Effects of Adding Polysaccharides and Citric Acid into Sodium Dihydrogen Phosphate Mixing Solution on the Material Properties of Gelatin-Hybridized Calcium-Phosphate Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of α-TCP Powder
2.2. Preparation of HAp Powder Surface-Modified with IP6
2.3. Preparation and Characterization of Gelatin Particles
2.4. Preparation of Mixing Solutions for Cement Fabrication
2.5. Preparation of the Cement Pastes Hybridized with the Gelatin Particles
2.6. Evaluation of the Cement Pastes and Set Specimens
3. Results and Discussion
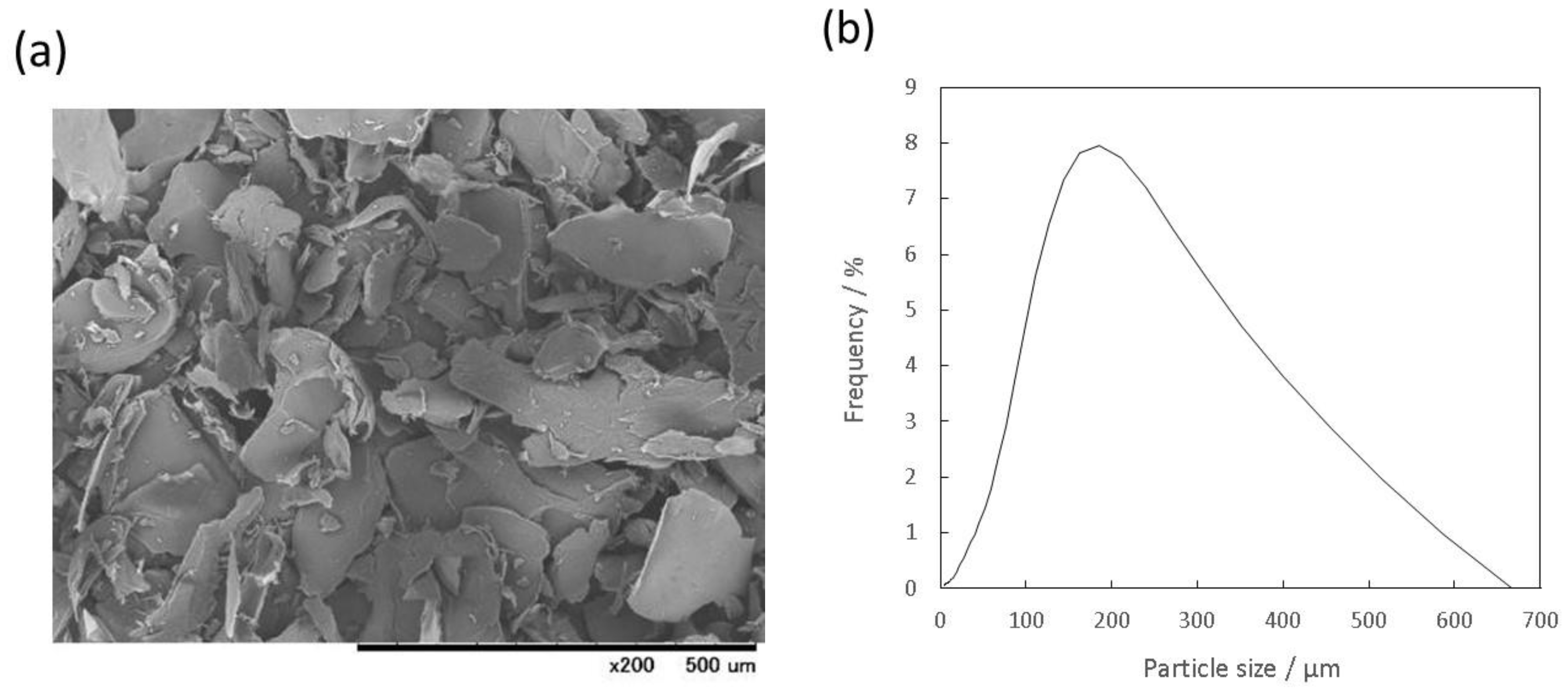
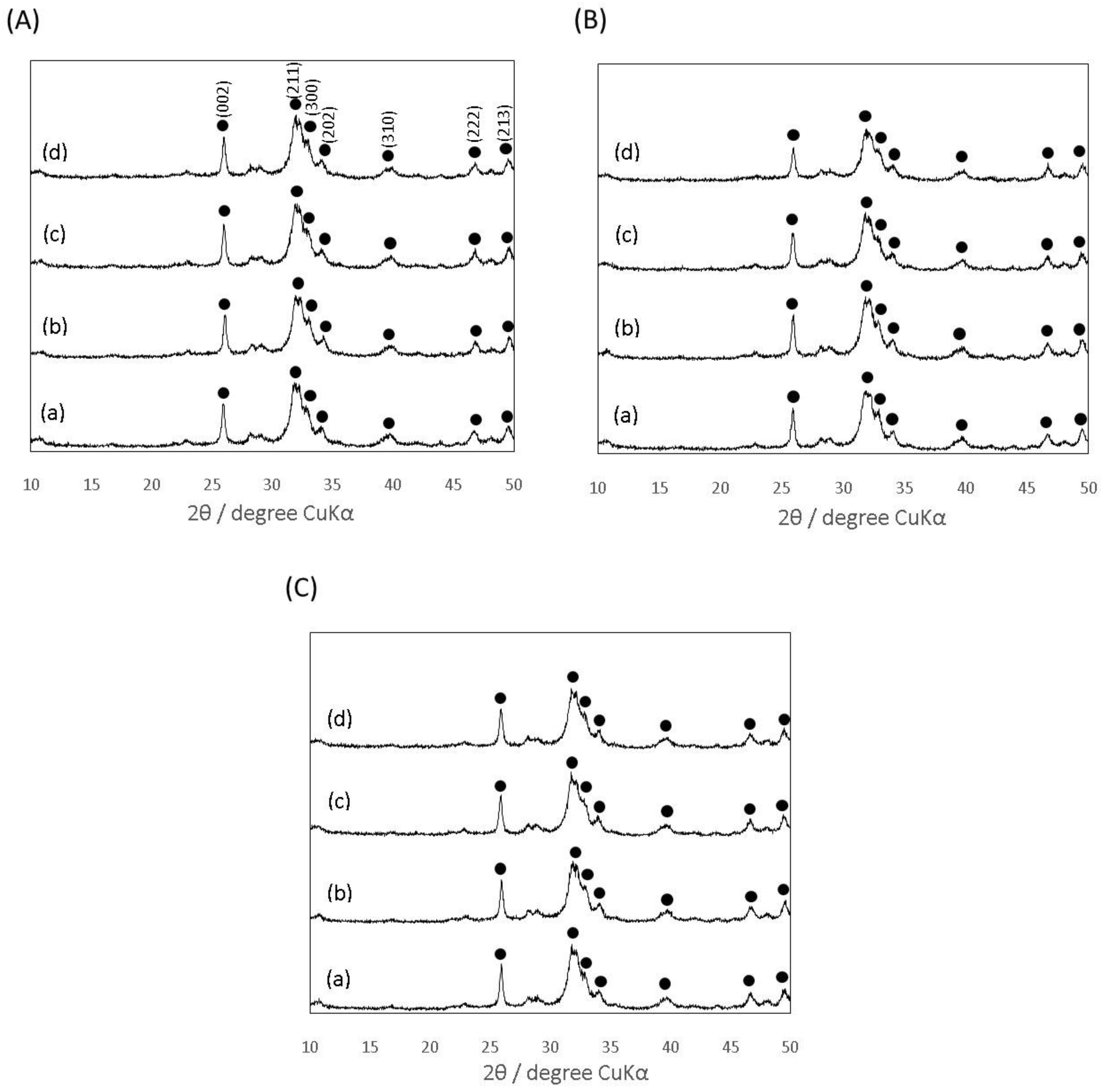
3.1. Characterization of the Prepared Gelatin Particles
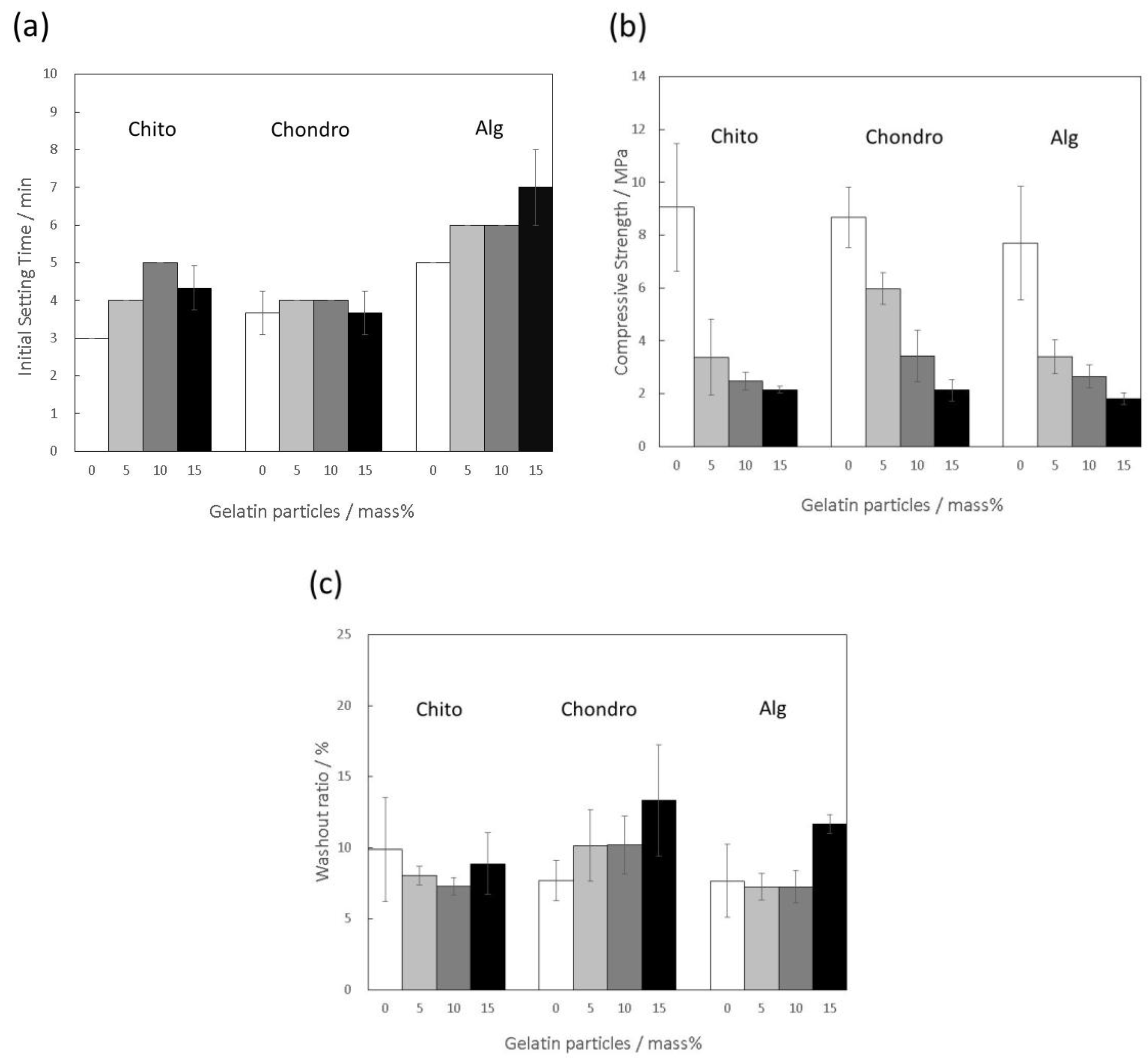
3.2. Effects of Additions of Polysaccharides into the Mixing Solution on Material Properties of the Gelatin-Hybridized Cement
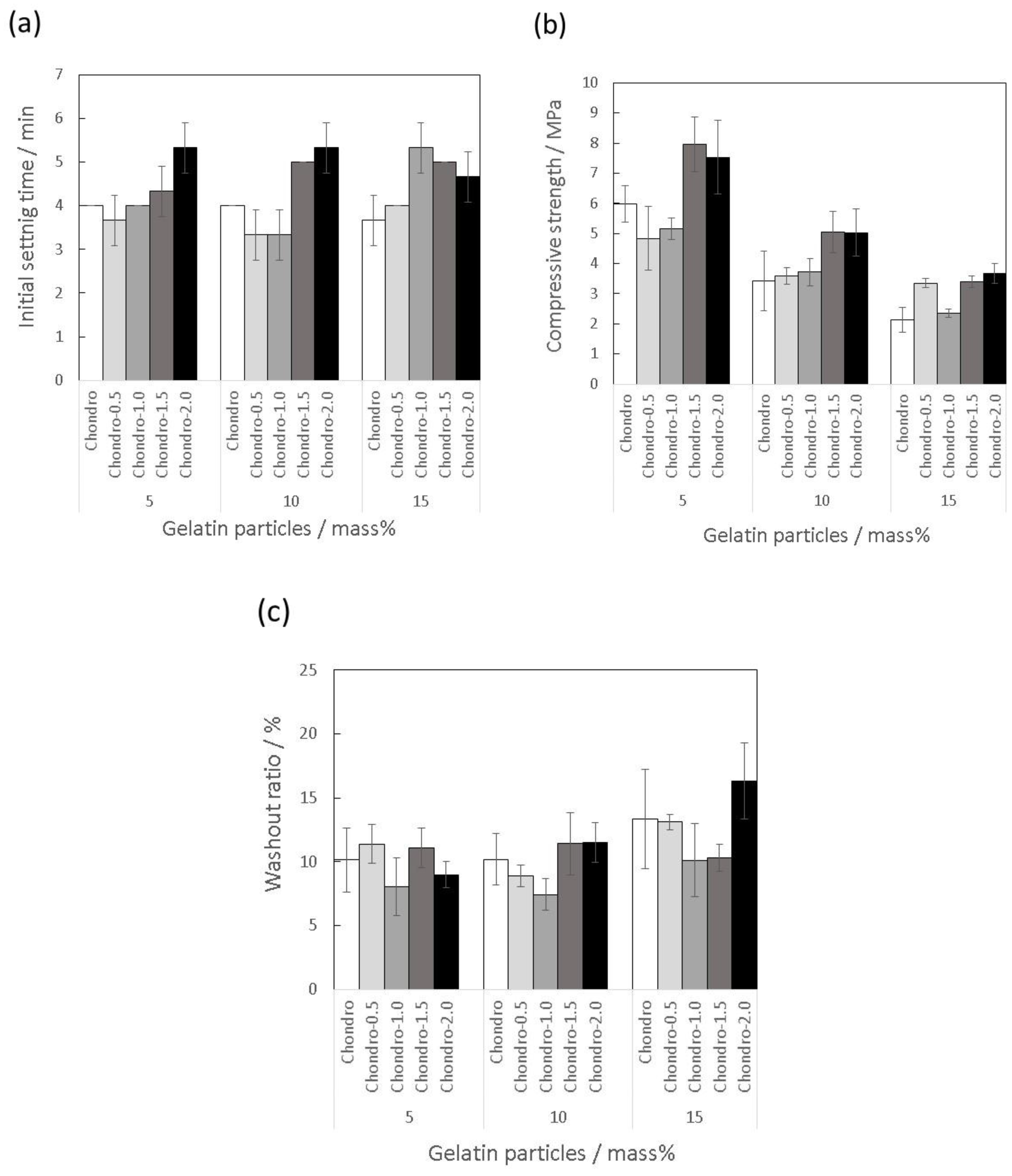
3.3. Effects of Addition of Citric Acid into the Mixing Solution Containing Chondroitin Sulfate on Material Properties of the Gelatin-Hybridized Cement
3.4. Summary
4. Discussion
Author Contributions
Conflicts of Interest
References
- Wang, X.; Chen, L.; Xiang, H.; Ye, J. Influence of anti-washout agents on the rheological properties and injectability of a calcium phosphate cement. J. Biomed. Mater. Res. Part B 2007, 81, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Miyamoto, Y.; Ishikawa, K.; Toh, T.; Yuasa, T.; Nagayama, M.; Suzuki, K. Initial histological evaluation of anti-washout type fast-setting calcium phosphate cement following subcutaneous implantation. Biomaterials 1998, 19, 2057–2063. [Google Scholar] [CrossRef]
- Aizawa, M.; Haruta, Y.; Okada, I. Development of novel cement processing using hydroxyapatite particles modified with inositol phosphate. Arch. Bioceram. Res. 2003, 3, 134–138. [Google Scholar]
- Kida, K.; Horiguchi, Y.; Oribe, K.; Morisue, H.; Matsumoto, M.; Toyama, Y.; Aizawa, M. Biological evaluation of chelate-setting apatite cement using inositol phosphate. Key Eng. Mater. 2008, 361, 335–338. [Google Scholar] [CrossRef]
- Kiminami, K.; Matsuoka, K.; Konishi, T.; Mizumoto, M.; Honda, M.; Arimura, H.; Aizawa, M. Effects of addition of α-tricalcium phosphate powders on material properties of the chelate-setting hydroxyapatite cement. Phosphorus Res. Bull. 2017, 33, 7–13. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.E.M.; Liao, H.B.; Zhang, Z.; Wolke, J.G.C.; Grijpma, D.W.; Mikos, A.G.; Feijen, J.; Jansen, J.A. In vivo degradation of calcium phosphate cement incorporated into biodegradable microspheres. Acta Biomater. 2010, 6, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Mikos, A.G.; Jansen, J.A. Injectable PLGA microsphere/calcium phosphate cements: Physical properties and degradation characteristics. J. Biomater. Sci. Polym. Ed. 2006, 17, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Ruhé, P.Q.; Hedberg-Dirk, E.L.; Padron, N.T.; Spauwen, P.H.M.; Jansen, J.A.; Mikos, A.G. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. J. Tissue Eng. 2006, 12, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Li, D.C.; He, J.K.; Wang, Z. Mechanical properties and in vivo performance of calcium phosphate cement-chitosan fibre composite. Proc. Inst. Mech. Eng. Part H 2008, 222, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, C.; Muzzarelli, R.A.A. Natural and artificial chitosan-inorganic composites. J. Inorg. Biochem. 2002, 92, 89–94. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Mikos, A.G.; Jansen, J.A. Porcine gelatin microsphere/calcium phosphate cement composites: An in vitro degradation study. J. Biomed. Mater. Res. Part B 2009, 91, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Link, D.P.; van den Dolder, J.; van den Beucken, J.J.J.P.; Habraken, W.; Soede, A.; Boerman, O.C.; Mikos, A.G.; Jansen, J.A. Evaluation of an orthotopically implanted calcium phosphate cement containing gelatin microparticles. J. Biomed. Mater. Res. Part A 2009, 90, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.E.M.; de Jonge, L.T.; Wolke, J.G.C.; Yubao, L.; Mikos, A.G.; Jansen, J.A. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J. Biomed. Mater. Res. Part A 2008, 87, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Panzavolta, S.; Sturba, L.; Torricelli, P.; Fini, M.; Giardino, R. Normal and osteopenic bone-derived osteoblast response to a biomimetic gelatin-calcium phosphate bone cement. J. Biomed. Mater. Res. Part A 2006, 78, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Valle, S.D.; Altankov, G.; Ginebra, M.P. Porous hydroxyapatite and gelatin/hydroxyapatite microspheres obtained by calcium phosphate cement emulsion. J. Biomed. Mater. Res. Part B 2011, 97, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, G.; Sugita, Y.; Kubo, K.; Yoshida, W.; Ikada, Y.; Sobajima, S.; Neo, M.; Maeda, H.; Kinoshita, Y. Gelatin powders accelerate the resorption of calcium phosphate cement and improve healing in the alveolar ridge. J. Biomater. Appl. 2013, 28, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Yomoda, M.; Sobajima, S.; Kasuya, A.; Neo, M. Calcium phosphate cement—Gelatin powder composite testing in canine models: Clinical implications for treatment of bone defects. J. Biomater. Appl. 2015, 29, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, A.; Sobajima, S.; Kinoshita, M. In vivo degradation and new bone formation of calcium phosphate cement-gelatin powder composite related to macroporosity after in situ gelatin degradation. J. Orthop. Res. 2012, 30, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.Y.; Ho, C.C.; Chen, D.C.H.; Lai, M.H.; Ding, S.J. Physicochemical properties and biocompatibility of chitosan oligosaccharide/gelatin/calcium phosphate hybrid cements. Mater. Chem. Phys. 2010, 120, 282–288. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Takahashi, K.; Sato, T. Preparation and compressive strength of α-tricalcium phosphate/gelatin gel composite cement. J. Biomed. Mater. Res. Part A 2001, 54, 525–530. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Takeuchi, M.; Toh, T.; Kon, M.; Nagayama, M.; Asaoka, K. Non-decay type fast-setting calcium phosphate cement: Hydroxyapatite putty containing an increased amount of sodium alginate. J. Biomed. Mater. Res. Part A 1997, 36, 393–399. [Google Scholar] [CrossRef]
- Tamimi-Mariño, F.; Mastio, J.; Rueda, C.; Blanco, L.; López-Cabarcos, E. Increase of the final setting time of brushite cements by using chondroitin 4-sulfate and silica gel. J. Mater. Sci. Mater. Med. 2007, 18, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Sarda, S.; Fernández, E.; Nilsson, M.; Balcells, M.; Planell, J.A. Kinetic study of citric acid influence on calcium phosphate bone cements as water-reducing agent. J. Biomed. Mater. Res. Part A 2002, 61, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Yamamoto, S.; Kawasaki, T.; Kohgo, T.; Nakatsu, M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials 2002, 23, 1091–1101. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous biphasic calcium phosphate ceramics: Influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar] [CrossRef]
- Schneiders, W.; Reinstorf, A.; Biewener, A.; Serra, A.; Grass, R.; Kinscher, M.; Heineck, J.; Rehberg, S.; Zwipp, H.; Rammelt, S. In vivo effects of modification of hydroxyapatite/collagen composites with and without chondroitin sulphate on bone remodeling in the sheep tibia. J. Orthop. Res. 2009, 27, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Self-setting calcium orthophosphate formulations: Cements, concretes, pastes and putties. Int. J. Mater. Chem. 2011, 1, 1–48. [Google Scholar] [CrossRef]
- Mosekilde, L.; Mosekilde, L. Normal vertebral body size and compressive strength: Relations to age and to vertebral and iliac trabecular bone compressive strength. Bone 1986, 7, 207–212. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfegheian, A.; Madihally, S.V. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Fernández, E.; de Maeyer, E.A.P.; Verbeeck, R.M.H.; Boltong, M.G.; Ginebra, J.; Driessens, F.C.M.; Planell, J.A. Setting reactions and hardening of an apatitic calcium phosphate cement. J. Dent. Res. 1997, 76, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Monma, H.; Goto, M.; Kohmura, T. Effect of additives on hydration and hardening of tricalcium phosphate. Gypsum Lime 1984, 188, 11–16. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ishikawa, K.; Takeuchi, M.; Toh, T.; Yuasa, T.; Nagayama, M.; Suzuki, K. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J. Biomed. Mater. Res. Part A 1999, 48, 36–42. [Google Scholar] [CrossRef]

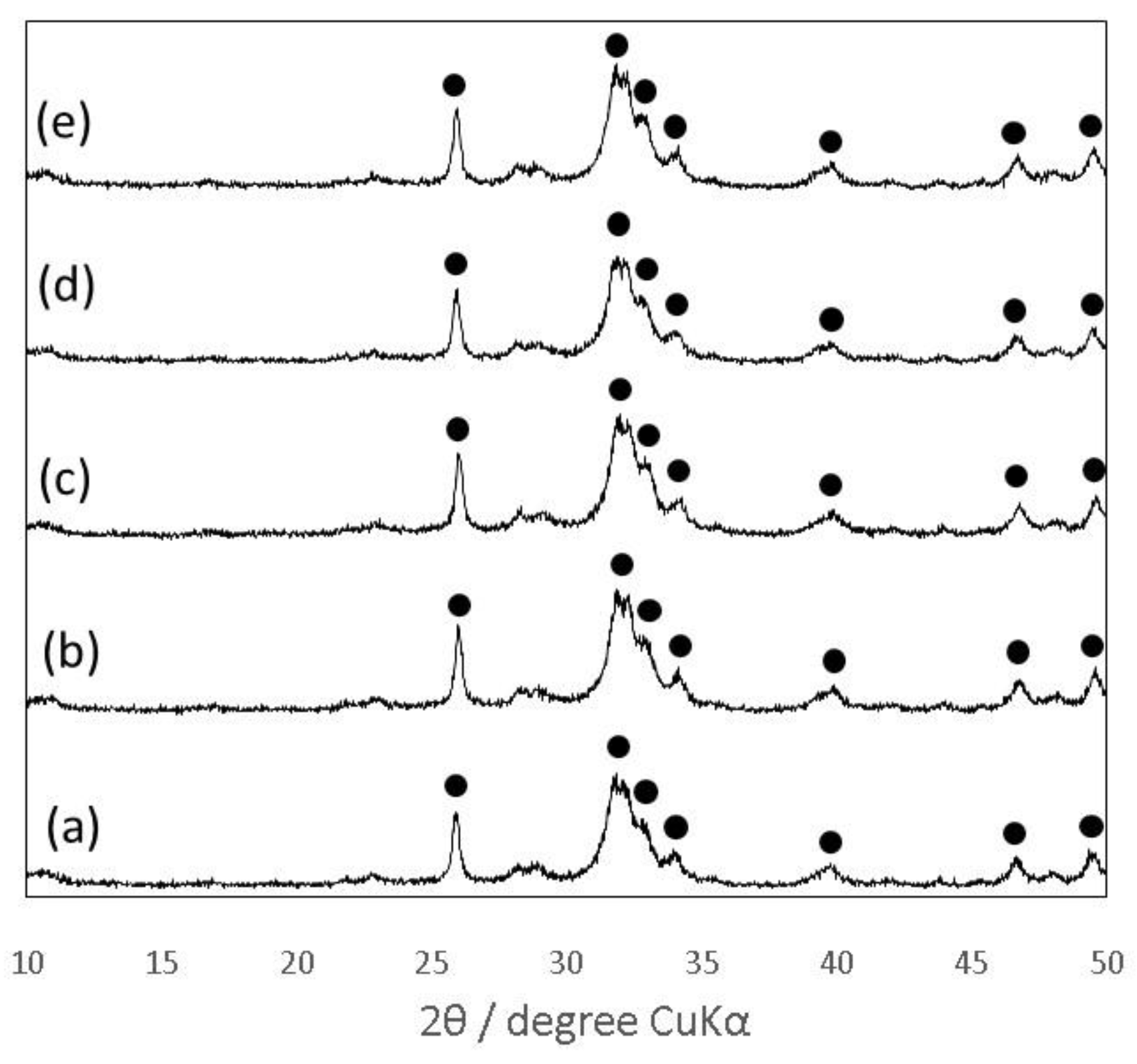
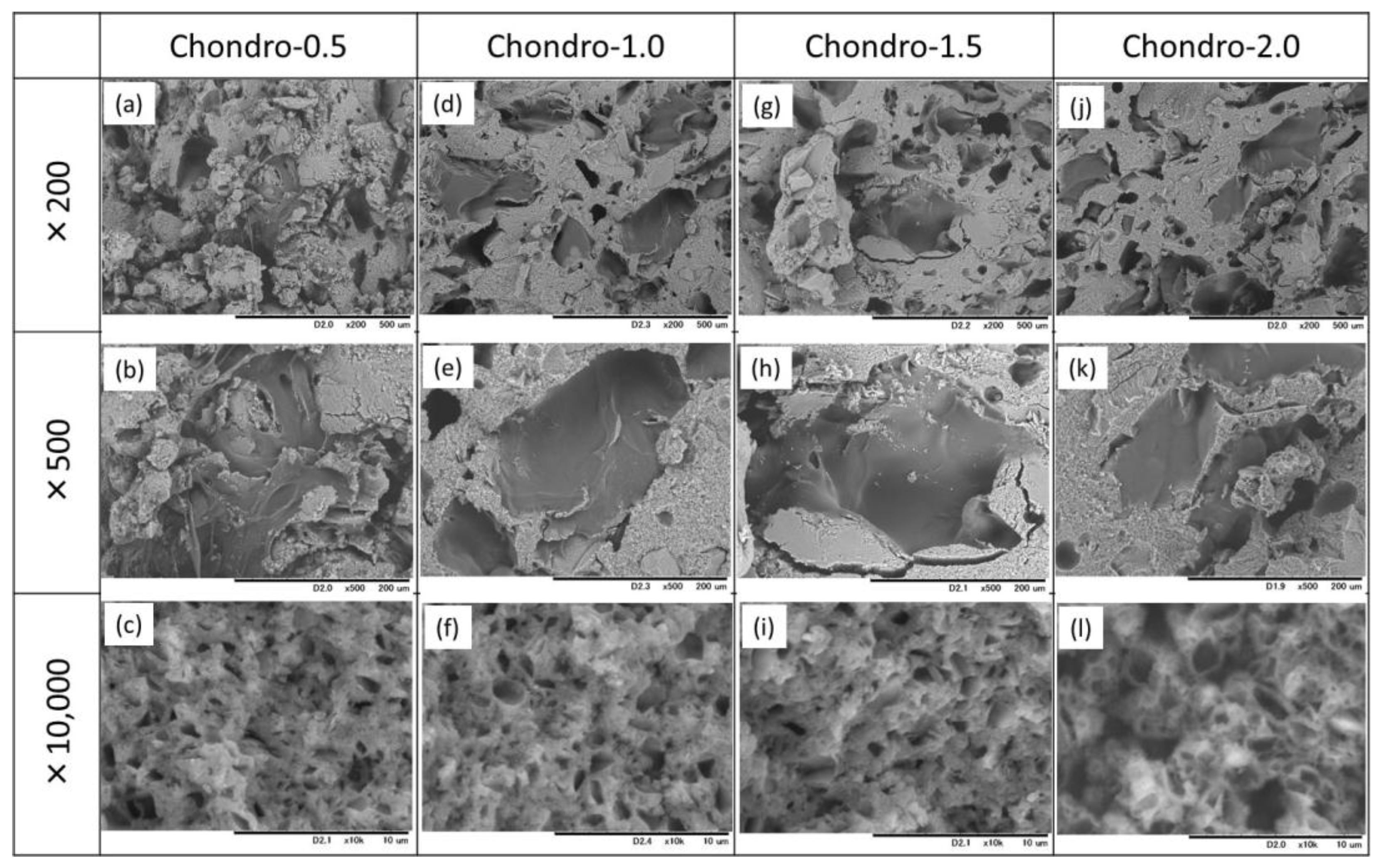

| Abbreviation | Chitosan (Mass%) | Sodium Alginate (Mass%) | Chondroitin 6-Sulfate Sodium Salt (Mass%) | Citric Acid (Mass%) | Sodium Dihydrogen Phosphate (Mass%) |
|---|---|---|---|---|---|
| Chito | 10 | 0 | 0 | 0 | 2.5 |
| Alg | 0 | 1.0 | 0 | 0 | 2.5 |
| Chondro | 0 | 0 | 15 | 0 | 2.5 |
| Chondro-0.5 | 0 | 0 | 15 | 0.5 | 2.5 |
| Chondro-1.0 | 0 | 0 | 15 | 1.0 | 2.5 |
| Chondro-1.5 | 0 | 0 | 15 | 1.5 | 2.5 |
| Chondro-2.0 | 0 | 0 | 15 | 2.0 | 2.5 |
| Abbreviation | Amount of the Hybridized Gelatin Particles (Mass%) | |||
|---|---|---|---|---|
| 0 | 5 | 10 | 15 | |
| Chito | 1/0.70 | 1/0.82 | 1/0.82 | 1/0.82 |
| Alg | 1/0.70 | 1/0.70 | 1/0.82 | 1/0.94 |
| Chondro | 1/0.70 | 1/0.70 | 1/0.70 | 1/0.70 |
| Chondro-0.5 | ----- | 1/0.70 | 1/0.70 | 1/0.74 |
| Chondro-1.0 | ----- | 1/0.70 | 1/0.70 | 1/0.70 |
| Chondro-1.5 | ----- | 1/0.62 | 1/0.62 | 1/0.70 |
| Chondro-2.0 | ----- | 1/0.62 | 1/0.62 | 1/0.60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiminami, K.; Konishi, T.; Mizumoto, M.; Nagata, K.; Honda, M.; Arimura, H.; Aizawa, M. Effects of Adding Polysaccharides and Citric Acid into Sodium Dihydrogen Phosphate Mixing Solution on the Material Properties of Gelatin-Hybridized Calcium-Phosphate Cement. Materials 2017, 10, 941. https://doi.org/10.3390/ma10080941
Kiminami K, Konishi T, Mizumoto M, Nagata K, Honda M, Arimura H, Aizawa M. Effects of Adding Polysaccharides and Citric Acid into Sodium Dihydrogen Phosphate Mixing Solution on the Material Properties of Gelatin-Hybridized Calcium-Phosphate Cement. Materials. 2017; 10(8):941. https://doi.org/10.3390/ma10080941
Chicago/Turabian StyleKiminami, Keishi, Toshiisa Konishi, Minori Mizumoto, Kohei Nagata, Michiyo Honda, Hidetoshi Arimura, and Mamoru Aizawa. 2017. "Effects of Adding Polysaccharides and Citric Acid into Sodium Dihydrogen Phosphate Mixing Solution on the Material Properties of Gelatin-Hybridized Calcium-Phosphate Cement" Materials 10, no. 8: 941. https://doi.org/10.3390/ma10080941




