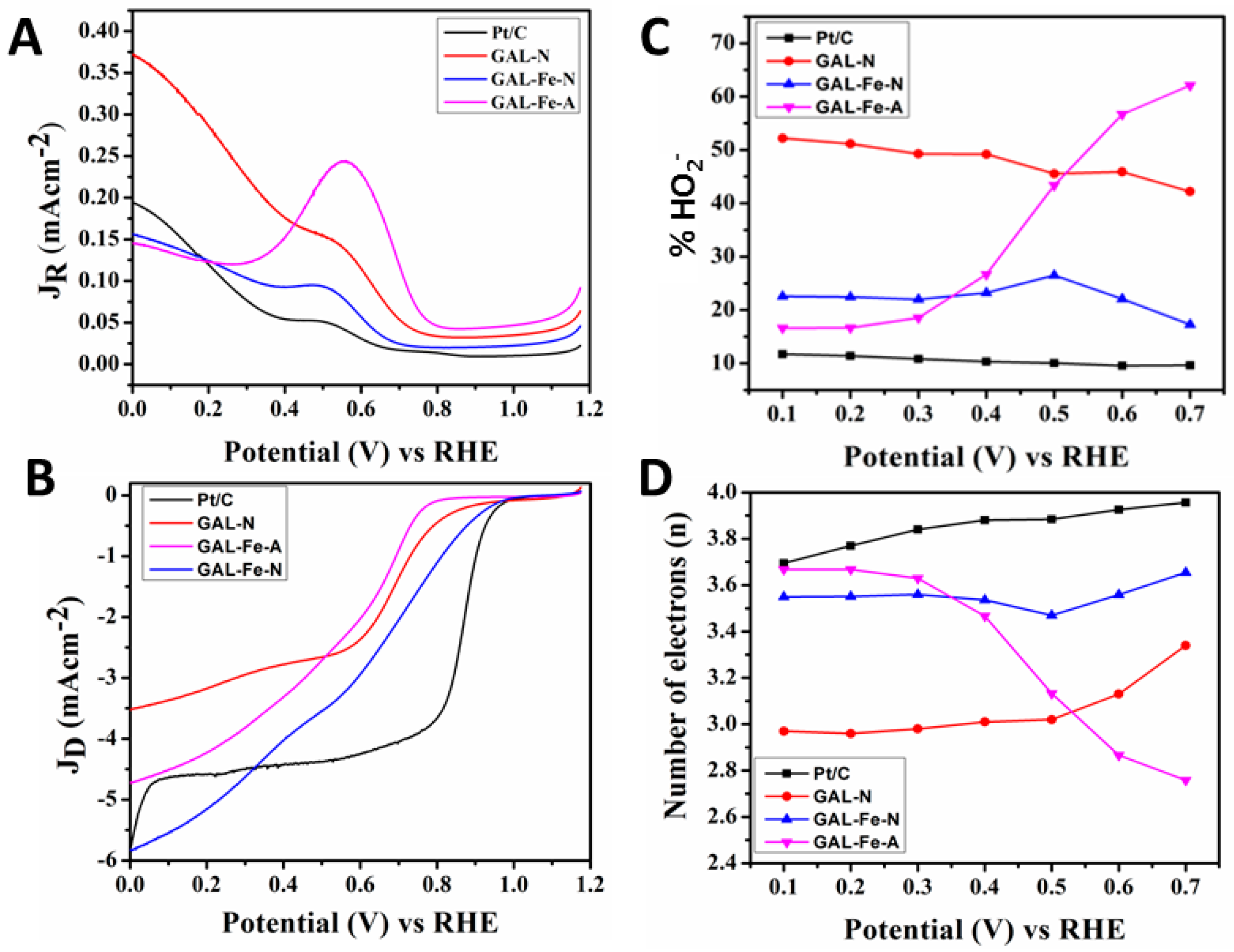

| Catalyst | Onset Overpotential (V) RHE | Number of Electrons (n) (0.1–0.7 V) RHE | % HO2− (0.1–0.7 V) RHE |
|---|---|---|---|
| GAL-N | 0.33 | 2.97–3.33 | 52.11–42.09 |
| GAL-Fe-A | 0.45 | 3.66–2.75 | 16.39–62.31 |
| GAL-Fe-N | 0.29 | 3.55–3.64 | 22.44–16.96 |
| Pt/C | 0.26 | 3.71–3.97 | 11.67–9.59 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunagaran, R.; Tung, T.T.; Shearer, C.; Tran, D.; Coghlan, C.; Doonan, C.; Losic, D. A Unique 3D Nitrogen-Doped Carbon Composite as High-Performance Oxygen Reduction Catalyst. Materials 2017, 10, 921. https://doi.org/10.3390/ma10080921
Karunagaran R, Tung TT, Shearer C, Tran D, Coghlan C, Doonan C, Losic D. A Unique 3D Nitrogen-Doped Carbon Composite as High-Performance Oxygen Reduction Catalyst. Materials. 2017; 10(8):921. https://doi.org/10.3390/ma10080921
Chicago/Turabian StyleKarunagaran, Ramesh, Tran Thanh Tung, Cameron Shearer, Diana Tran, Campbell Coghlan, Christian Doonan, and Dusan Losic. 2017. "A Unique 3D Nitrogen-Doped Carbon Composite as High-Performance Oxygen Reduction Catalyst" Materials 10, no. 8: 921. https://doi.org/10.3390/ma10080921